Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review
Abstract
:1. Introduction
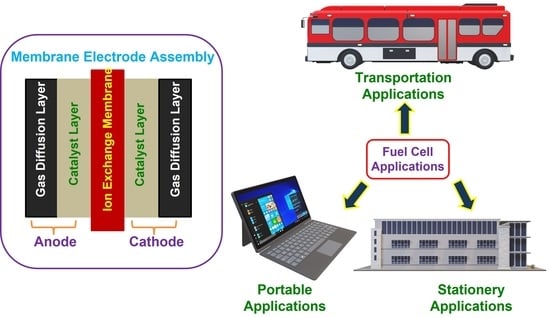
1.1. Fuel Cells
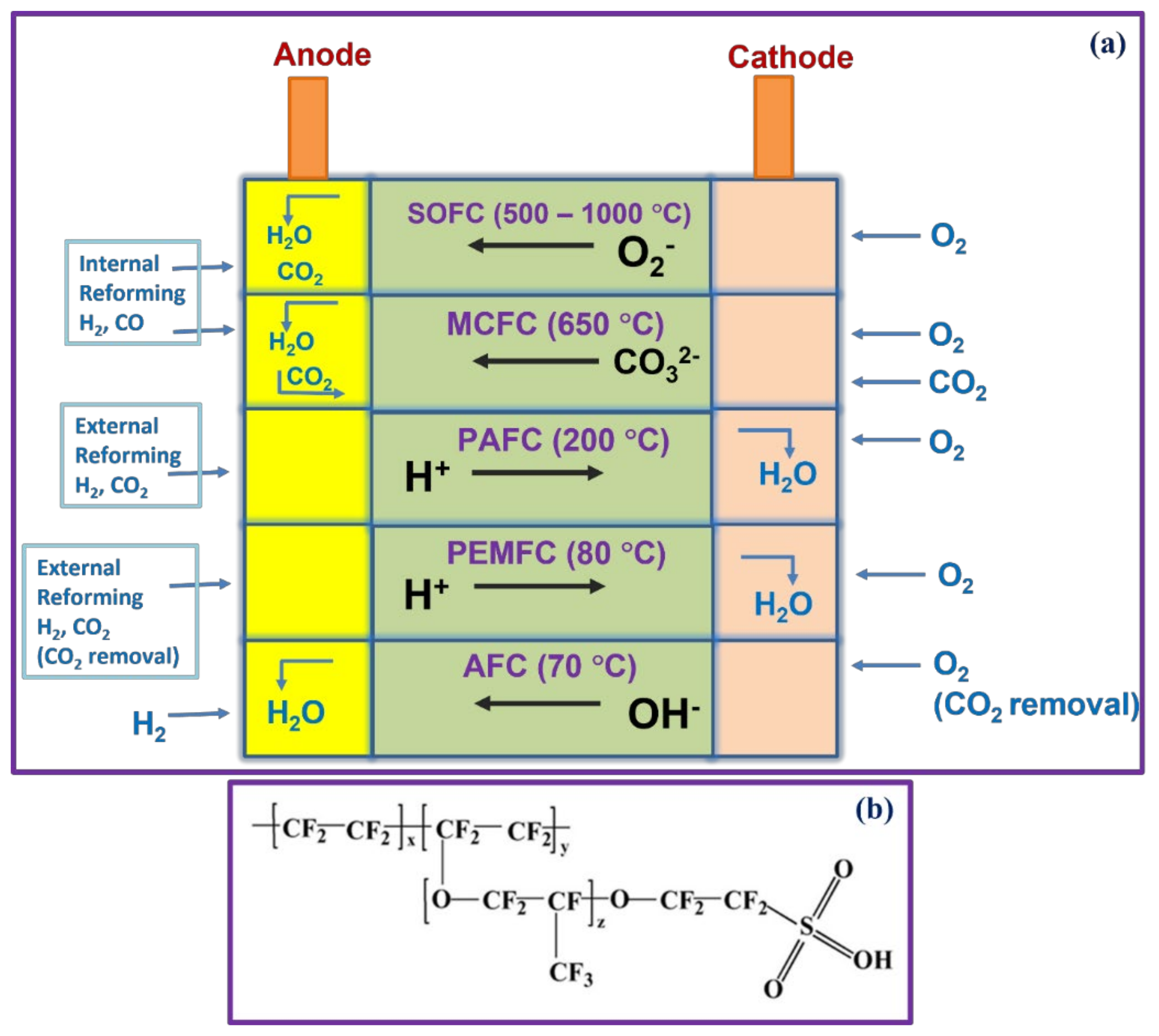
1.2. Types of FCs
1.2.1. PEMFCs
1.2.2. DMFCs
1.2.3. AMFCs
1.3. Ion Exchange Membranes
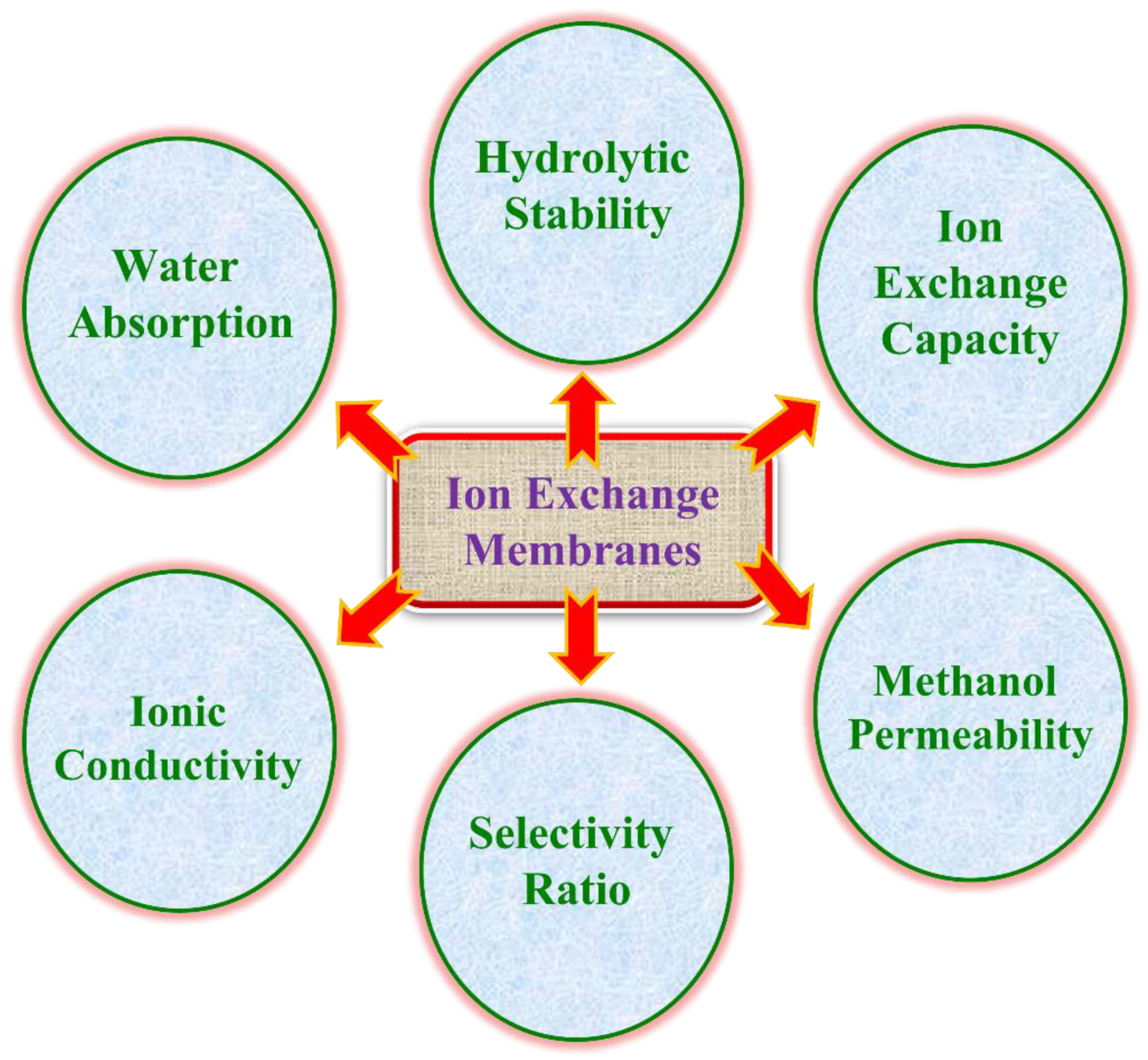
1.4. Preliminary Characteristic of IEM
1.4.1. Water Uptake and Schroeder’s Paradox
1.4.2. Ion Exchange Capacity
1.4.3. Ionic Conductivity
1.4.4. Methanol Permeability
1.4.5. Selectivity Ratio
1.4.6. Oxidative Stability
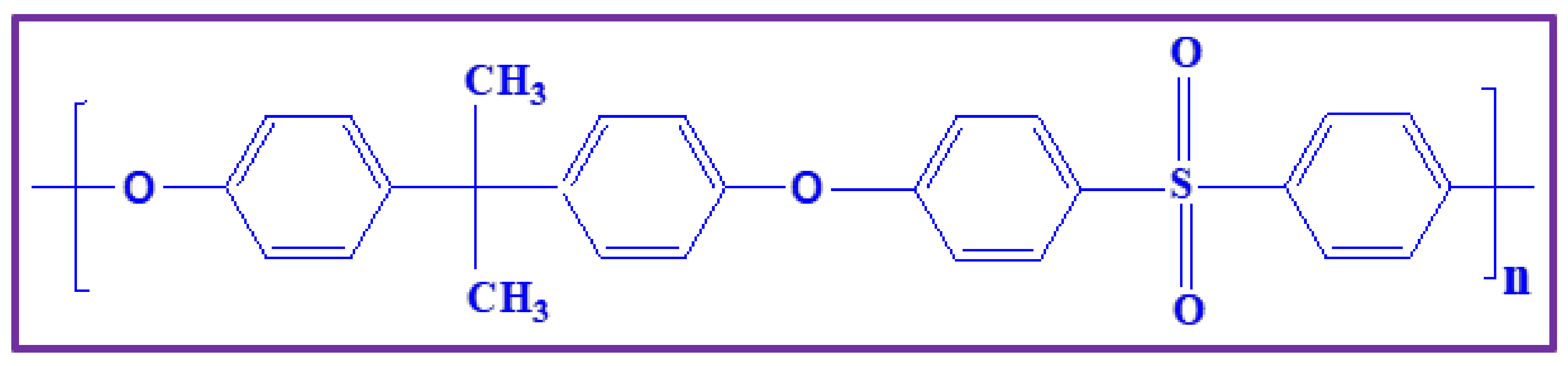
2. Polysulfone
2.1. Membranes Derived from Polysulfone and Its Composites for PEMFCs Application
2.2. Polysulfone and Its Composites for DMFCs
2.3. Alkaline Based Polysulfone and Its Composites for AMFCs
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kordesch, K.V.; Simader, G.R. Environmental impact of fuel cell technology. Chem. Rev. 1995, 95, 191–207. [Google Scholar] [CrossRef]
- Yan, Q.; Toghiani, H.; Causey, H. Steady state and dynamic performance of proton exchange membrane fuel cells (PEMFCs) under various operating conditions and load changes. J. Power Sources 2006, 161, 492–502. [Google Scholar] [CrossRef]
- Barbir, F.; Gómez, T. Efficiency and economics of proton exchange membrane (PEM) fuel cells. Int. J. Hydrogen Energy 1997, 22, 1027–1037. [Google Scholar] [CrossRef]
- Kacprzak, A. Hydroxide electrolyte direct carbon fuel cells-technology review. Int. J. Energy Res. 2018, 43, 65–85. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S. Membranes for direct ethanol fuel cells: An overview. Appl. Energy 2016, 163, 334–342. [Google Scholar] [CrossRef]
- Steele, B.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel cells-fundamentals and applications. Fuel Cells 2001, 1, 5–39. [Google Scholar] [CrossRef]
- Araya, S.S.; Andreasen, S.J.; Nielsen, H.V.; Kaer, S.K. Investigating the effects of methanol-water vapor mixture on a PBI-based high temperature PEM fuel cell. Int. J. Hydrogen Energy 2012, 37, 18231–18242. [Google Scholar] [CrossRef]
- Haile, S.M. Fuel cell materials and components. Acta Mater. 2003, 51, 5981–6000. [Google Scholar] [CrossRef]
- Kordesch, K.; Simader, G. Fuel Cells and Their Applications; VCH: Weinheim, Germany, 1996. [Google Scholar]
- Pineri, M.; Eisenberg, A. Structure and Properties of Ionomers; Springer: Dordrecht, The Netherlands, 1987. [Google Scholar]
- Samms, S.R.; Wasmus, S.; Savinell, R.F. Thermal stability of nafion® in simulated fuel cell environments. J. Electrochem. Soc. 1996, 143, 1498. [Google Scholar] [CrossRef]
- Uosaki, K.; Okazaki, K.; Kita, H. Conductivity of Nation membranes at low temperatures. J. Electroanal. Chem. Interfacial. Electrochem. 1990, 287, 163–169. [Google Scholar] [CrossRef]
- Cappadonia, M.; Erning, J.W.; Niaki, S.M.S.; Stimming, U. Conductance of Nafion 117 membranes as a function of temperature and water content. Solid State Ion. 1995, 77, 65–69. [Google Scholar] [CrossRef]
- Adjemian, K.T.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Investigation of PEMFC operation above 100 °C employing perfluorosulfonic acid silicon oxide composite membranes. J. Power Sources 2002, 109, 356–364. [Google Scholar] [CrossRef]
- Kim, Y.M.; Choi, S.H.; Lee, H.C.; Hong, M.Z.; Kim, K.; Lee, H.-I. Organic-inorganic composite membranes as addition of SiO2 for high temperature-operation in polymer electrolyte membrane fuel cells (PEMFCs). Electrochim. Acta 2004, 49, 4787–4796. [Google Scholar] [CrossRef]
- Antonucci, P.L.; Arico, A.S.; Creti, P.; Ramunni, E.; Antonucci, V. Investigation of a direct methanol fuel cell based on a composite Nafion®-silica electrolyte for high temperature operation. Solid State Ion. 1999, 125, 431–437. [Google Scholar] [CrossRef]
- Seyed, H.-S.; Gholamreza, B.; Mohammad, S.L. Performance of the sulfonated poly ether ether ketone proton exchange membrane modified with sulfonated polystyrene and phosphotungstic acid for microbial fuel cell applications. J. Appl. Polym. Sci. 2021, 138, 50430. [Google Scholar]
- Martina, P.; Gayathri, R.; Raja Pugalenthi, M.; Cao, G.; Liu, C.; Ramesh Prabhu, M. Nanosulfonated silica incorporated SPEEK/SPVdF-HFP polymer blend membrane for PEM fuel cell application. Ionics 2020, 26, 3447–3458. [Google Scholar] [CrossRef]
- Mader, J.A.; Benicewicz, B.C. Sulfonated Polybenzimidazoles for High Temperature PEM Fuel Cells. Macromolecules 2010, 43, 6706–6715. [Google Scholar] [CrossRef]
- Singha, S.; Jana, T.; Modestra, J.A.; Kumar, A.N.; Mohan, S.V. Highly efficient sulfonated polybenzimidazole as a proton exchange membrane for microbial fuel cells. J. Power Sources 2016, 317, 143–152. [Google Scholar] [CrossRef]
- Lufrano, F.; Squadrito, G.; Patti, A.; Passalacqua, E. Sulfonated polysulfone as promising membranes for polymer electrolyte fuel cells. J. Appl. Polym. Sci. 2000, 77, 1250–1256. [Google Scholar] [CrossRef]
- Zhao, T.S.; Kreuer, K.D.; Nguyen, T.V. Advances in Fuel Cells; Elsevier Ltd.: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Srinivasan, S. Fuel Cells: From Fundamentals to Applications; Springer: Berlin, Germany, 2006. [Google Scholar]
- Kamarudin, S.K.; Daud, W.R.W.; Ho, S.L.; Hasran, U.A. Overview on the challenges and developments of micro-direct methanol fuel cells (DMFC). J. Power Sources 2007, 163, 743–754. [Google Scholar] [CrossRef]
- Zaidi, S.M.; Matsuura, T. Polymer Membranes for Fuel Cells; Springer Science: Berlin, Germany, 2009. [Google Scholar]
- Li, N.; Fane, A.; Ho, W.S.; Matsuura, T. Advanced Membrane Technology and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Zhao, T.S.; Xu, C.; Chen, R.; Yang, W.W. Mass transport phenomena in direct methanol fuel cells. Prog. Energy Combust. Sci. 2009, 35, 275–292. [Google Scholar] [CrossRef]
- Dai, J.; He, G.; Ruan, X.; Zheng, W.; Pan, Y.; Yan, X. Constructing a rigid crosslinked structure for enhanced conductivity of imidazolium functionalized polysulfone hydroxide exchange membrane. Int. J. Hydrogen Energy 2016, 41, 10923–10934. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Tignor, S.E.; Krause, J.A.; Choe, Y.K.; Bae, C. Systematic alkaline study of polymer backbones for anion exchange membrane applications. Macromolecules 2016, 49, 3361–3372. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, T.Y.; Yan, X.M.; Xu, X.W.; Zhang, Q.D.; Zhao, B.L.; El Hamouti, I.; Hao, C.; He, G.H. Novel benzimidazolium functionalized polysulfone-based anion exchange membranes with improved alkaline stability. Chin. J. Polym. Sci. 2018, 36, 129–138. [Google Scholar] [CrossRef]
- Vinodh, R.; Ilakkiya, A.; Elamathi, S.; Sangeetha, D. A novel anion exchange membrane from polystyrene (ethylene butylene) polystyrene: Synthesis and characterization. Mater. Sci. Eng. B 2010, 167, 43–50. [Google Scholar] [CrossRef]
- Liu, L.; Chu, X.; Liao, J.; Huang, Y.; Li, Y.; Ge, Z.; Hickner, M.A.; Li, N. Tuning the properties of Poly(2,6-dimethyl-1,4-phenylene oxide) anion exchange membranes and their performance in H2/O2 fuel cells. Energy Environ. Sci. 2018, 11, 435–446. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Horan, J.L.; Jin, Y.; Ren, X.; Ertem, S.P.; Seifert, S.; Liberatore, M.W.; Herring, A.M.; Coughlin, E.B. Crosslinked anion exchange membranes with connected cations. J. Polym. Sci. Part A Polym. Chem. 2017, 56, 618–625. [Google Scholar] [CrossRef]
- Shukla, G.; Shahi, V.K. Poly(arylene ether ketone) copolymer grafted with amine groups containing long alkyl chain by chloroacetylation for improved alkaline stability and conductivity of anion exchange membrane. ACS Appl. Energy Mater. 2018, 1, 1175–1182. [Google Scholar] [CrossRef]
- Zuo, D.; Gong, Y.; Yan, Q.; Zhang, H. Preparation and characterization of hydroxyl ion-conducting interpenetrating polymer network based on PVA and PEI. J. Polym. Res. 2016, 23, 126–132. [Google Scholar] [CrossRef]
- Guo, D.; Lai, A.N.; Lin, C.X.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Imidazolium-functionalized poly(arylene ether sulfone) anion-exchange membranes densely grafted with flexible side chains for fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 25279–25288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Prior, M.T.; Várez, A.; Levenfeld, B. Synthesis and characterization of benzimidazolium-functionalized polysulfones as anion-exchange membranes. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2363–2373. [Google Scholar] [CrossRef]
- Yan, X.; Gu, S.; He, G.; Wu, X.; Zheng, W.; Ruan, X. Quaternary phosphonium-functionalized poly(ether ether ketone) as highly conductive and alkali-stable hydroxide exchange membrane for fuel cells. J. Membr. Sci. 2014, 466, 220–228. [Google Scholar] [CrossRef]
- Jangu, C.; Long, T.E. Phosphonium cation-containing polymers: From ionic liquids to polyelectrolytes. Polymer 2014, 55, 3298–3304. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Dai, J.; Wang, H.; Jin, B.; Bai, R. A facile strategy for the synthesis of guanidinium-funcionalized polymer as alkaline anion exchange membrane with improved alkaline stability. J. Membr. Sci. 2014, 453, 52–60. [Google Scholar] [CrossRef]
- Kim, D.S.; Fujimoto, C.H.; Hibbs, M.R.; Labouriau, A.; Choe, Y.-K.; Kim, Y.S. Resonance stabilized perfluorinated ionomers for alkaline membrane fuel cells. Macromolecules 2013, 46, 7826–7833. [Google Scholar] [CrossRef]
- Cha, M.S.; Lee, J.Y.; Kim, T.-H.; Jeong, H.Y.; Shin, H.Y.; Oh, S.-G.; Hong, Y.T. Preparation and characterization of crosslinked anion exchange membrane (AEM) materials with poly(phenylene ether)-based short hydrophilic block for use in electrochemical applications. J. Membr. Sci. 2017, 530, 73–83. [Google Scholar] [CrossRef]
- Huang, X.L.; Lin, C.X.; Hu, E.N.; Soyekwo, F.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Imidazolium-functionalized anion exchange membranes using poly(ether sulfone)s as macrocrosslinkers for fuel cells. RCS Adv. 2017, 7, 27342–27353. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Yan, X.; Li, T.; Wu, X.; Chen, W.; Huang, S.; Wu, Y.; Zhen, D.; He, G. Design of pendent imidazolium side with flexible ether-containing spacer for alkaline anion exchange membrane. J. Membr. Sci. 2017, 523, 216–224. [Google Scholar] [CrossRef]
- Hugar, K.M.; Kostalik, H.A., IV; Coates, G.W. Imidazolium cations with exceptional alkaline stability: A systematic study of structure-stability relationships. J. Am. Chem. Soc. 2015, 137, 8730–8737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grew, K.N.; Chiu, W.K.S. A dusty fluid model for predicting hydroxyl anion conductivity in alkaline anion exchange membranes. J. Electrochem. Soc. 2010, 157, B327. [Google Scholar] [CrossRef]
- Hren, M.; Božič, M.; Fakin, D.; Kleinschek, K.S.; Gorgieva, S. Alkaline membrane fuel cells: Anion exchange membranes and fuels. Sustain. Energy Fuels 2021, 5, 604–637. [Google Scholar] [CrossRef]
- Sun, C.; Zlotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Noto, V.D. [Nafion/(WO3)x] hybrid membranes for vanadium redox flow batteries. Solid State Ion. 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Vinodh, R.; Sangeetha, D. Quaternized Poly(Styrene Ethylene Butylene Poly Styrene)/Multiwalled Carbon Nanotube Composites for Alkaline Fuel Cell Applications. J. Nanosci. Nanotechnol. 2013, 13, 5522–5533. [Google Scholar] [CrossRef]
- Vinodh, R.; Sangeetha, D. Efficient utilization of anion exchange composites using silica filler for low temperature alkaline membrane fuel cells. Int. J. Plast. Technol. 2013, 17, 35–50. [Google Scholar] [CrossRef]
- Di, S.; Yan, L.; Han, S.; Yue, B.; Feng, Q.; Xie, L.; Chen, J.; Zhang, D.; Sun, C. Enhancing the high-temperature proton conductivity of phosphoric acid doped poly(2,5-benzimidazole) by preblending boron phosphate nanoparticles to the raw materials. J. Power Sources 2012, 211, 161–168. [Google Scholar] [CrossRef]
- Vinodh, R.; Sangeetha, D. Comparative study of composite membranes from nano-metal-oxide-incorporated polymer electrolytes for direct methanol alkaline membrane fuel cells. J. Appl. Polym. Sci. 2013, 128, 1930–1938. [Google Scholar] [CrossRef]
- Santoyo, A.B.; Carraso, J.L.G.; Gomez, E.G.; Martin, F.M.; Montesinos, A.M.H. Application of reverse osmosis to reduce pollutants present in industrial waste water. Desalination 2003, 155, 101–108. [Google Scholar] [CrossRef]
- Nechifor, G.; Voicu, S.I.; Nechifor, A.C.; Garea, S. Nanostructured hybrid membrane polysulfone-carbon nanotubes for hemodialysis. Desalination 2009, 241, 342–348. [Google Scholar] [CrossRef]
- Arico, A.S.; Srinivasan, S.; Antonucci, V. DMFCs from fundamental aspects to technology development. Fuel Cells 2001, 1, 133–161. [Google Scholar] [CrossRef]
- Dillon, R.; Srinivasan, S.; Arico, A.S.; Antonucci, V. International activities in DMFC R&D: Status of technologies and potential applications. J. Power Sources 2004, 127, 112–126. [Google Scholar]
- Kariduraganavar, M.Y.; Munavalli, B.B.; Torvi, A.I. Proton conducting polymer electrolytes for fuel cells via electrospinning technique. In Organic-Inorganic Composite Polymer Electrolyte Membranes: Preparation, Properties and Fuel Cell Applications; Inamuddin, Mohammad, A., Asiri, A.M., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 421–458. [Google Scholar]
- Kraytsberg, A.; Eli, Y.E. A review of advanced, materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Muller, F.; Ferreira, C.A.; Azambuja, D.S.; Aleman, C.; Armelin, E. Measuring the proton conductivity of ion-exchange membranes using electrochemical impedance spectroscopy and through-plane cell. J. Phys. Chem. B 2014, 118, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Munavalli, B.; Torvi, A.; Kariduraganavar, M. A facile route for the preparation of proton exchange membranes using sulfonated side chain graphite oxides and crosslinked sodium alginate for fuel cell. Polymer 2018, 142, 293–309. [Google Scholar] [CrossRef]
- Li, Z.; He, G.; Zhao, Y.; Cao, Y.; Wu, H.; Li, Y.; Jiang, Z. Enhanced proton conductivity of proton exchange membranes by incorporating sulfonated metal-organic frameworks. J. Power Sources 2014, 262, 372–379. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, F.; Feng, W.; Zou, X.; Zhao, C.; Na, H.; Liu, C.; Sun, F.; Zhu, G. From metal-organic framework (MOF) to MOF-polymer composite membrane: Enhancement of low-humidity proton conductivity. Chem. Sci. 2013, 4, 983–992. [Google Scholar] [CrossRef]
- Ren, Y.; Chia, G.H.; Gao, Z. Metal-organic frameworks in fuel cell technologies. Nano Today 2013, 8, 577–597. [Google Scholar] [CrossRef]
- Horike, S.; Umeyama, D.; Kitagawa, S. Ion conductivity and transport by porous coordination polymers and metal-organic frameworks. Acc. Chem. Res. 2013, 46, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian-Alam, L.; Mahdavi, H. A novel polysulfone-based ternary nanocomposite membrane consisting of metal-organic framework and silica nanoparticles: As proton exchange membrane for polymer electrolyte fuel cells. Renew. Energy 2018, 126, 630–639. [Google Scholar] [CrossRef] [Green Version]
- Nor, N.A.M.; Nakao, H.; Jaafar, J.; Kim, J.-D. Crosslinked carbon nanodots with highly sulfonated polyphenylsulfone as proton exchange membrane for fuel cell applications. Int. J. Hydrogen Energy 2020, 45, 9979–9988. [Google Scholar]
- Munavalli, B.B.; Kariduraganavar, M.Y. Development of novel sulfonic acid functionalized zeolites incorporated composite proton exchange membranes for fuel cell application. Electrochim. Acta 2017, 296, 294–307. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Cao, L.; He, X.; Shi, B.; Li, Y.; Xu, M.; Jiang, Z. Enhanced proton conductivity of sulfonated polysulfone membranes under low humidity via the incorporation of multifunctional graphene oxide. ACS Appl. Nano Mater. 2019, 2, 4734–4743. [Google Scholar] [CrossRef]
- Simari, C.; Lufrano, E.; Brunetti, A.; Barbieri, G.; Nicotera, I. Highly-performing and low-cost nanostructured membranes based on Polysulfone and layered doubled hydroxide for high-temperature proton exchange membrane fuel cells. J. Power Sources 2020, 471, 228440. [Google Scholar] [CrossRef]
- Pan, T.; Yue, B.; Yan, L.; Zeng, G.; Hu, Y.; He, S.; Lu, W.; Zhao, H.; Zhang, J. N,N-bis(sulfopropyl)aminyl-4-phenyl polysulfone and O,O’-bis(sulfopropyl)resorcinol-5-yl-4-phenyl polysulfone composite membrane for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2020, 45, 23490–23503. [Google Scholar] [CrossRef]
- Mahimai, B.M.; Kulasekaran, P.; Deivanayagam, P. Novel polysulfone/sulfonated polyaniline/niobium pentoxide polymer blend nanocomposite membranes for fuel cell applications. J. Appl. Polym. Sci. 2021, 138, 51207. [Google Scholar] [CrossRef]
- Han, S.; Yue, B.; Yan, L. Research progress in the development of high-temperature proton exchange membranes based on phosphonic acid group. Acta Phys. Chim. Sin. 2014, 30, 8–21. [Google Scholar]
- Stone, C.; Daynard, T.; Hu, L.; Mah, C.; Steck, A. Phosphonic acid functionalized proton exchange membranes for PEM fuel cells. J. New Mat. Electr. Sys. 2000, 3, 43–50. [Google Scholar]
- Bock, T.; Möhwald, H.; Mülhaupt, R. Arylphosphonic acid-functionalized polyelectrolytes as fuel cell membrane material. Macromol. Chem. Phys. 2007, 208, 1324–1340. [Google Scholar] [CrossRef]
- Herath, M.B.; Creager, S.E.; Kitaygorodskiy, A.; DesMarteau, D.D. Perfluoroalkyl phosphonic and phosphinic acids as proton conductors for anhydrous proton-exchange membranes. Chem. Phys. Chem. 2010, 11, 2871–2878. [Google Scholar] [CrossRef]
- Paddison, S.J.; Kreuer, K.-D.; Maier, J. About the choice of the protogenic group in polymer electrolyte membranes: Ab initio modelling of sulfonic acid, phosphonic acid, and imidazole functionalized alkanes. Phys. Chem. Chem. Phys. 2006, 8, 4530–4542. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yue, B.; Yan, L.; Zhao, H.; Zhang, J. Proton conducting composite membranes based on sulfonated polysulfone and polysulfone-g-(phosphonated polystyrene) via controlled atom-transfer radical polymerization for fuel cell applications. Solid State Ion. 2019, 338, 103–112. [Google Scholar] [CrossRef]
- Yang, J.; Li, Q.; Cleemann, L.N.; Jensen, J.O.; Pan, C.; Bjerrum, N.J.; He, R. Crosslinked hexafluoropropylidene polybenzimidazole membranes with chloromethyl polysulfone for fuel cell applications. Adv. Energy Mater. 2013, 3, 622–630. [Google Scholar] [CrossRef]
- Bai, H.; Wang, H.; Zhang, J.; Wu, C.; Zhang, J.; Xiang, Y.; Lu, S. Simultaneously enhancing ionic conduction and mechanical strength of poly(ether sulfones)-poly(vinyl pyrrolidone) membrane by introducing graphitic carbon nitride nanosheets for high temperature proton exchange membrane fuel cell application. J. Membr. Sci. 2018, 558, 26–33. [Google Scholar] [CrossRef]
- Tang, H.; Geng, K.; Hu, Y.; Li, N. Synthesis and properties of phosphonated polysulfones for durable high-temperature proton exchange membranes fuel cell. J. Membr. Sci. 2020, 605, 118107. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Bai, H.; Tan, Q.; Wang, H.; He, B.; Xiang, Y.; Lu, S. A new high temperature polymer electrolyte membrane based on trifunctional group grafted polysulfone for fuel cell application. J. Membr. Sci. 2019, 572, 496–503. [Google Scholar] [CrossRef]
- Bai, H.; Wang, H.; Zhang, J.; Zhang, J.; Lu, S.; Xiang, Y. High temperature polymer electrolyte membrane achieved by grafting poly (1-vinylimidazole) on polysulfone for fuel cells application. J. Membr. Sci. 2019, 592, 117395. [Google Scholar] [CrossRef]
- Devi, A.U.; Muthumeenal, A.; Sabarathinam, R.; Nagendran, A. Fabrication and electrochemical properties of SPVdF-co-HFP/SPES blend proton exchange membranes for direct methanol fuel cells. Renew. Energy 2017, 102, 258–265. [Google Scholar] [CrossRef]
- Azimi, M.; Peighambardoust, S. Methanol crossover and selectivity of nafion/heteropolyacid/. montmorillonite nanocomposite proton exchange membranes for DMFC Applications. Iran. J. Chem. Eng. 2017, 14, 65–81. [Google Scholar]
- Yılmaz, E.; Can, E. Cross-linked poly (aryl ether sulfone) membranes for direct methanol fuel cell applications. J. Polym. Sci. B Polym. Phys. 2018, 56, 558–575. [Google Scholar] [CrossRef]
- Bhavani, P.; Sangeetha, D. Blend membranes for direct methanol and proton exchange membrane fuel cells. Chin. J. Polym. Sci. 2012, 30, 548–560. [Google Scholar] [CrossRef]
- Muthumeenal, A.; Neelakandan, S.; Kanagaraj, P.; Nagendran, A. Synthesis and properties of novel proton exchange membranes based on sulfonated polyethersulfone and N-phthaloyl chitosan blends for DMFC applications. Renew. Energy 2016, 86, 922–929. [Google Scholar] [CrossRef]
- Clarizia, G.; Tasselli, F.; Simari, C.; Nicotera, I.; Bernardo, P. Solution casting blending: An effective way for tailoring gas transport and mechanical properties of poly(vinyl butyral) and Pebax2533. J. Phys. Chem. C 2019, 123, 11264–11272. [Google Scholar] [CrossRef]
- Simari, C.; Lufrano, E.; Coppola, L.; Nicotera, I. Composite gel polymer electrolytes based on organo-modified nanoclays: Investigation on lithium-ion transport and mechanical properties. Membranes 2018, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Nohara, T.; Koseki, K.; Tabata, K.; Shimada, R.; Suzuki, Y.; Umemoto, K.; Takeda, M.; Sato, R.; Rodbuntum, S.; Arita, T.; et al. Core size-dependent proton conductivity of silica filler-functionalized polymer electrolyte membrane. ACS Sustain. Chem. Eng. 2020, 8, 14674–14678. [Google Scholar] [CrossRef]
- Devrim, Y.; Erkan, S.; Bac, N.; Eroğlu, I. Preparation and characterization of sulfonated polysulfone/titanium dioxide composite membranes for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 3467–3475. [Google Scholar] [CrossRef]
- Borduin, R.; Li, W. Fabrication of foamed polyethersulfone–zeolite mixed matrix membranes for polymer electrolyte membrane fuel cell humidification. J. Manuf. Sci. Eng. 2017, 139, 021004. [Google Scholar] [CrossRef]
- Sakamoto, M.; Nohara, S.; Miyatake, K.; Uchida, M.; Watanabe, M.; Uchida, H. Effects of incorporation of SiO2 nanoparticles into sulfonated polyimide electrolyte membranes on fuel cell. performance under low humidity conditions. Electrochim. Acta 2014, 137, 213–218. [Google Scholar] [CrossRef]
- de Bonis, C.; Simari, C.; Kosma, V.; Mecheri, B.; D’Epifanio, A.; Allodi, V.; Mariotto, G.; Brutti, S.; Suarez, S.; Pilar, K.; et al. Enhancement of proton mobility and mitigation of methanol crossover in sPEEK fuel cells by an organically modified titania nanofiller. J. Solid State Electrochem. 2016, 20, 1585–1598. [Google Scholar] [CrossRef]
- Simari, C.; Stallworth, P.; Peng, J.; Coppola, L.; Greenbaum, S.; Nicotera, I. Graphene oxide and sulfonated-derivative: Proton transport properties and electrochemical behavior of Nafion based nanocomposites. Electrochim. Acta 2019, 297, 240–249. [Google Scholar] [CrossRef]
- Simari, C.; Potsi, G.; Policicchio, A.; Perrotta, I.; Nicotera, I. Clay carbon nanotubes hybrid materials for nanocomposite membranes: Advantages of branched structure for proton transport under low humidity conditions in PEMFCs. J. Phys. Chem. C 2016, 120, 2574–2584. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Boutsika, L.G.; Coppola, L.; Spyrou, K.; Enotiadis, A. NMR investigation on nanocomposite membranes based on organosilica layered materials bearing different functional groups for PEMFCs. Int. J. Hydrogen Energy 2017, 42, 7940–27949. [Google Scholar] [CrossRef]
- Nicotera, I.; Kosma, V.; Simari, C.; Angioni, S.; Mustarelli, P.; Quartarone, E. Ion dynamics and mechanical properties of sulfonated polybenzimidazole membranes for high temperature proton exchange membrane fuel cells. J. Phys. Chem. C 2015, 119, 9745–9753. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Borau, V.; Jiménez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Catalytic transfer hydrogenation of citral on calcined layered double hydroxides. Appl. Catal. Gen. 2001, 206, 95–101. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. Sci. Eng. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Primo, J. Base catalysis for fine chemical production: Claisen-schmidt condensation on zeolites and hydrocalcites for the production of chalcones and flavanones of pharmaceutical interest. J. Catal. 1995, 151, 60–66. [Google Scholar] [CrossRef]
- Lufrano, E.; Simari, C.; Lo Vecchio, C.; Arico, A.S.; Baglio, V.; Nicotera, I. Barrier properties of sulfonated polysulfone/layered double hydroxides nanocomposite membrane for direct methanol fuel cell operating at high methanol concentrations. Int. J. Hydrogen Energy 2020, 45, 20647–20658. [Google Scholar] [CrossRef]
- Xua, X.; Zhaoa, G.; Wang, H.; Li, X.; Feng, X.; Cheng, B.; Shi, L.; Kang, W.; Zhuang, X.; Yin, Y. Bio-inspired amino-acid-functionalized cellulose whiskers incorporated into sulfonated polysulfone for proton exchange membrane. J. Power Sources 2019, 409, 123–131. [Google Scholar] [CrossRef]
- Ozden, A.; Ercelik, M.; Devrim, Y.; Colpan, C.O.; Hamdullahpur, F. Evaluation of sulfonated polysulfone/zirconium hydrogen phosphate composite membranes for direct methanol fuel cells. Electrochim. Acta 2017, 256, 196–210. [Google Scholar] [CrossRef]
- Krathumkhet, N.; Vongjitpimol, K.; Chuesutham, T.; Changkhamchom, S.; Phasuksom, K.; Sirivat, A.; Wattanakul, K. Preparation of sulfonated zeolite ZSM-5/sulfonated polysulfone composite membranes as PEM for direct methanol fuel cell application. Solid State Ion. 2018, 319, 278–284. [Google Scholar] [CrossRef]
- Simari, C.; Lo Vecchio, C.; Baglio, V.; Nicotera, I. Sulfonated polyethersulfone/polyetheretherketone blend as high performing and cost-effective electrolyte membrane for direct methanol fuel cells. Renew. Energy 2020, 159, 336–345. [Google Scholar] [CrossRef]
- Altaf, F.; Gill, R.; Batool, R.; Rehman, Z.-U.; Majeed, H.; Abbas, G.; Jacob, K. Synthesis and applicability study of novel poly(dopamine)-modified carbon nanotubes based polymer electrolyte membranes for direct methanol fuel cell. J. Environ. Chem. Eng. 2020, 8, 104118. [Google Scholar] [CrossRef]
- McLean, G.F.; Niet, T.; Prince-Richard, S.; Djilali, N. An assessment of alkaline fuel cell technology. Int. J. Hydrogen Energy 2002, 27, 507–526. [Google Scholar] [CrossRef]
- Lu, S.; Pan, J.; Huang, A.; Zhuang, L.; Lu, J. Alkaline polymer electrolyte fuel cells completely free from noble metal catalysts. Proc. Natl. Acad. Sci. USA 2008, 105, 20611–20614. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Li, G.; Pan, J.; Tan, L.; Lu, J.; Zhuang, L. Alkaline polymer electrolyte fuel cell with Ni-based anode and Co-based cathode. Int. J. Hydrogen Energy 2013, 38, 16264–16268. [Google Scholar] [CrossRef]
- Ureña, N.; Pérez-Prior, M.T.; del Rio, C.; Várez, A.; Levenfeld, B. New amphiphilic semi-interpenetrating networks based on polysulfone for anion-exchange membrane fuel cells with improved alkaline and mechanical stabilities. Polymer 2021, 226, 123824. [Google Scholar] [CrossRef]
- Bai, Y.; Yuan, Y.; Miao, L.; Lü, C. Functionalized rGO as covalent crosslinkers for constructing chemically stable polysulfone-based anion exchange membranes with enhanced ion conductivity. J. Membr. Sci. 2019, 570–571, 481–493. [Google Scholar] [CrossRef]
- Li, T.; Yan, X.; Liu, J.; Wu, X.; Gong, X.; Zhen, D.; Sun, S.; Chen, W.; He, G. Friedel-Crafts alkylation route for preparation of pendent side chain imidazolium-functionalized polysulfone anion exchange membranes for fuel cells. J. Membr. Sci. 2019, 573, 157–166. [Google Scholar] [CrossRef]
- Ma, L.; Qaisrani, N.A.; Hussain, M.; Li, L.; Jia, Y.; Ma, S.; Zhou, R.; Bai, L.; He, G.; Zhang, F. Cyclodextrin modified, multication cross-linked high performance anion exchange membranes for fuel cell application. J. Membr. Sci. 2020, 607, 118190. [Google Scholar] [CrossRef]
- Iravaninia, M.; Azizi, S.; Rowshanzamir, S. A comprehensive study on the stability and ion transport in cross-linked anion exchange membranes based on polysulfone for solid alkaline fuel cells. Int. J. Hydrogen Energy 2017, 42, 17229–17241. [Google Scholar] [CrossRef]
- Bai, Y.; Yuan, Y.; Yang, Y.; Lu, C. A facile fabrication of functionalized rGO crosslinked chemically stable polysulfone-based anion exchange membranes with enhanced performance. Int. J. Hydrogen Energy 2019, 44, 6618–6630. [Google Scholar] [CrossRef]
- Liu, W.; Liang, N.; Peng, P.; Qu, R.; Chen, D.; Zhang, H. Anion-exchange membranes derived from quaternized polysulfone and exfoliated layered double hydroxide for fuel cells. J. Solid State Chem. 2017, 246, 324–328. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Pan, J.; Sotto, A.; Shen, J. Constructing an internally cross-linked structure for polysulfone to improve dimensional stability and alkaline stability of high performance anion exchange membranes. Int. J. Hydrogen Energy 2019, 44, 8279–8289. [Google Scholar] [CrossRef]
- Teresa Perez-Prior, M.; Urena, N.; Tannenberg, M.; del Rio, C.; Levenfeld, B. DABCO-Functionalized Polysulfones as Anion-Exchange Membranes for Fuel Cell Applications: Effect of Crosslinking. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1326–1336. [Google Scholar] [CrossRef]
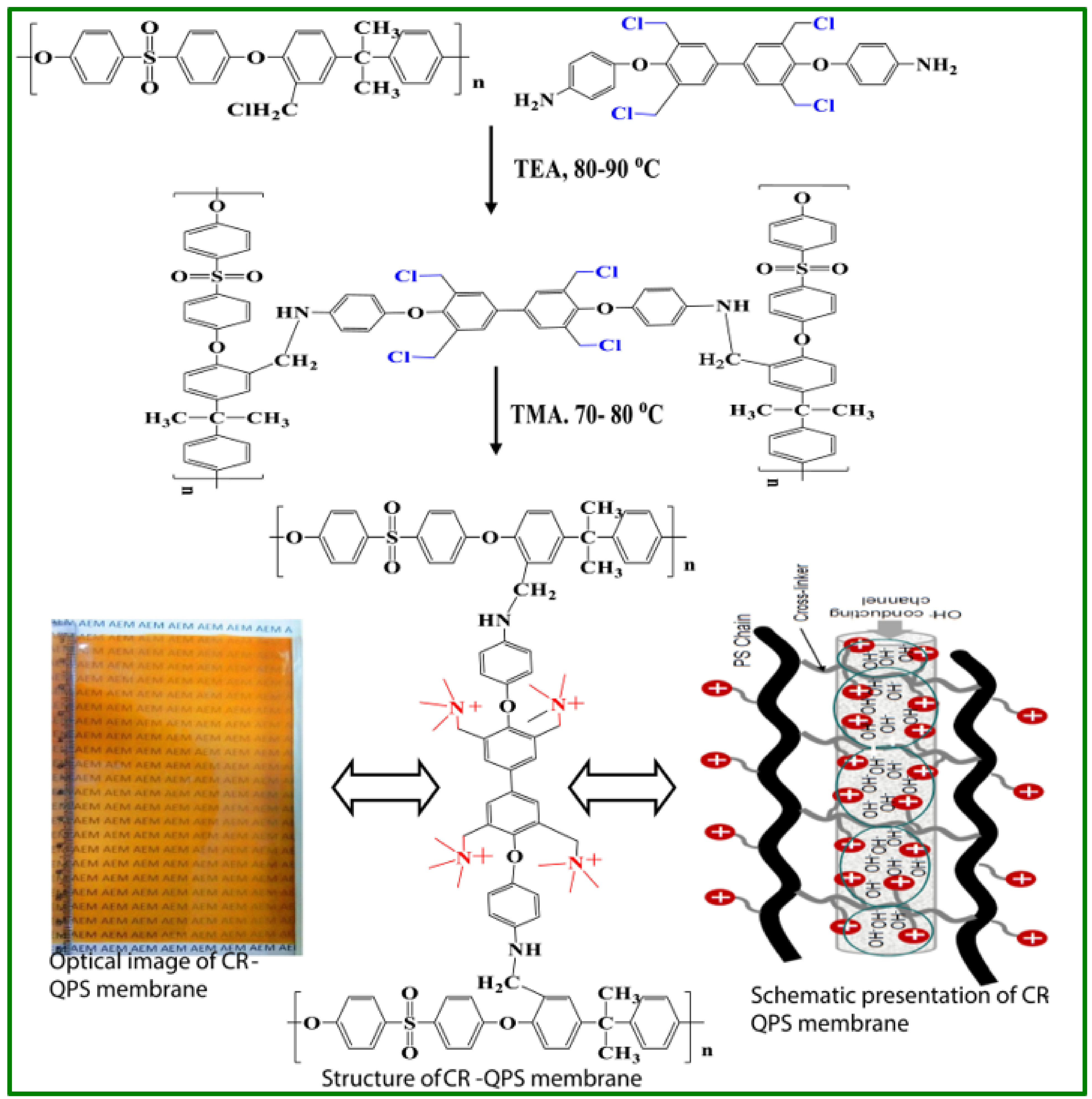
- Sharma, P.; Manohar, M.; Kumar, S.; Shahi, V.K. Highly charged and stable cross-linked polysulfone alkaline membrane for fuel cell applications: 4,4,-((3,3′-bis(chloromethyl)-(1,1′-biphenyl)-4,4-diyl) bis(oxy))dianiline (BCBD) a novel cross-linker. Int. J. Hydrogen Energy 2020, 45, 18693–18703. [Google Scholar] [CrossRef]
- Msomi, P.F.; Nonjola, P.T.; Ndungu, P.G.; Ramontja, J. Poly (2, 6-dimethyl-1, 4-phenylene)/polysulfone anion exchange membrane blended with TiO2 with improved water uptake for alkaline fuel cell application. Int. J. Hydrogen Energy 2020, 45, 29465–29476. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Arangadi, A.F.; Velu, S.; Banat, F.; Show, P.L. ZrO2 incorporated polysulfone anion exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2020, 45, 29668–29680. [Google Scholar] [CrossRef]


| IEM | CEM | AEM |
|---|---|---|
| Counter ion | H+ conductive | OH− conductive |
| Ion-exchange group | -SO3−; -PO4−; -CO2− | Quaternary ammonium cation, 1-methyl pyridinium |
| Features | High ionic conductivity, excellent ionomer solution | Non-noble metal catalyst can be used. Oxygen reduction reaction and methanol oxidation reaction are more facile. |
| Issues | High-cost materials, fuel crossover, chemical, and mechanical stability, practical lifetime | Low ionic conductivity, low thermostability, influence of CO2, durability, chemical, and mechanical stability |
| Membrane | Membrane Characteristics | Fuel Cell Performance | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| WA (%) | IEC (meq. g−1) | Ionic Conductivity (S cm−1) | Methanol Permeability | Selectivity Ratio | Oxidative Stability | |||
| PSF/MOF/Si nanocomposite | 16.50 | 0.86 | 0.017 @ 70°C | --- | --- | --- | OCV: 0.90 V; PD: 40.80 mW cm−2 @ 160 °C | [67] |
| Crosslinked CNDs-SPPSU | 134 | 1.67 | 0.0563 @ 80 °C | --- | --- | --- | OCV: 1.0224 V @ 100% RH | [68] |
| SPEESSA/sulfonic acid zeolite composite | 29.12 | 3.189 | 0.124 | --- | --- | --- | OCV: 0.91 V; PD: 0.45 W cm−2 @ 1.1 A cm−2 | [69] |
| SPSU/NIMs-GO composite | 34.1 | 1.49 | 0.23 @ 75 °C | --- | --- | --- | OCV: 1.038 V; PD: 167.6 mW cm−2 @ 60 °C | [70] |
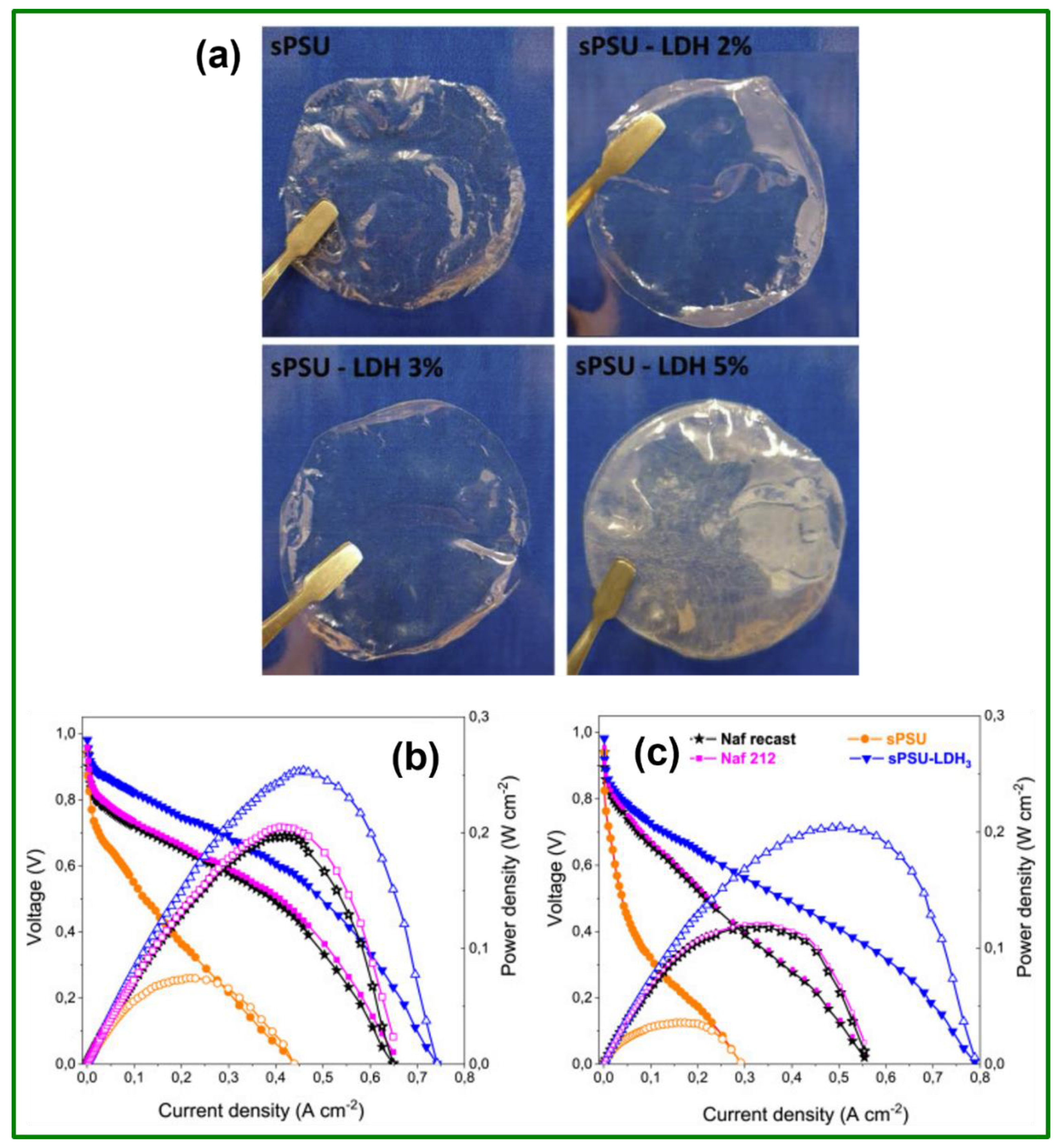
| SPSU-LDH composite | 31 | 1.49 | 0.0137 @ 120 °C | --- | --- | --- | PD: 204.5 mW cm−2 @ 110 °C | [71] |
| PSF-N-C3H6SO3H/ PSF-O-C3H6SO3H | 60 | 2.03 | 0.04666 | 2.65 × 10−8 cm2 s−1 | --- | 94.12% residual mass remains at 80 °C for 1 h in Fenton’s solution | --- | [72] |
| PSU/SPANI/Nb2O5 nanocomposite | 17.6 | 1.50 | 0.0674 | --- | --- | 98.6% residual mass remains in Fenton’s solution | --- | [73] |
| PSU-g-phosphonated polystyrene/SPSU composite | 23.07 | --- | 0.0172 @ 95 °C | 0.96 × 10−8 cm2 s−1 | --- | >95% residual mass remains at 25 °C for 120 h in Fenton’s solution | --- | [79] |
| Phosphonated PSU | 6.6 | 2.75 | 0.0003 @ 160 °C | --- | --- | 87.7% residual mass remains for 70 h in Fenton’s solution | --- | [82] |
| PA doped TDAP-g-PSU | --- | --- | 0.056 @ 160 °C | --- | --- | --- | OCV: 0.92 V; PD: 453 mW cm−2 @ 150 °C | [83] |
| Poly(1-vinylimidazole)-g-PSU | 220.3 | --- | 0.127 @ 160 °C | --- | --- | --- | OCV: 0.98 V; PD: 559 mW cm−2 @ 160 °C | [84] |
| Membrane | Membrane Characteristics | Fuel Cell Performance | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| WA (%) | IEC (meq.g−1) | Ionic Conductivity (S cm−1) | Methanol Permeability (cm2 s−1) | Selectivity Ratio (sS cm−3) | Oxidative Stability | |||
| SPSU/LDH nanocomposite | 29 | 1.49 | 0.102 @ 120 °C | 116 mA cm−2 | --- | --- | OCV: 0.82 V; PD: 150 mW cm−2 @ 80 °C in 5 M CH3OH | [105] |
| Amino-acid functionalized cellulose whiskers/SPSU | 68 | --- | 0.234 @ 80 °C | 7.6 × 10−7 | --- | --- | OCV: 0.73 V; PD: 73.757 mW cm−2 @ 60 °C in 2 M CH3OH | [106] |
| SPSU/ZrP | 38 | --- | 0.156 @ 80 °C | --- | --- | 96.66% of weight retention after Fenton test | OCV: 0.75 V; PD: 119 mW cm−2 @ 80 °C | [107] |
| Sulfonated ZSM-5/SPSU | 45.41 | 1.03 | 0.00965 @ RT | 2.24 × 10−6 | 4309.03 | --- | --- | [108] |
| SPSU/SPEEK | 34 | --- | 0.073 @ 120 °C | --- | --- | --- | OCV: 0.81 V; PD: 130 mW cm−2 @ 80 °C in 4 M CH3OH | [109] |
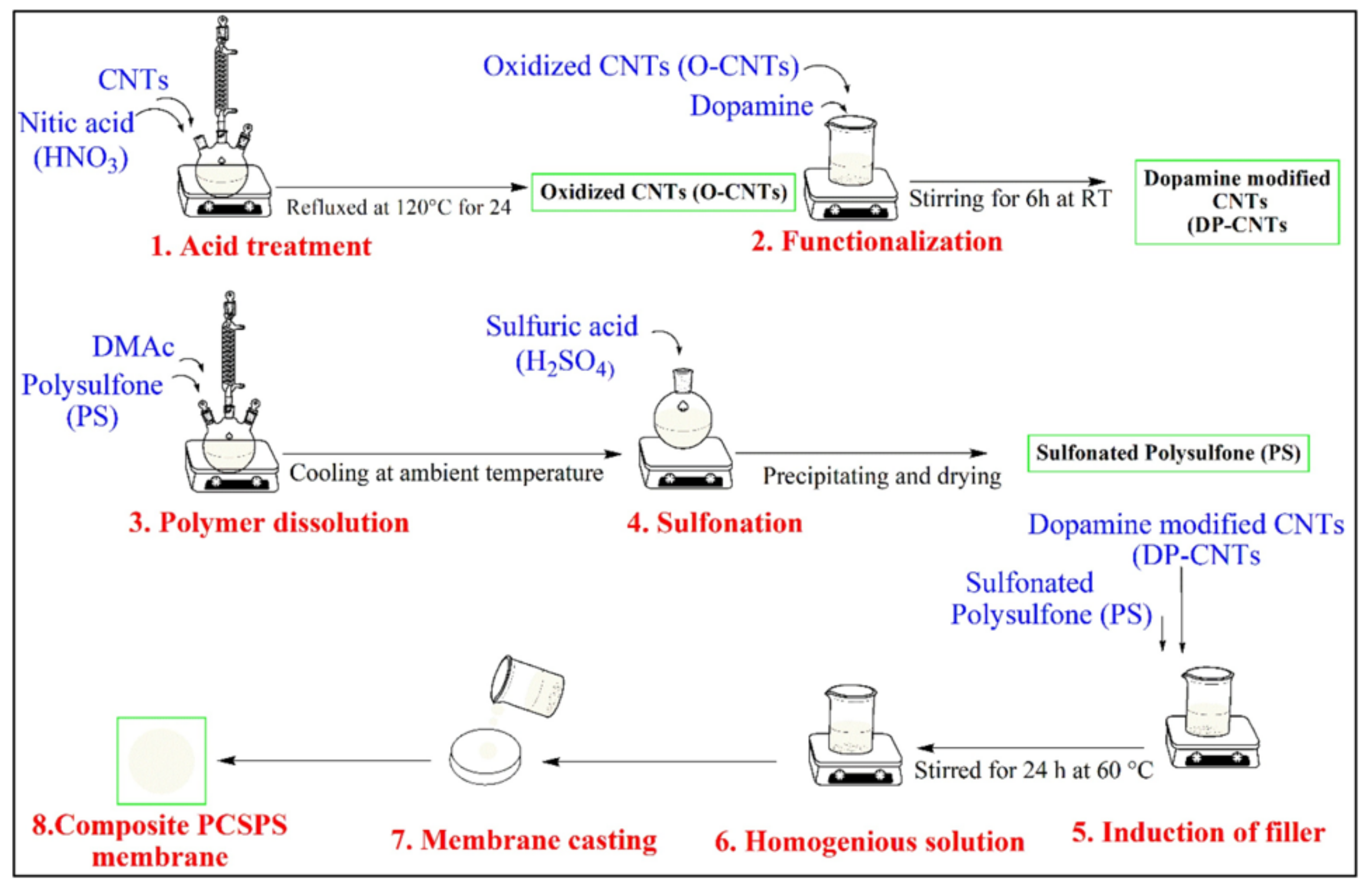
| PD-CNT/SPSU composite | 32 | --- | 0.1216 @ 80 °C | 5.68 × 10−7 | --- | --- | --- | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinodh, R.; Atchudan, R.; Kim, H.-J.; Yi, M. Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review. Polymers 2022, 14, 300. https://doi.org/10.3390/polym14020300
Vinodh R, Atchudan R, Kim H-J, Yi M. Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review. Polymers. 2022; 14(2):300. https://doi.org/10.3390/polym14020300
Chicago/Turabian StyleVinodh, Rajangam, Raji Atchudan, Hee-Je Kim, and Moonsuk Yi. 2022. "Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review" Polymers 14, no. 2: 300. https://doi.org/10.3390/polym14020300
APA StyleVinodh, R., Atchudan, R., Kim, H.-J., & Yi, M. (2022). Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review. Polymers, 14(2), 300. https://doi.org/10.3390/polym14020300