Stimulus-Responsive, Gelatin-Containing Supramolecular Nanofibers as Switchable 3D Microenvironments for Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
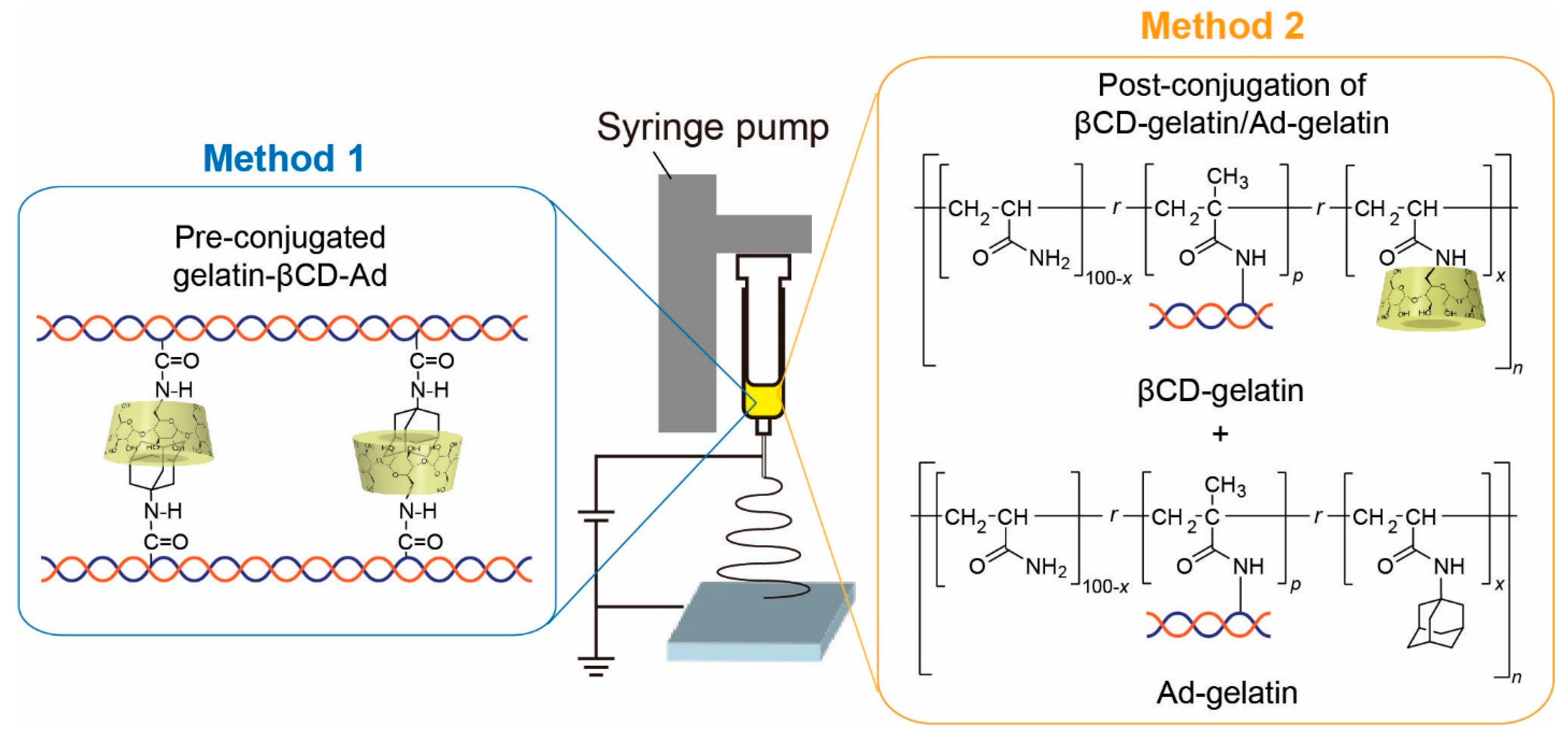
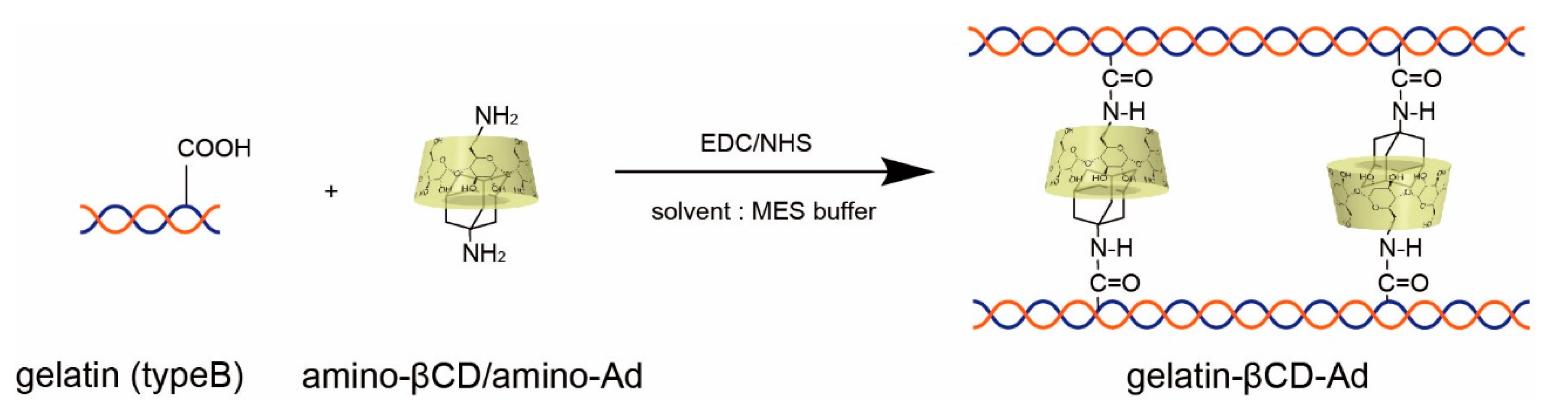
2.2.1. Pre-Conjugated Gelatin-βCD-Ad (Method 1)
2.2.2. Post-Conjugation of βCD-Gelatin and Ad-Gelatin (Method 2)
2.3. Gel Permeation Chromatography (GPC)
2.4. Electrospinning of Gelatin-Containing Fibers
2.4.1. Electrospinning of Gelatin-βCD-Ad Fibers (Method 1)
2.4.2. Electrospinning of βCD-Gelatin/Ad-Gelatin Fibers (Method 2)
2.5. Microscopic Imaging of Electrospun Nanofibers
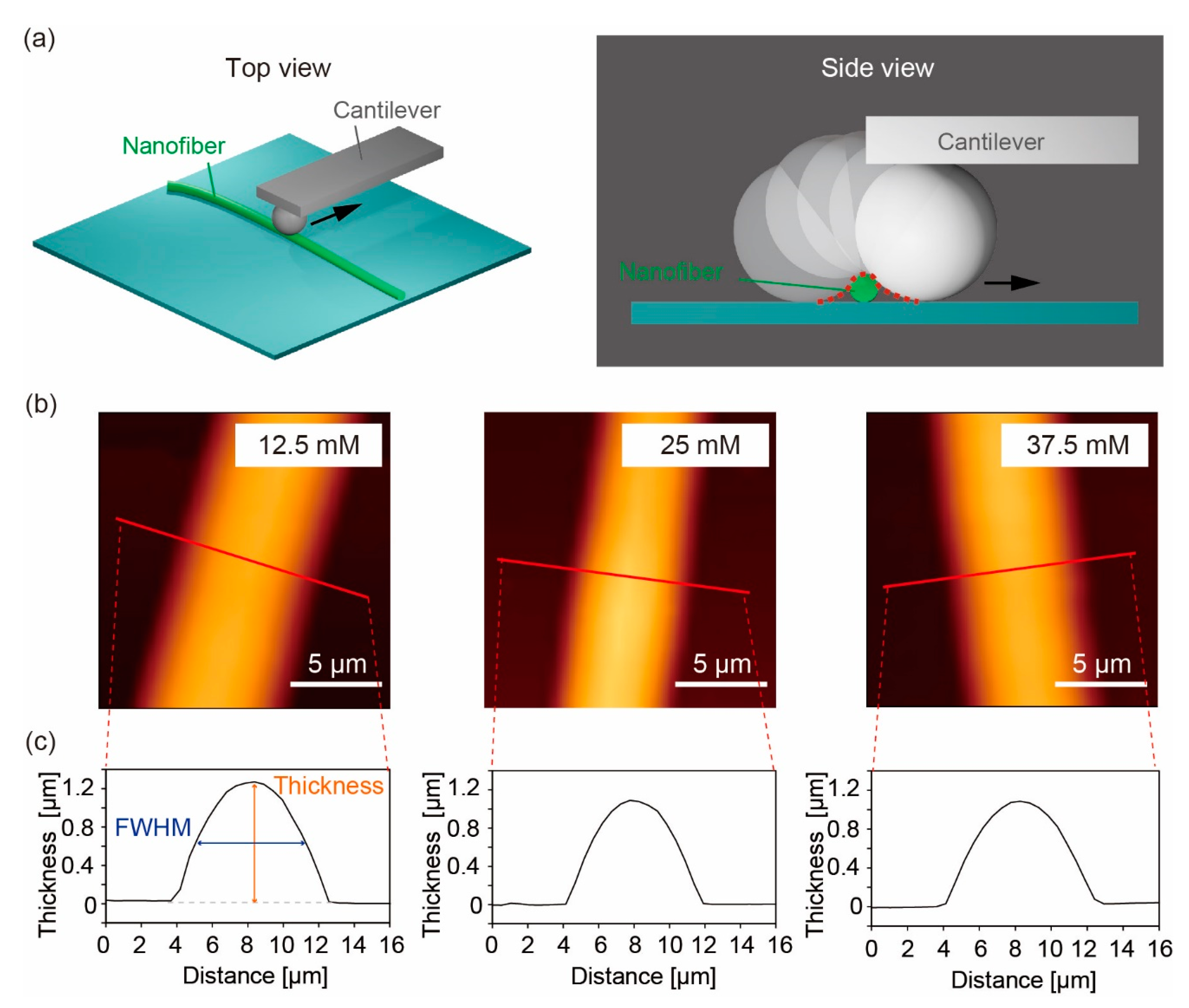
2.6. AFM Nano-Indentation
3. Results and Discussion
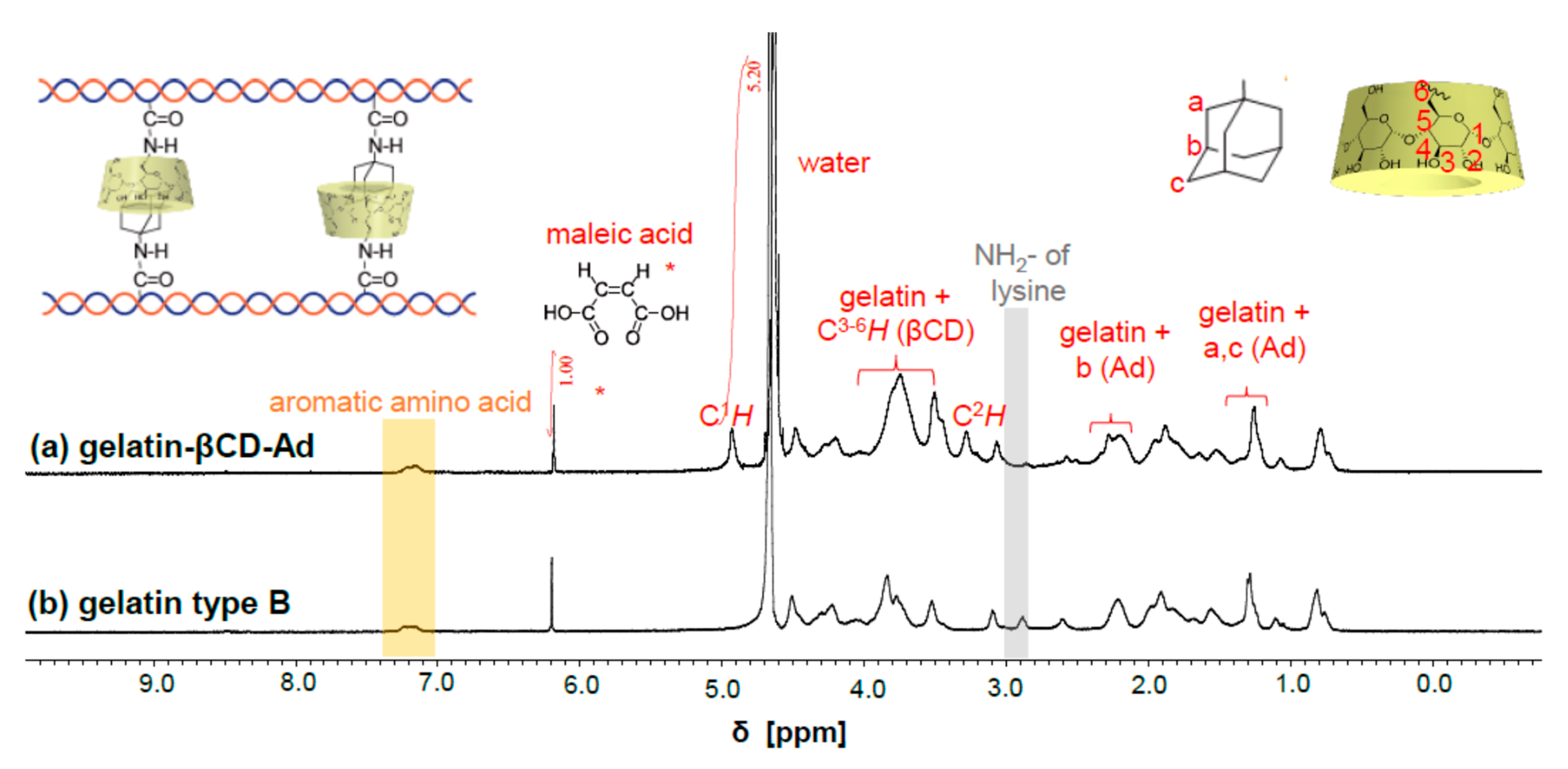
3.1. Characterization of Chemical Components of Gelatin-βCD-Ad
3.2. Characterization of Chemical Components of βCD-Gelatin and Ad-Gelatin
3.3. Optical Microscopy Images of Gelatin-βCD-Ad Fibers (Method 1)
3.4. Topography and Mechanical Properties of Pre-Conjugated Gelatin-βCD (Method 1)
3.5. Topography and Mechanical Properties of Post-Conjugated βCD-Gelatin and Ad-Gelatin (Method 2)
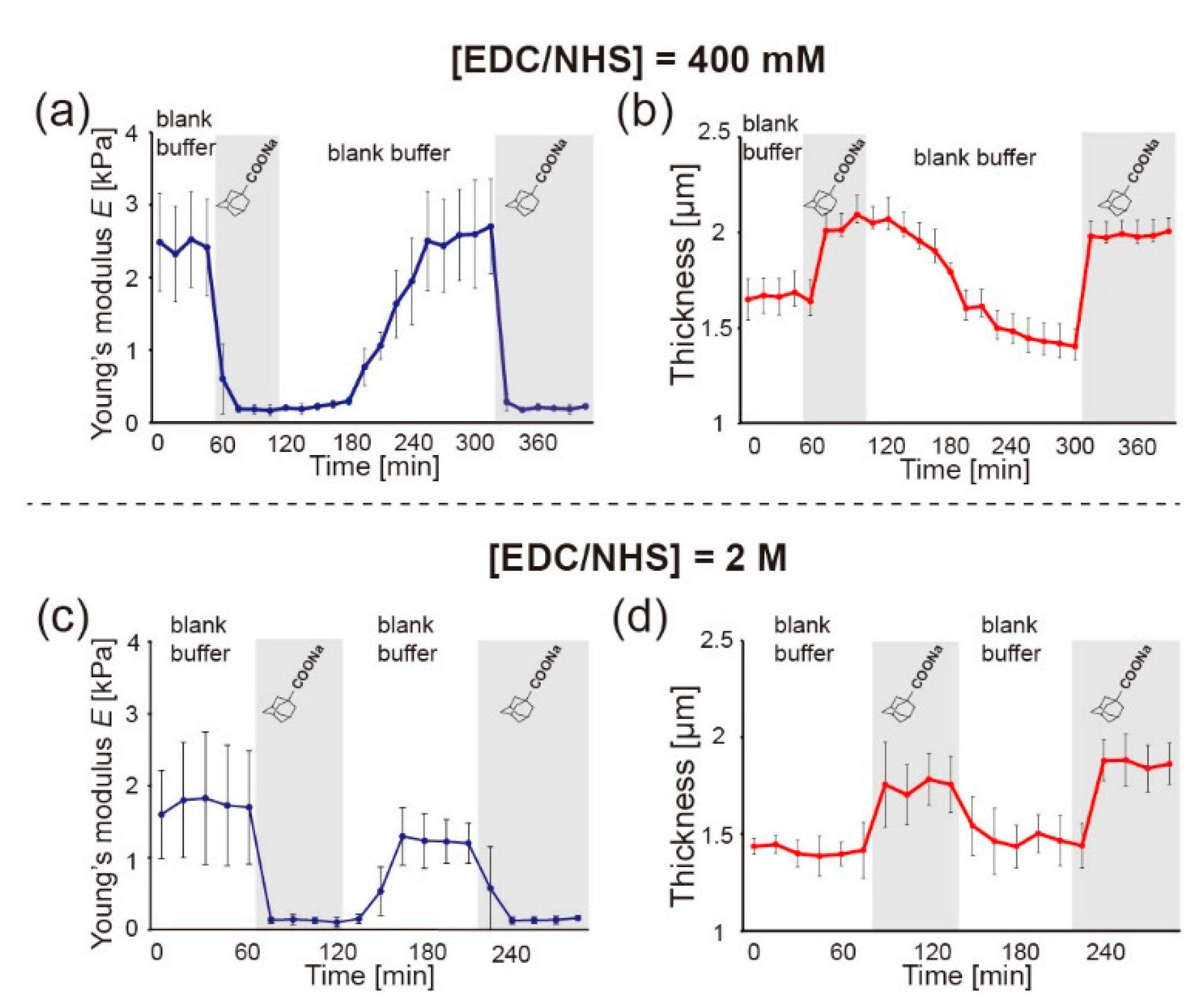
3.6. Reversible Switching of Young’s Modulus of βCD-Gelatin/Ad-Gelatin Fibers (Method 2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, K.; Mazo, I.; Leung, H.; Engelke, K.; von Andrian, U.H.; Deryugina, E.I.; Strongin, A.Y.; Brocker, E.-B.; Friedl, P. Compensation mechanism in tumor cell migration: Mesenchymal–amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 2003, 160, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Fattet, L.; Jung, H.-Y.; Matsumoto, M.W.; Aubol, B.E.; Kumar, A.; Adams, J.A.; Chen, A.C.; Sah, R.L.; Engler, A.J.; Pasquale, E.B.; et al. Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex. Dev. Cell 2020, 54, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.S.; Günay, K.A.; Cutler, A.A.; Vogler, T.O.; Brown, T.E.; Pawlikowski, B.T.; Bednarski, O.J.; Bannister, K.L.; Rogowski, C.J.; Mckay, A.G.; et al. Injury-mediated stiffening persistently activates muscle stem cells through YAP and TAZ mechanotransduction. Sci. Adv. 2021, 7, eabe4501. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bonnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Recent Progress in Cartilage Lubrication. Adv. Mater. 2021, 33, 2005513. [Google Scholar] [CrossRef]
- Tonti, O.R.; Larson, H.; Lipp, S.N.; Luetkemeyer, C.M.; Makam, M.; Vargas, D.; Wilcox, S.M.; Calve, S. Tissue-specific parameters for the design of ECM-mimetic biomaterials. Acta Biomater. 2021, 132, 83–102. [Google Scholar] [CrossRef]
- Leclercq, B.; Mejlachowicz, D.; Behar-Cohen, F. Ocular Barriers and Their Influence on Gene Therapy Products Delivery. Pharmaceutics 2022, 14, 998. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, W.; Lu, Q.; Ma, Q.; Kaplan, D.L.; Zhu, H. Silk nanofiber hydrogels with tunable modulus to regulate nerve stem cell fate. J. Mater. Chem. B 2014, 2, 6590–6600. [Google Scholar] [CrossRef]
- Bentele, T.; Amadei, F.; Kimmle, E.; Veschgini, M.; Linke, P.; Sontag-González, M.; Tennigkeit, J.; Ho, A.D.; Özbek, S.; Tanaka, M. New Class of Crosslinker-Free Nanofiber Biomaterials from Hydra Nematocyst Proteins. Sci. Rep. 2019, 9, 19116–19119. [Google Scholar] [CrossRef]
- Xue, J.; Pisignano, D.; Xia, Y. Maneuvering the Migration and Differentiation of Stem Cells with Electrospun Nanofibers. Adv. Sci. 2020, 7, 2000735. [Google Scholar] [CrossRef]
- Ehrmann, A. Non-Toxic Crosslinking of Electrospun Gelatin Nanofibers for Tissue Engineering and Biomedicine—A Review. Polymers 2021, 13, 1973. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Chang, W.-H.; Dong, G.-C.; Chen, K.-Y.; Chen, Y.-S.; Yao, C.-H. Cell adhesion and proliferation enhancement by gelatin nanofiber scaffolds. J. Bioact. Compat. Polym. 2011, 26, 565–577. [Google Scholar] [CrossRef]
- Liu, L.; Yoshioka, M.; Nakajima, M.; Ogasawara, A.; Liu, J.; Hasegawa, K.; Li, S.; Zou, J.; Nakatsuji, N.; Kamei, K.-I.; et al. Nanofibrous gelatin substrates for long-term expansion of human pluripotent stem cells. Biomaterials 2014, 35, 6259–6267. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, J.; Hong, J.; Takashima, Y.; Fujimoto, N.; Nakajima, M.; Yamamoto, A.; Dong, X.; Dang, Y.; Hou, Y.; et al. Low cell-matrix adhesion reveals two subtypes of human pluripotent stem cells. Stem Cell Rep. 2018, 11, 142–156. [Google Scholar] [CrossRef]
- Huang, D.; Nakamura, Y.; Ogata, A.; Kidoaki, S. Characterization of 3D matrix conditions for cancer cell migration with elasticity/porosity-independent tunable microfiber gels. Polym. J. 2020, 52, 333–344. [Google Scholar] [CrossRef]
- Wang, W.Y.; Davidson, C.D.; Lin, D.; Baker, B.M. Actomyosin contractility-dependent matrix stretch and recoil induces rapid cell migration. Nat. Commun. 2019, 10, 1186. [Google Scholar] [CrossRef]
- Gharib, S.A.; Manicone, A.M.; Parks, W.C. Matrix metalloproteinases in emphysema. Matrix Biol. 2018, 73, 34–51. [Google Scholar] [CrossRef]
- Lambertenghi-Deliliers, G.; Orazi, A.; Luksch, R.; Annaloro, C.; Soligo, D. Myelodysplastic syndrome with increased marrow fibrosis: A distinct clinico-pathological entity. Br. J. Haematol. 1991, 78, 161–166. [Google Scholar] [CrossRef]
- Palmquist, K.H.; Tiemann, S.F.; Ezzeddine, F.L.; Yang, S.; Pfeifer, C.R.; Erzberger, A.; Rodrigues, A.R.; Shyer, A.E. Reciprocal cell-ECM dynamics generate supracellular fluidity underlying spontaneous follicle patterning. Cell 2022, 185, 1960–1973. [Google Scholar] [CrossRef]
- Yoshikawa, H.Y.; Rossetti, F.F.; Kaufmann, S.; Kaindl, T.; Madsen, J.; Engel, U.; Lewis, A.L.; Armes, S.P.; Tanaka, M. Quantitative Evaluation of Mechanosensing of Cells on Dynamically Tunable Hydrogels. J. Am. Chem. Soc. 2011, 133, 1367–1374. [Google Scholar] [CrossRef]
- Frank, V.; Kaufmann, S.; Wright, R.; Horn, P.; Yoshikawa, H.Y.; Wuchter, P.; Madsen, J.; Lewis, A.L.; Armes, S.P.; Ho, A.D.; et al. Frequent mechanical stress suppresses proliferation of mesenchymal stem cells from human bone marrow without loss of multipotency. Sci. Rep. 2016, 6, 24264. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Preorganized Hydrogel: Self-Healing Properties of Supramolecular Hydrogels Formed by Polymerization of Host–Guest-Monomers that Contain Cyclodextrins and Hydrophobic Guest Groups. Adv. Mater. 2013, 25, 2849–2853. [Google Scholar] [CrossRef] [PubMed]
- Hörning, M.; Nakahata, M.; Linke, P.; Yamamoto, A.; Veschgini, M.; Kaufmann, S.; Takashima, Y.; Harada, A.; Tanaka, M. Dynamic mechano-regulation of myoblast cells on supramolecular hydrogels cross-linked by reversible host-guest interactions. Sci. Rep. 2017, 7, 7660. [Google Scholar] [CrossRef] [PubMed]
- Hippler, M.; Weißenbruch, K.; Richler, K.; Lemma, E.D.; Nakahata, M.; Richter, B.; Barner-Kowollik, C.; Takashima, Y.; Harada, A.; Blasco, E. Mechanical stimulation of single cells by reversible host-guest interactions in 3D microscaffolds. Sci. Adv. 2020, 6, eabc2648. [Google Scholar] [CrossRef]
- Hayashi, K.; Matsuda, M.; Mitake, N.; Nakahata, M.; Munding, N.; Harada, A.; Kaufmann, S.; Takashima, Y.; Tanaka, M. One-Step Synthesis of Gelatin-Conjugated Supramolecular Hydrogels for Dynamic Regulation of Adhesion Contact and Morphology of Myoblasts. ACS Appl. Polym. Mater. 2022, 4, 2595–2603. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Iványi, R. Catalytic transfer hydrogenation of sugar derivatives. Carbohydr. Polym. 2001, 45, 139–145. [Google Scholar] [CrossRef]
- Melton, L.D.; Slessor, K.N. Synthesis of monosubstituted cyclohexaamyloses. Carbohydr. Res. 1971, 18, 29–37. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef]
- Harada, A.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H. Macroscopic self-assembly through molecular recognition. Nat. Chem. 2011, 3, 34–37. [Google Scholar] [CrossRef]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Supramolecular self-healing materials from non-covalent cross-linking host–guest interactions. Chem. Commun. 2020, 56, 4381–4395. [Google Scholar] [CrossRef]
- Amonpattaratkit, P.; Khunmanee, S.; Kim, D.H.; Park, H. Synthesis and Characterization of Gelatin-Based Crosslinkers for the Fabrication of Superabsorbent Hydrogels. Materials 2017, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhao, M.; Li, L.; Sun, S.; Yu, H.; Cheng, Y.; Yang, Y.; Fan, Y.; Sun, Y. Preparation and Properties of Double-Crosslinked Hydroxyapatite Composite Hydrogels. Int. J. Mol. Sci. 2022, 23, 9962. [Google Scholar] [CrossRef] [PubMed]
- Baur, B.; Steinhoff, G.; Hernando, J.; Purrucker, O.; Tanaka, M.; Nickel, B.; Stutzmann, M.; Eickhoff, M. Chemical functionalization of GaN and AlN surfaces. Appl. Phys. Lett. 2005, 87, 263901. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.M.; Krijgsveld, J.; Zaat, S.A.J.; Dankert, J.; Feijen, J. Cross-linking and characterisation of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. Ed. 2000, 11, 225–243. [Google Scholar] [CrossRef]
- Johnson, K.L. Contact Mechanics; Cambridge University Press: Cambridge, MA, USA, 1985. [Google Scholar]
- de Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Erencia, M.; Cano, F.; Tornero, J.A.; Fernandes, M.M.; Tzanov, T.; Macanás, J.; Carrillo, F. Electrospinning of gelatin fibers using solutions with low acetic acid concentration: Effect of solvent composition on both diameter of electrospun fibers and cytotoxicity. J. Appl. Polym. Sci. 2015, 132, 42115. [Google Scholar] [CrossRef]
- Van Hoorick, J.; Gruber, P.; Markovic, M.; Tromayer, M.; Van Erps, J.; Thienpont, H.; Liska, R.; Ovsianikov, A.; Dubruel, P.; Van Vlierberghe, S. Cross-Linkable Gelatins with Superior Mechanical Properties through Carboxylic Acid Modification: Increasing the Two-Photon Polymerization Potential. Biomacromolecules 2017, 18, 3260–3272. [Google Scholar] [CrossRef]
- Hippler, M.; Blasco, E.; Qu, J.; Tanaka, M.; Barner-Kowollik, C.; Wegener, M.; Bastmeyer, M. Controlling the shape of 3D microstructures by temperature and light. Nat. Commun. 2019, 10, 232. [Google Scholar] [CrossRef]





| EDC [mM] | NHS [mM] | D [µm] | W [µm] | E [kPa] | |
|---|---|---|---|---|---|
| Gelatin-βCD-Ad12.5 | 12.5 | 12.5 | 1.3 ± 0.1 | 1.6 ± 0.3 | 16.3 ± 0.2 |
| Gelatin-βCD-Ad25.0 | 25.0 | 25.0 | 1.1 ± 0.1 | 1.2 ± 0.1 | 31.6 ± 3.0 |
| Gelatin-βCD-Ad37.5 | 37.5 | 37.5 | 1.1 ± 0.0 | 1.6 ± 0.1 | 42.0 ± 1.2 |
| EDC [mM] | NHS [mM] | D [µm] | W [µm] | E [kPa] | |
|---|---|---|---|---|---|
| βCD-gelatin/Ad-gelatin200 | 200 | 200 | 1.9 ± 0.4 | 3.0 ± 0.6 | 1.0 ± 0.4 |
| βCD-gelatin/Ad-gelatin400 | 400 | 400 | 1.1 ± 0.3 | 3.3 ± 0.7 | 2.3 ± 0.8 |
| βCD-gelatin/Ad-gelatin2000 | 2000 | 2000 | 1.3 ± 0.4 | 2.8 ± 1.3 | 1.6 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, K.; Matsuda, M.; Nakahata, M.; Takashima, Y.; Tanaka, M. Stimulus-Responsive, Gelatin-Containing Supramolecular Nanofibers as Switchable 3D Microenvironments for Cells. Polymers 2022, 14, 4407. https://doi.org/10.3390/polym14204407
Hayashi K, Matsuda M, Nakahata M, Takashima Y, Tanaka M. Stimulus-Responsive, Gelatin-Containing Supramolecular Nanofibers as Switchable 3D Microenvironments for Cells. Polymers. 2022; 14(20):4407. https://doi.org/10.3390/polym14204407
Chicago/Turabian StyleHayashi, Kentaro, Mami Matsuda, Masaki Nakahata, Yoshinori Takashima, and Motomu Tanaka. 2022. "Stimulus-Responsive, Gelatin-Containing Supramolecular Nanofibers as Switchable 3D Microenvironments for Cells" Polymers 14, no. 20: 4407. https://doi.org/10.3390/polym14204407
APA StyleHayashi, K., Matsuda, M., Nakahata, M., Takashima, Y., & Tanaka, M. (2022). Stimulus-Responsive, Gelatin-Containing Supramolecular Nanofibers as Switchable 3D Microenvironments for Cells. Polymers, 14(20), 4407. https://doi.org/10.3390/polym14204407








