A Multifunctional Conjugated Polymer Developed as an Efficient System for Differentiation of SH-SY5Y Tumour Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis of the Poly Vinyl-Acetate
2.4. Synthesis of Copoly-Vinyl Acetate-Vinyl Alcohol
2.5. Synthesis of Polyvinyl Alcohol
2.6. Synthesis of the Copolymer CoVAc-VA Functionalised with Rhodamine B
2.7. One-Pot Synthesis of the Water-Soluble Polymer Conjugates
2.8. Cell Cultures
2.9. Confocal Microscopy Analysis and Differentiation of SH-SY5H Cells
2.10. Cell Viability Assay
3. Results and Discussion
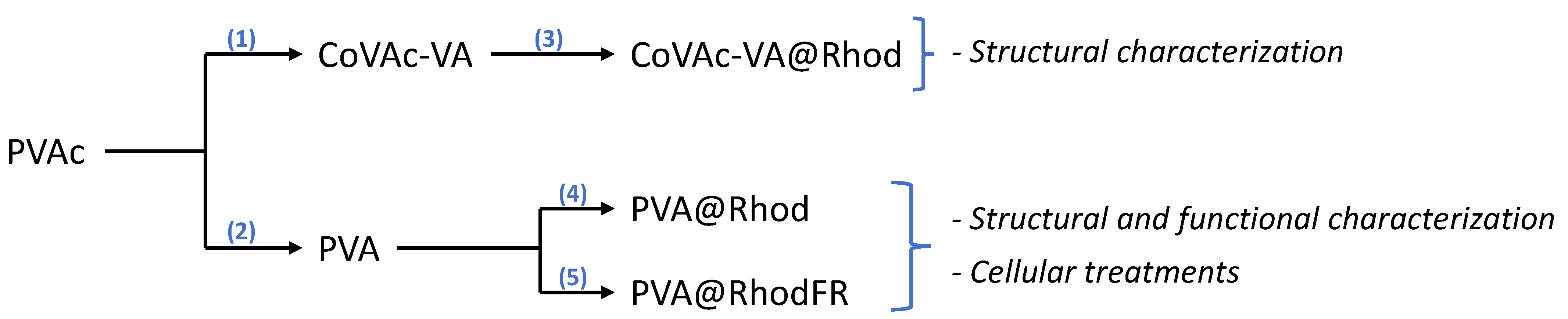
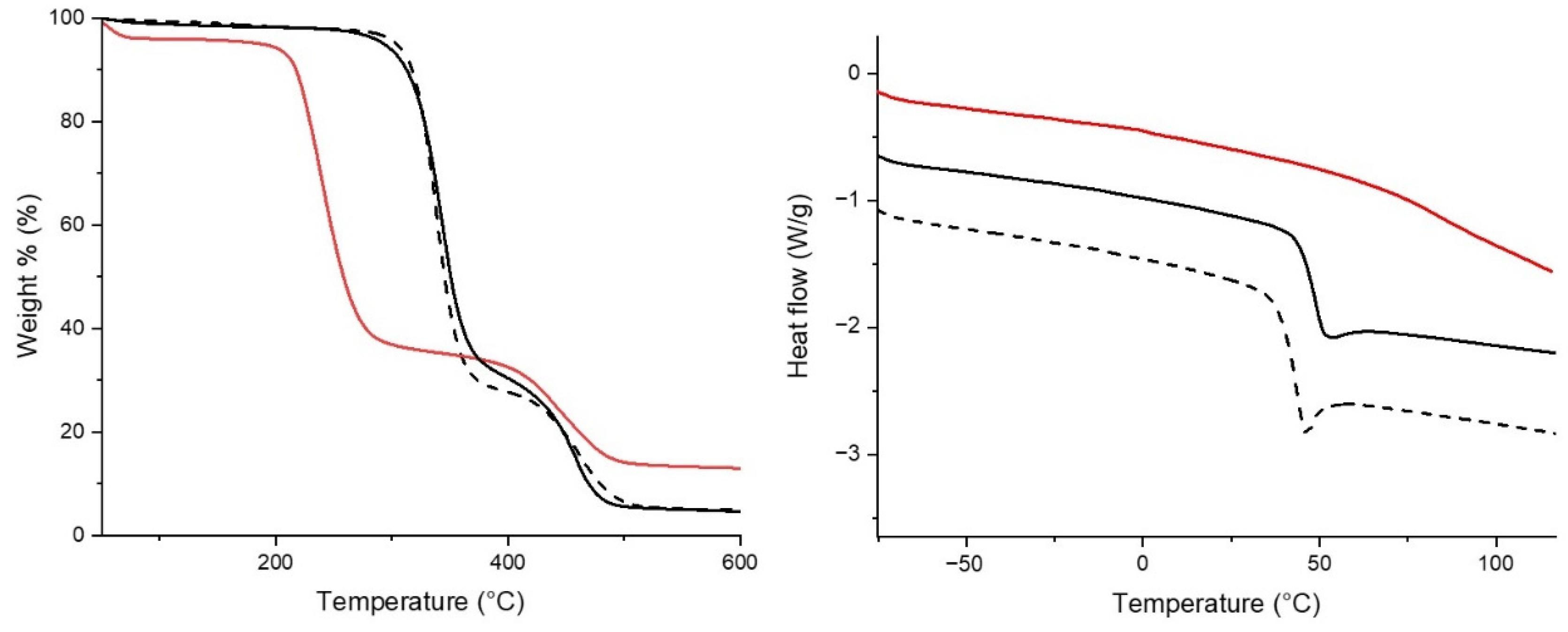
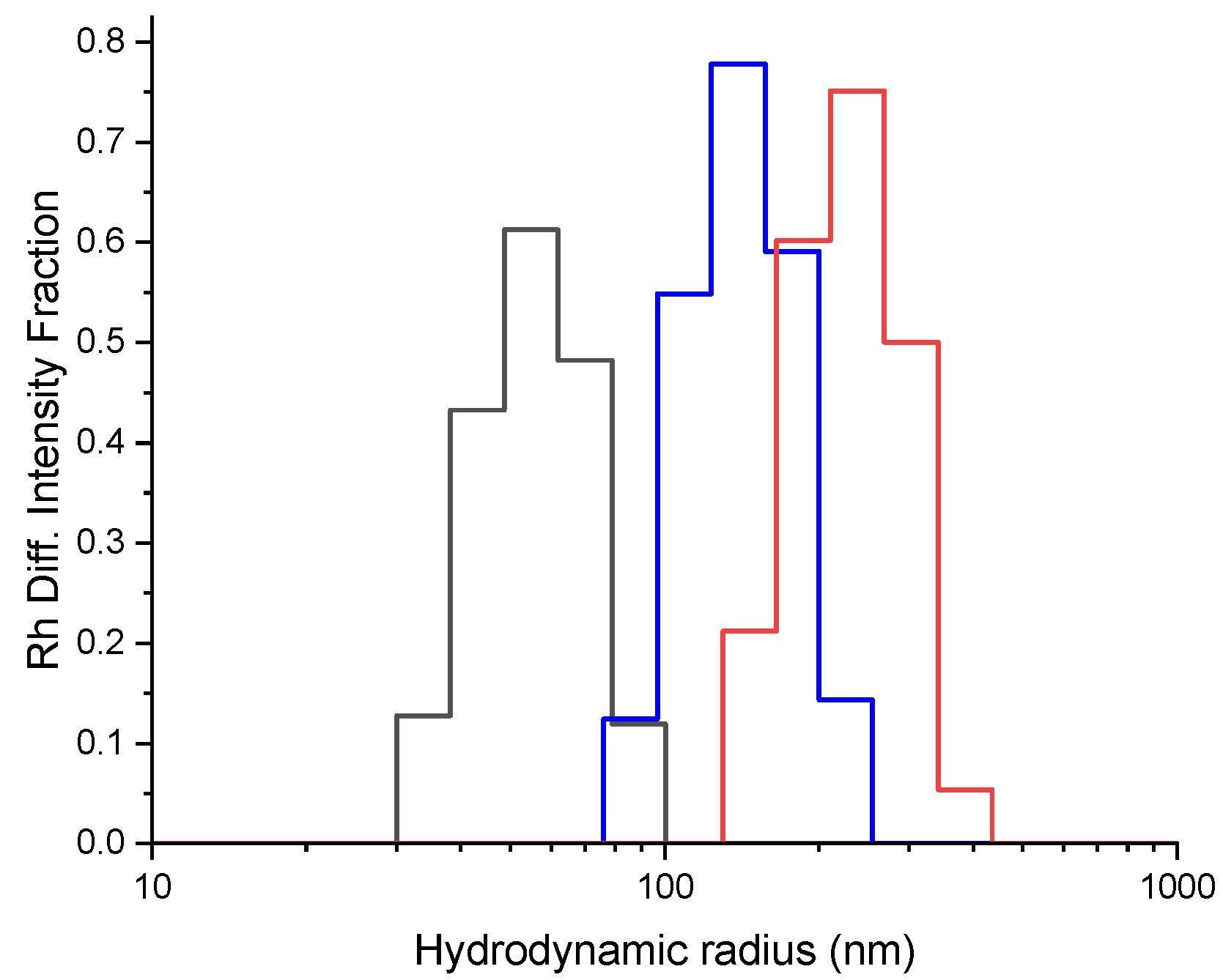
3.1. Synthesis and Characterisation of the Polymer Conjugate
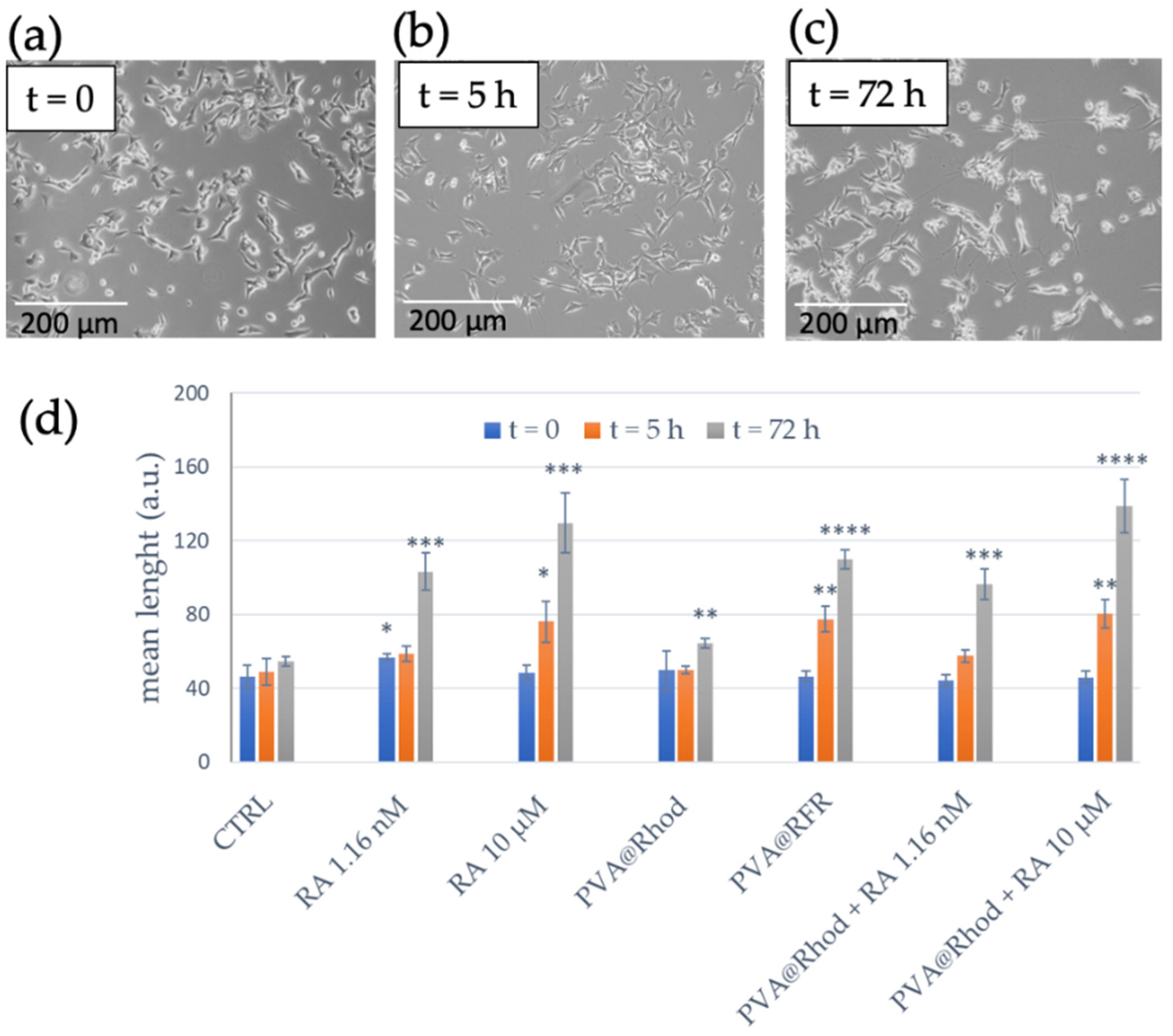
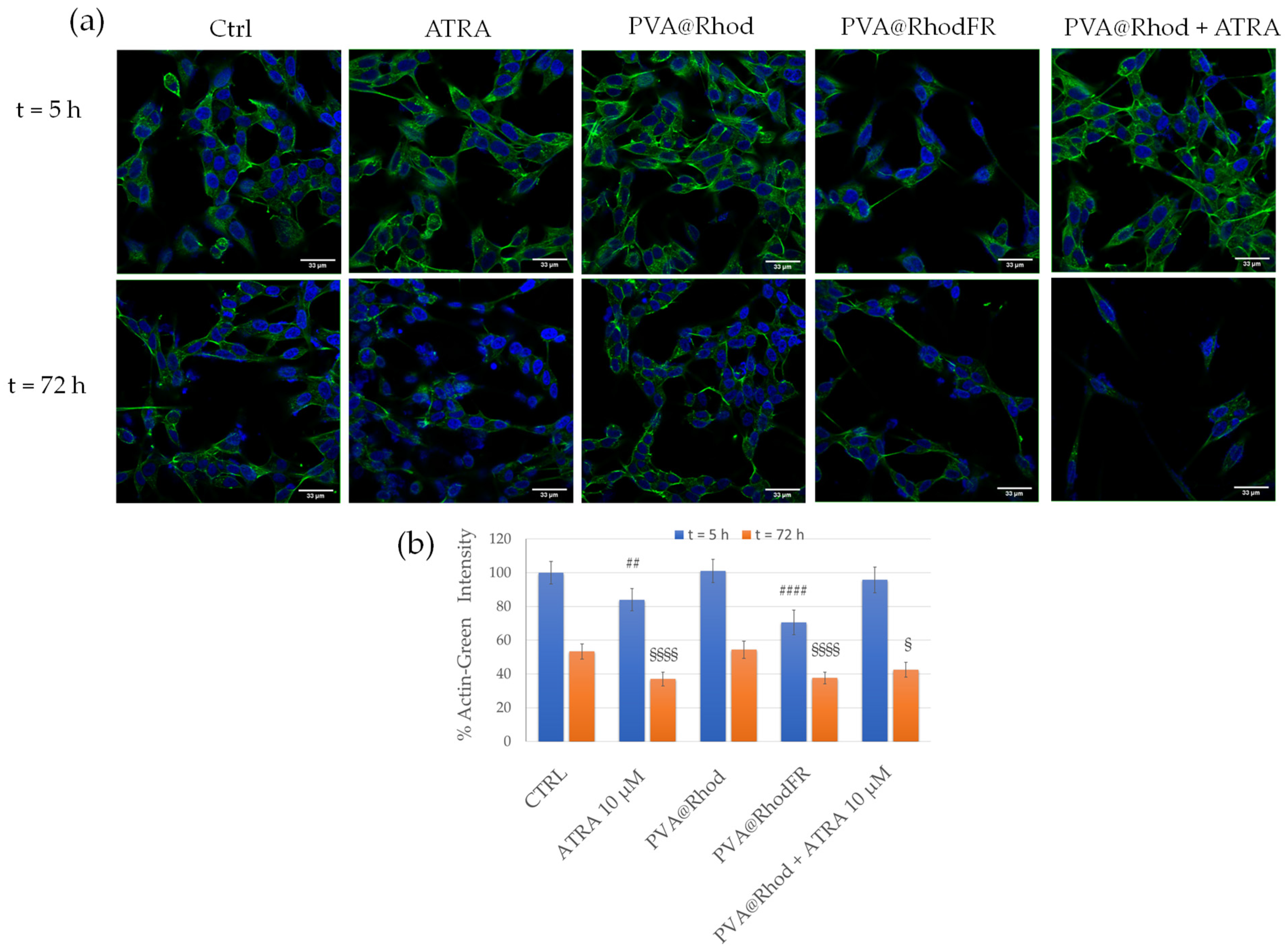
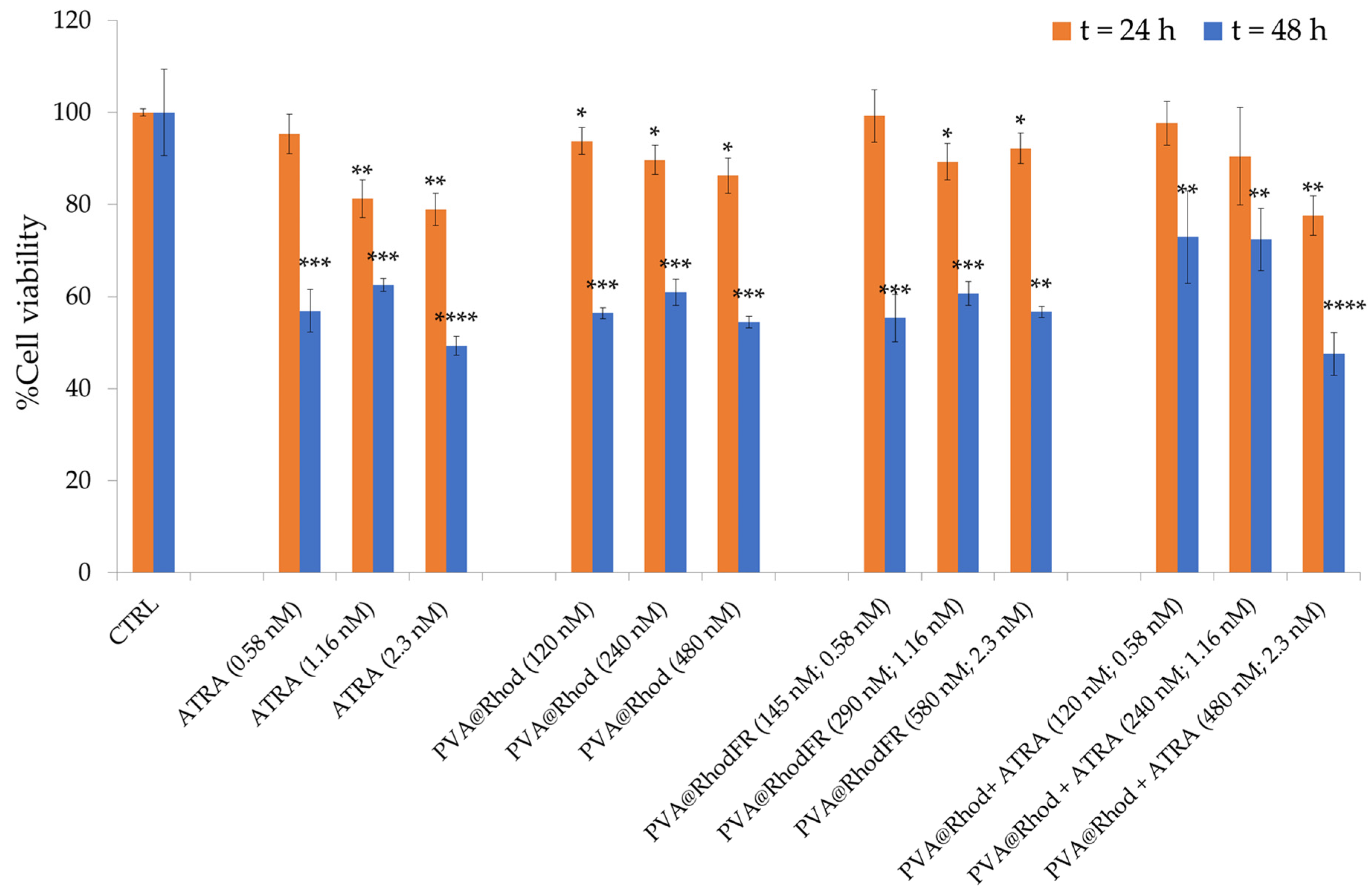
3.2. Differentiation of SH-SY5Y Tumour Cells to Neuron-like Cells and Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Wu, H.; Feng, C.; Xiao, K.; Yang, X.; Liu, Q.; Lin, T.-Y.; Zhang, H.; Walton, J.H.; Ajena, Y.; et al. “One-Pot” Fabrication of Highly Versatile and Biocompatible Poly(vinyl alcohol)-porphyrin-based Nanotheranostics. Theranostics 2017, 7, 3901–3914. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, P.; Strano, G.; Zuccarello, L.; Satriano, C. Gold and Silver Nanoparticles for Applications in Theranostics. Curr. Top. Med. Chem. 2016, 16, 3069–3102. [Google Scholar] [CrossRef]
- Tomasella, P.; Sanfilippo, V.; Bonaccorso, C.; Cucci, L.M.; Consiglio, G.; Nicosia, A.; Mineo, P.G.; Forte, G.; Satriano, C. Theranostic Nanoplatforms of Thiolated Reduced Graphene Oxide Nanosheets and Gold Nanoparticles. Appl. Sci. 2020, 10, 5529. [Google Scholar] [CrossRef]
- Mineo, P.G.; Abbadessa, A.; Rescifina, A.; Mazzaglia, A.; Nicosia, A.; Scamporrino, A.A. PEGylate porphyrin-gold nanoparticles conjugates as removable pH-sensor nano-probes for acidic environments. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 40–47. [Google Scholar] [CrossRef]
- Mineo, P.; Abbadessa, A.; Mazzaglia, A.; Gulino, A.; Villari, V.; Micali, N.; Millesi, S.; Satriano, C.; Scamporrino, E. Gold nanoparticles functionalized with PEGylate uncharged porphyrins. Dye. Pigment. 2017, 141, 225–234. [Google Scholar] [CrossRef]
- Piperno, A.; Mazzaglia, A.; Scala, A.; Pennisi, R.; Zagami, R.; Neri, G.; Torcasio, S.M.; Rosmini, C.; Mineo, P.G.; Potara, M.; et al. Casting Light on Intracellular Tracking of a New Functional Graphene-Based MicroRNA Delivery System by FLIM and Raman Imaging. ACS Appl. Mater. Interfaces 2019, 11, 46101–46111. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Demetzos, C. Promising Nanotechnology Approaches in Treatment of Autoimmune Diseases of Central Nervous System. Brain Sci. 2020, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, A.; Abbadessa, A.; Vento, F.; Mazzaglia, A.; Mineo, P.G. Silver Nanoparticles Decorated with PEGylated Porphyrins as Potential Theranostic and Sensing Agents. Materials 2021, 14, 2764. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Palomba, F.; Rampazzo, E.; Zaccheroni, N.; Malferrari, M.; Rapino, S.; Greco, V.; Satriano, C.; Genovese, D.; Prodi, L. Specific, Surface-Driven, and High-Affinity Interactions of Fluorescent Hyaluronan with PEGylated Nanomaterials. ACS Appl. Mater. Interfaces 2020, 12, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, A.; Vento, F.; Satriano, C.; Villari, V.; Micali, N.; Cucci, L.M.; Sanfilippo, V.; Mineo, P.G. Light-Triggered Polymeric Nanobombs for Targeted Cell Death. ACS Appl. Nano Mater. 2020, 3, 1950–1960. [Google Scholar] [CrossRef]
- Mineo, P.; Faggio, C.; Micali, N.; Scamporrino, E.; Villari, V. A star polymer based on a polyethylene glycol with a porphyrinic core as a photosensitizing agent for application in photodynamic therapy: Tests in vitro on human erythrocytes. RSC Adv. 2014, 4, 19389–19395. [Google Scholar] [CrossRef]
- Sanfilippo, V.; Caruso, V.C.L.; Cucci, L.M.; Inturri, R.; Vaccaro, S.; Satriano, C. Hyaluronan-Metal Gold Nanoparticle Hybrids for Targeted Tumor Cell Therapy. Int. J. Mol. Sci. 2020, 21, 3085. [Google Scholar] [CrossRef]
- Duncan, R.; Spreafico, F. Polymer Conjugates. Clin. Pharmacokinet. 1994, 27, 290–306. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Vicent, M.J.; Duncan, R. Polymer conjugates: Nanosized medicines for treating cancer. Trends Biotechnol. 2006, 24, 39–47. [Google Scholar] [CrossRef]
- Xu, B.; Watkins, R.; Wu, L.; Zhang, C.; Davis, R. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055. [Google Scholar] [CrossRef]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Impallomeni, G.; Mineo, P.G.; Vento, F.; Ballistreri, A. Novel pranoprofen-poly(ε-caprolactone) conjugates: Microwave-assisted synthesis and structural characterization. Polym. Int. 2020, 70, 604–611. [Google Scholar] [CrossRef]
- Scala, A.; Piperno, A.; Torcasio, S.M.; Nicosia, A.; Mineo, P.G.; Grassi, G. “Clickable” polylactic acids obtained by solvent free intra-chain amidation. Eur. Polym. J. 2018, 109, 341–346. [Google Scholar] [CrossRef]
- Scala, A.; Piperno, A.; Micale, N.; Mineo, P.G.; Abbadessa, A.; Risoluti, R.; Castelli, G.; Bruno, F.; Vitale, F.; Cascio, A.; et al. “Click” on PLGA-PEG and hyaluronic acid: Gaining access to anti-leishmanial pentamidine bioconjugates. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2778–2785. [Google Scholar] [CrossRef] [PubMed]
- Hyon, S.-H.; Cha, W.-I.; Ikada, Y.; Kita, M.; Ogura, Y.; Honda, Y. Poly(vinyl alcohol) hydrogels as soft contact lens material. J. Biomater. Sci. Polym. Ed. 1994, 5, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Yamamuro, T.; Oka, M.; Kumar, P.; Kotoura, Y.; Hyonyt, S.-H.; Ikadat, Y. Poly(vinyl alcohol) hydrogel as an artificial articular cartilage: Evaluation of biocompatibility. J. Appl. Biomater. 1991, 2, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, Y.; Kim, Y.; Hwang, Y.; Hwang, N. Biomimetically Reinforced Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Polymers 2017, 9, 655. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Balasubramaniam, M.P.; Murugan, P.; Chenthamara, D.; Ramakrishnan, S.G.; Salim, A.; Lin, F.-H.; Robert, B.; Subramaniam, S. Synthesis of chitosan-ferulic acid conjugated poly(vinyl alcohol) polymer film for an improved wound healing. Mater. Today Commun. 2020, 25, 101510. [Google Scholar] [CrossRef]
- Castleberry, S.A.; Quadir, M.A.; Sharkh, M.A.; Shopsowitz, K.E.; Hammond, P.T. Polymer conjugated retinoids for controlled transdermal delivery. J. Control. Release 2017, 262, 1–9. [Google Scholar] [CrossRef]
- Kaneo, Y.; Tanaka, T.; Yamamoto, S.; Kikkawa, C. Preparation and properties of acid-cleavable poly(vinyl alcohol)cis-aconityl-antitumor anthracycline conjugates. J. Drug Deliv. Sci. Technol. 2013, 23, 143–149. [Google Scholar] [CrossRef]
- Li, M.; Xu, X.; Lu, F.; Guo, S. Primaryin vitroandin vivoevaluation of norcantharidin-chitosan/poly (vinyl alcohol) for cancer treatment. Drug Deliv. 2013, 21, 293–301. [Google Scholar] [CrossRef]
- Kakinoki, A.; Kaneo, Y.; Ikeda, Y.; Tanaka, T.; Fujita, K. Synthesis of Poly(vinyl alcohol)-Doxorubicin Conjugates Containing cis-Aconityl Acid-Cleavable Bond and Its Isomer Dependent Doxorubicin Release. Biol. Pharm. Bull. 2008, 31, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, A.; Vento, F.; Pellegrino, A.L.; Ranc, V.; Piperno, A.; Mazzaglia, A.; Mineo, P. Polymer-Based Graphene Derivatives and Microwave-Assisted Silver Nanoparticles Decoration as a Potential Antibacterial Agent. Nanomaterials 2020, 10, 2269. [Google Scholar] [CrossRef]
- Li, J.K.; Wang, N.; Wu, X.S. Poly(vinyl alcohol) nanoparticles prepared by freezing–thawing process for protein/peptide drug delivery. J. Control. Release 1998, 56, 117–126. [Google Scholar] [CrossRef]
- Leamon, C.P.; Low, P.S. Delivery of macromolecules into living cells: A method that exploits folate receptor endocytosis. Proc. Natl. Acad. Sci. USA 1991, 88, 5572–5576. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Super pH-Sensitive Multifunctional Polymeric Micelle. Nano Lett. 2005, 5, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Sadhukha, T.; Ma, L.; Panyam, J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J. Control. Release 2009, 136, 21–29. [Google Scholar] [CrossRef]
- Fan, Z.-Y.; Zhao, Y.-L.; Zhu, X.-Y.; Luo, Y.; Shen, M.-W.; Shi, X.-Y. Folic acid modified electrospun poly(vinyl alcohol)/polyethyleneimine nanofibers for cancer cell capture applications. Chin. J. Polym. Sci. 2016, 34, 755–765. [Google Scholar] [CrossRef]
- Wang, D.-H.; Zhu, D.-J.; Ding, W.; Xue, M.; Yang, Y. Tuning the fluorescence of tetraphenylethylene in dilute solutions via modulating multiple-hydrogen-bonding interactions between a Hamilton receptor and cyanuric acid. Org. Biomol. Chem. 2018, 16, 4429–4432. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Villari, V.; Micali, N.; Nicosia, A.; Mineo, P. Water-Soluble Non-Ionic PEGylated Porphyrins: A Versatile Category of Dyes for Basic Science and Applications. Top. Curr. Chem. 2021, 379, 1–25. [Google Scholar] [CrossRef]
- Hama, Y.; Urano, Y.; Koyama, Y.; Bernardo, M.; Choyke, P.L.; Kobayashi, H. A Comparison of the Emission Efficiency of Four Common Green Fluorescence Dyes after Internalization into Cancer Cells. Bioconjug. Chem. 2006, 17, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Roy, I.; Ohulchanskyy, T.Y.; Goswami, L.N.; Bonoiu, A.C.; Bergey, E.J.; Tramposch, K.M.; Maitra, A.; Prasad, P.N. Covalently Dye-Linked, Surface-Controlled, and Bioconjugated Organically Modified Silica Nanoparticles as Targeted Probes for Optical Imaging. ACS Nano 2008, 2, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Napoli, J.; Enver, T.; Bernardino, L.; Ferreira, L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sell, S. Stem cell origin of cancer and differentiation therapy. Crit. Rev. Oncol./Hematol. 2004, 51, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, B.; Yañez, Y.; Palanca, S.; Cañete, A.; Burks, D.J.; Castel, V.; Font de Mora, J. Targeting Neuroblastoma Stem Cells with Retinoic Acid and Proteasome Inhibitor. PLoS ONE 2013, 8, e76761. [Google Scholar] [CrossRef]
- Reynolds, C.P.; Matthay, K.K.; Villablanca, J.G.; Maurer, B.J. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003, 197, 185–192. [Google Scholar] [CrossRef]
- Peinemann, F.; van Dalen, E.C.; Kahangire, D.A.; Berthold, F.; Peinemann, F. Retinoic acid post consolidation therapy for high-risk neuroblastoma patients treated with autologous hematopoietic stem cell transplantation. In Cochrane Database of Systematic Reviews; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Giuli, M.V.; Hanieh, P.N.; Giuliani, E.; Rinaldi, F.; Marianecci, C.; Screpanti, I.; Checquolo, S.; Carafa, M. Current Trends in ATRA Delivery for Cancer Therapy. Pharmaceutics 2020, 12, 707. [Google Scholar] [CrossRef]
- Shah, K.; Date, A.; Joshi, M.; Patravale, V. Solid lipid nanoparticles (SLN) of tretinoin: Potential in topical delivery. Int. J. Pharm. 2007, 345, 163–171. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nagasawa, T.; Nakamura, N.; Takenaga, M.; Mizoguchi, M.; Kawai, S.-I.; Mizushima, Y.; Igarashi, R. Successful treatment of photo-damaged skin of nano-scale atRA particles using a novel transdermal delivery. J. Control. Release 2005, 104, 29–40. [Google Scholar] [CrossRef]
- Tran, T.H.; Bae, B.-C.; Lee, Y.-K.; Na, K.; Huh, K.M. Heparin-folate-retinoic acid bioconjugates for targeted delivery of hydrophobic photosensitizers. Carbohydr. Polym. 2013, 92, 1615–1624. [Google Scholar] [CrossRef]
- Hou, L.; Yao, J.; Zhou, J.; Zhang, Q. Pharmacokinetics of a paclitaxel-loaded low molecular weight heparin-all-trans-retinoid acid conjugate ternary nanoparticulate drug delivery system. Biomaterials 2012, 33, 5431–5440. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Sadeghi-aliabadi, H.; Ghasemi, S.; Behdadfar, B. Use of Magnetic Folate-Dextran-Retinoic Acid Micelles for Dual Targeting of Doxorubicin in Breast Cancer. BioMed Res. Int. 2013, 2013, 24381941. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.; Subramanian, V. PA6 Stromal Cell Co-Culture Enhances SH-SY5Y and VSC4.1 Neuroblastoma Differentiation to Mature Phenotypes. PLoS ONE 2016, 11, e0159051. [Google Scholar] [CrossRef]
- Mineo, P.; Vitalini, D.; Scamporrino, E.; Bazzano, S.; Alicata, R. Effect of delay time and grid voltage changes on the average molecular mass of polydisperse polymers and polymeric blends determined by delayed extraction matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2773–2779. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, D.; Mineo, P.; Scamporrino, E. Effect of combined changes in delayed extraction time and potential gradient on the mass resolution and ion discrimination in the analysis of polydisperse polymers and polymer blends by delayed extraction matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1999, 13, 2511–2517. [Google Scholar] [CrossRef]
- Scamporrino, E.; Vitalini, D.; Mineo, P. Synthesis and MALDI-TOF MS characterization of high molecular weight poly(1,2-dihydroxybenzene phthalates) obtained by uncatalyzed bulk polymerization of O,O’-phthalid-3-ylidenecatechol or 4-methyl-O,O’-phthalid-3-ylidenecatechol. Macromolecules 1996, 29, 5520–5528. [Google Scholar] [CrossRef]
- Scamporrino, E.; Maravigna, P.; Vitalini, D.; Mineo, P. A new procedure for quantitative correction of matrix-assisted laser desorption/ionization time-of-flight mass spectrometric response. Rapid Commun. Mass Spectrom. 1998, 12, 646–650. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Preparation of a Mini-Library of Thermo-Responsive Star (NVCL/NVP-VAc) Polymers with Tailored Properties Using a Hexafunctional Xanthate RAFT Agent. Polymers 2017, 10, 20. [Google Scholar] [CrossRef]
- Menges, F. Spectragryph-Optical Spectroscopy Software, Version 1.2.11. 2019. Available online: https://www.effemm2.de/spectragryph/ (accessed on 24 February 2022).
- Lopes, F.M.; Londero, G.F.; de Medeiros, L.M.; da Motta, L.L.; Behr, G.A.; de Oliveira, V.A.; Ibrahim, M.; Moreira, J.C.F.; de Oliveira Porciúncula, L.; da Rocha, J.B.T.; et al. Evaluation of the Neurotoxic/Neuroprotective Role of Organoselenides Using Differentiated Human Neuroblastoma SH-SY5Y Cell Line Challenged with 6-Hydroxydopamine. Neurotox. Res. 2012, 22, 138–149. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 1–11. [Google Scholar] [CrossRef]
- Ballistreri, A.; Foti, S.; Montaudo, G.; Scamporrino, E. Evolution of aromatic compounds in the thermal decomposition of vinyl polymers. J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 1147–1153. [Google Scholar] [CrossRef]
- Tian, Z.; Tian, B.; Zhang, J. Synthesis and characterization of new rhodamine dyes with large Stokes shift. Dye. Pigment. 2013, 99, 1132–1136. [Google Scholar] [CrossRef]
- O’Neill, L.; Byrne, H.J. Structure−Property Relationships for Electron−Vibrational Coupling in Conjugated Organic Oligomeric Systems. J. Phys. Chem. B 2005, 109, 12685–12690. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Qi, W.; Su, R.; He, Z.; Peng, X. Mechanistic and conformational studies on the interaction of human serum albumin with rhodamine B by NMR, spectroscopic and molecular modeling methods. J. Mol. Liq. 2020, 316, 113889. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Ye, N.; Tang, X.-F.; Khan, M.I.; Liu, H.-L.; Shi, N.; Hang, L.-F. Synergistic Effect of Retinoic Acid Polymeric Micelles and Prodrug for the Pharmacodynamic Evaluation of Tumor Suppression. Front. Pharmacol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Hassanzadeh, F.; Sadeghi Aliabadi, H.; Nayebsadrian, M.; Banitalebi, M.; Rostami, M. Synthesis and Characterization of Folate-Targeted Dextran/Retinoic Acid Micelles for Doxorubicin Delivery in Acute Leukemia. BioMed Res. Int. 2014, 2014, 525684. [Google Scholar] [CrossRef]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble Polymer Carriers for the Treatment of Cancer: The Importance of Molecular Architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef]
- Lewandowska, K.; Staszewska, D.U.; Bohdanecký, M. The Huggins viscosity coefficient of aqueous solution of poly(vinyl alcohol). Eur. Polym. J. 2001, 37, 25–32. [Google Scholar] [CrossRef]
- Caracciolo, G. Clinically approved liposomal nanomedicines: Lessons learned from the biomolecular corona. Nanoscale 2018, 10, 4167–4172. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Constantinescu, R.; Constantinescu, A.T.; Reichmann, H.; Janetzky, B. Neuronal differentiation and long-term culture of the human neuroblastoma line SH-SY5Y. In Neuropsychiatric Disorders An Integrative Approach; Springer: Berlin/Heidelberg, Germany, 2007; pp. 17–28. [Google Scholar]
- Serdar, B.S.; Erkmen, T.; Ergür, B.U.; Akan, P.; Koçtürk, S. Which Medium and Ingredients Provide Better Morphological Differentiation of SH-SY5Y Cells? Proceedings 2018, 2, 1577. [Google Scholar] [CrossRef]
- Maden, M.; Hind, M. Retinoic acid, a regeneration-inducing molecule. Dev. Dyn. 2003, 226, 237–244. [Google Scholar] [CrossRef]
- Nicolini, G.; Miloso, M.; Zoia, C.; Di Silvestro, A.; Cavaletti, G.; Tredici, G. Retinoic acid differentiated SH-SY5Y human neuroblastoma cells: An in vitro model to assess drug neurotoxicity. Anticancer Res. 1998, 18, 2477–2481. [Google Scholar]
- Maruyama, W.; Strolin Benedetti, M.; Takahashi, T.; Naoi, M. A neurotoxin N-methyl(R)salsolinol induces apoptotic cell death in differentiated human dopaminergic neuroblastoma SH-SY5Y cells. Neurosci. Lett. 1997, 232, 147–150. [Google Scholar] [CrossRef]
- Croft, A.P.; Przyborski, S.A. Formation of Neurons by Non-Neural Adult Stem Cells: Potential Mechanism Implicates an Artifact of Growth in Culture. Stem Cells 2006, 24, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.; Zintl, F. Effects of retinoic acid on proliferation, apoptosis, cytotoxicity, migration, and invasion of neuroblastoma cells. Med. Pediatr. Oncol. 2003, 40, 205–213. [Google Scholar] [CrossRef]
- Tabata, Y. Tumor accumulation of poly(vinyl alcohol) of different sizes after intravenous injection. J. Control. Release 1998, 50, 123–133. [Google Scholar] [CrossRef]
- Kurtoglu, M.; Lampidis, T.J. From delocalized lipophilic cations to hypoxia: Blocking tumor cell mitochondrial function leads to therapeutic gain with glycolytic inhibitors. Mol. Nutr. Food Res. 2009, 53, 68–75. [Google Scholar] [CrossRef]
- Magut, P.K.S.; Das, S.; Fernand, V.E.; Losso, J.; McDonough, K.; Naylor, B.M.; Aggarwal, S.; Warner, I.M. Tunable Cytotoxicity of Rhodamine 6G via Anion Variations. J. Am. Chem. Soc. 2013, 135, 15873–15879. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicosia, A.; La Perna, G.; Cucci, L.M.; Satriano, C.; Mineo, P. A Multifunctional Conjugated Polymer Developed as an Efficient System for Differentiation of SH-SY5Y Tumour Cells. Polymers 2022, 14, 4329. https://doi.org/10.3390/polym14204329
Nicosia A, La Perna G, Cucci LM, Satriano C, Mineo P. A Multifunctional Conjugated Polymer Developed as an Efficient System for Differentiation of SH-SY5Y Tumour Cells. Polymers. 2022; 14(20):4329. https://doi.org/10.3390/polym14204329
Chicago/Turabian StyleNicosia, Angelo, Giuseppe La Perna, Lorena Maria Cucci, Cristina Satriano, and Placido Mineo. 2022. "A Multifunctional Conjugated Polymer Developed as an Efficient System for Differentiation of SH-SY5Y Tumour Cells" Polymers 14, no. 20: 4329. https://doi.org/10.3390/polym14204329
APA StyleNicosia, A., La Perna, G., Cucci, L. M., Satriano, C., & Mineo, P. (2022). A Multifunctional Conjugated Polymer Developed as an Efficient System for Differentiation of SH-SY5Y Tumour Cells. Polymers, 14(20), 4329. https://doi.org/10.3390/polym14204329








