Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
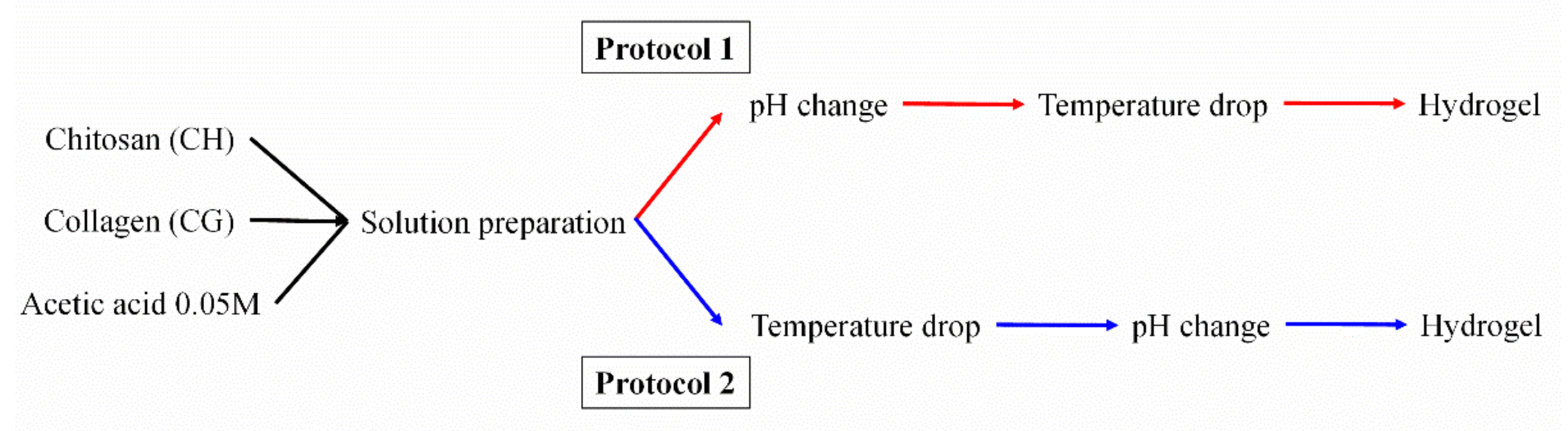
2.2. Processing of Hydrogels
2.3. Rheological Characterization
- -
- Strain sweep tests: these trials were performed to evaluate the linear viscoelastic region, in which stress and strain have a linear relationship without altering the structure of the sample, and the critical strain (last strain in the linear viscoelastic region). These tests were carried out at between 0.1 and 100% of strain and at a constant frequency of 1 Hz and 4 °C.
- -
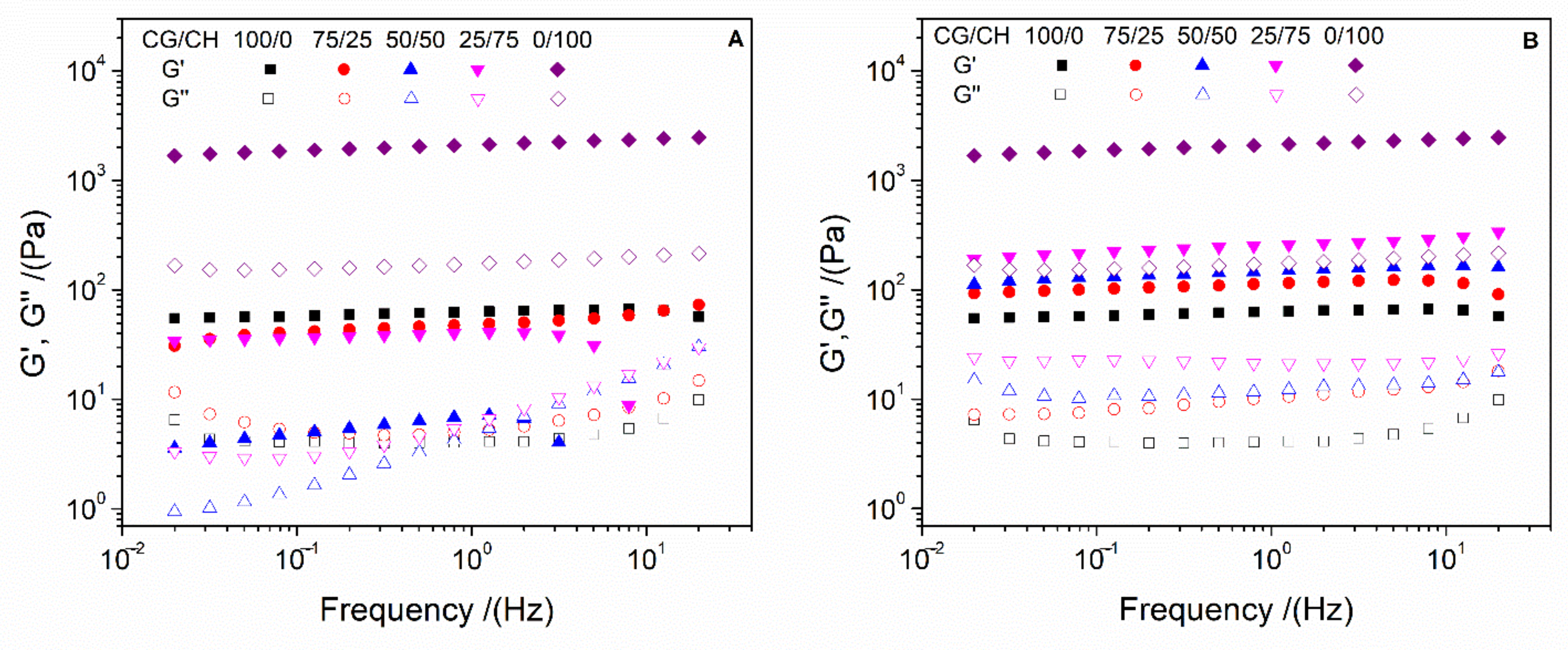
- Frequency sweep tests: These analyses were performed at between 0.02 and 20 Hz and at a constant strain of 2% (below of the critical strain) and 4 °C. Thus, the behavior of the elastic (G′) and viscous (G″) moduli was evaluated in the entire frequency range studied. In addition, G′ and loss tangent (tan δ = G″/G′) at 1 Hz (G′1 and tan δ1, respectively) were evaluated to improve the comparison between the systems.
- -
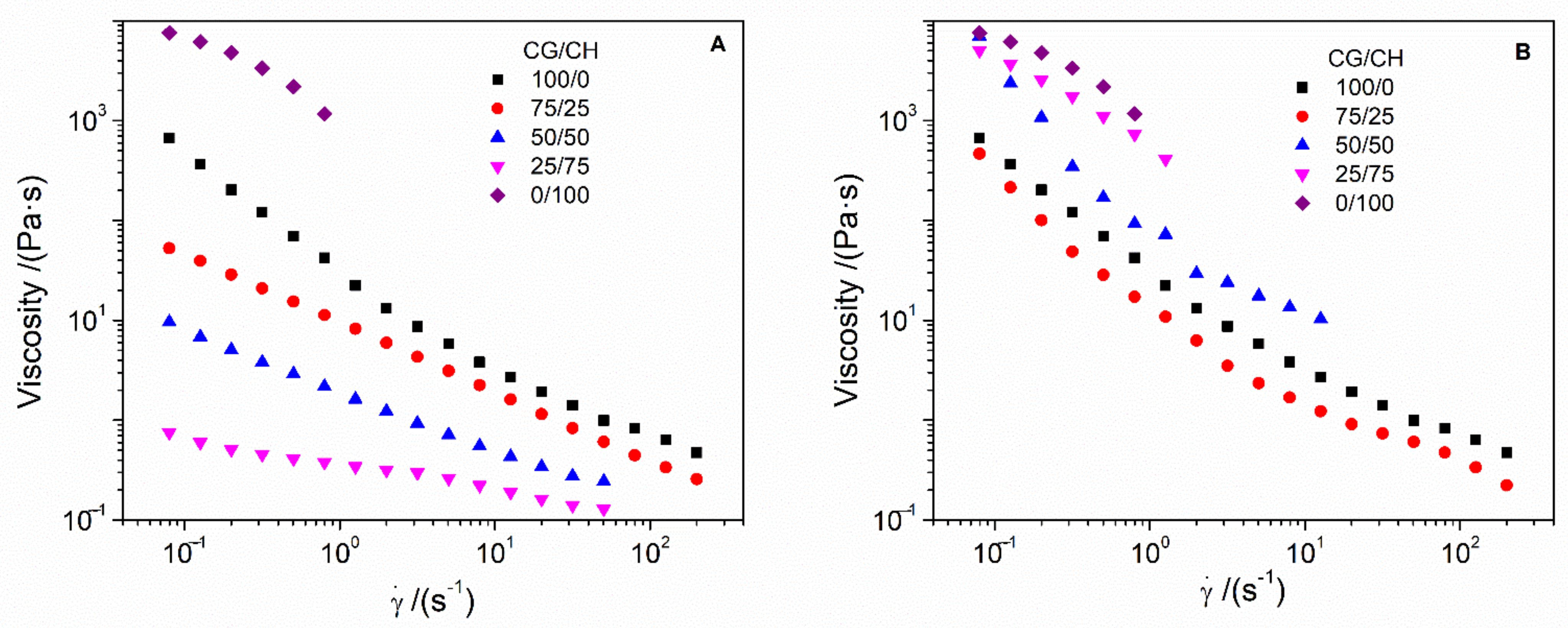
- Flow curves: These tests were carried out at between 0.02 and 200 s−1 of shear rate (γ) and at a constant strain of 2% and 4 °C. In this way, these tests allow the systems to be categorized as Newtonian, dilatant or pseudoplastic according to the dependence of viscosity on shear rate.
- -
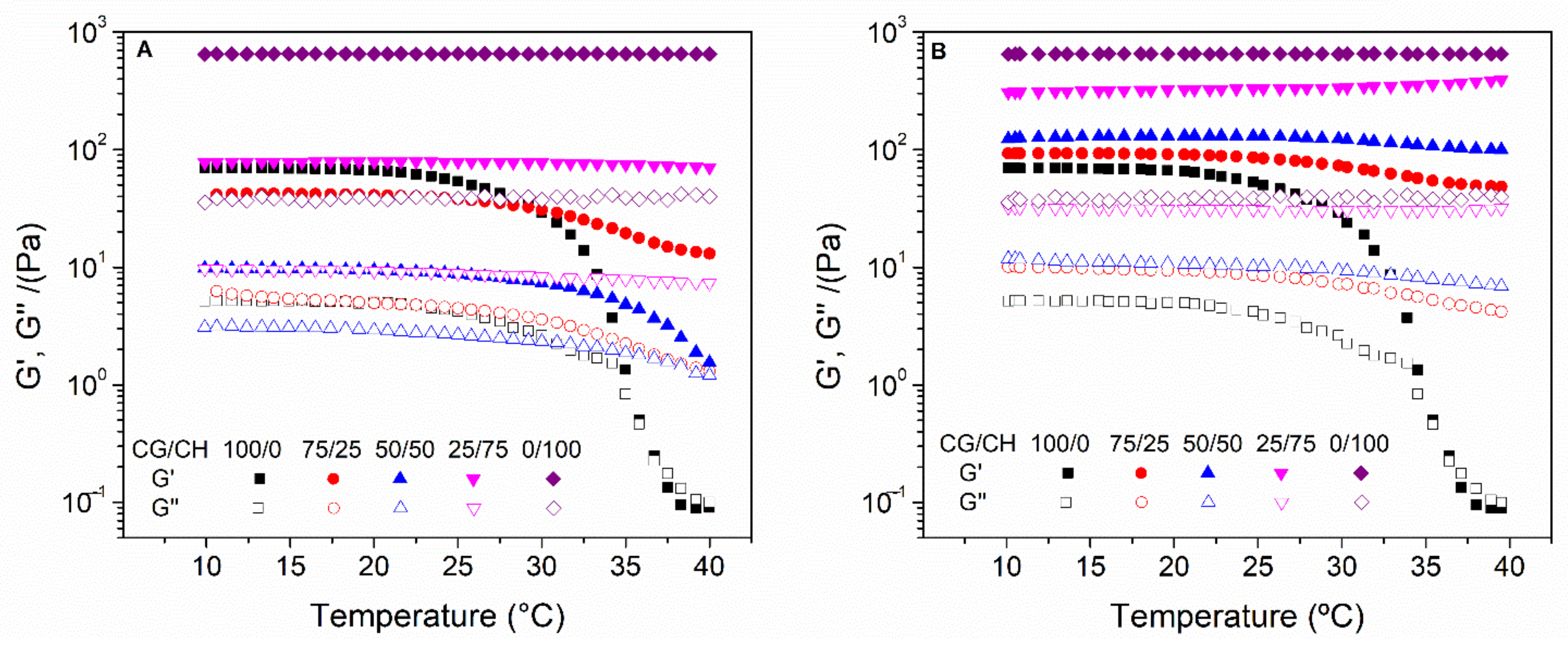
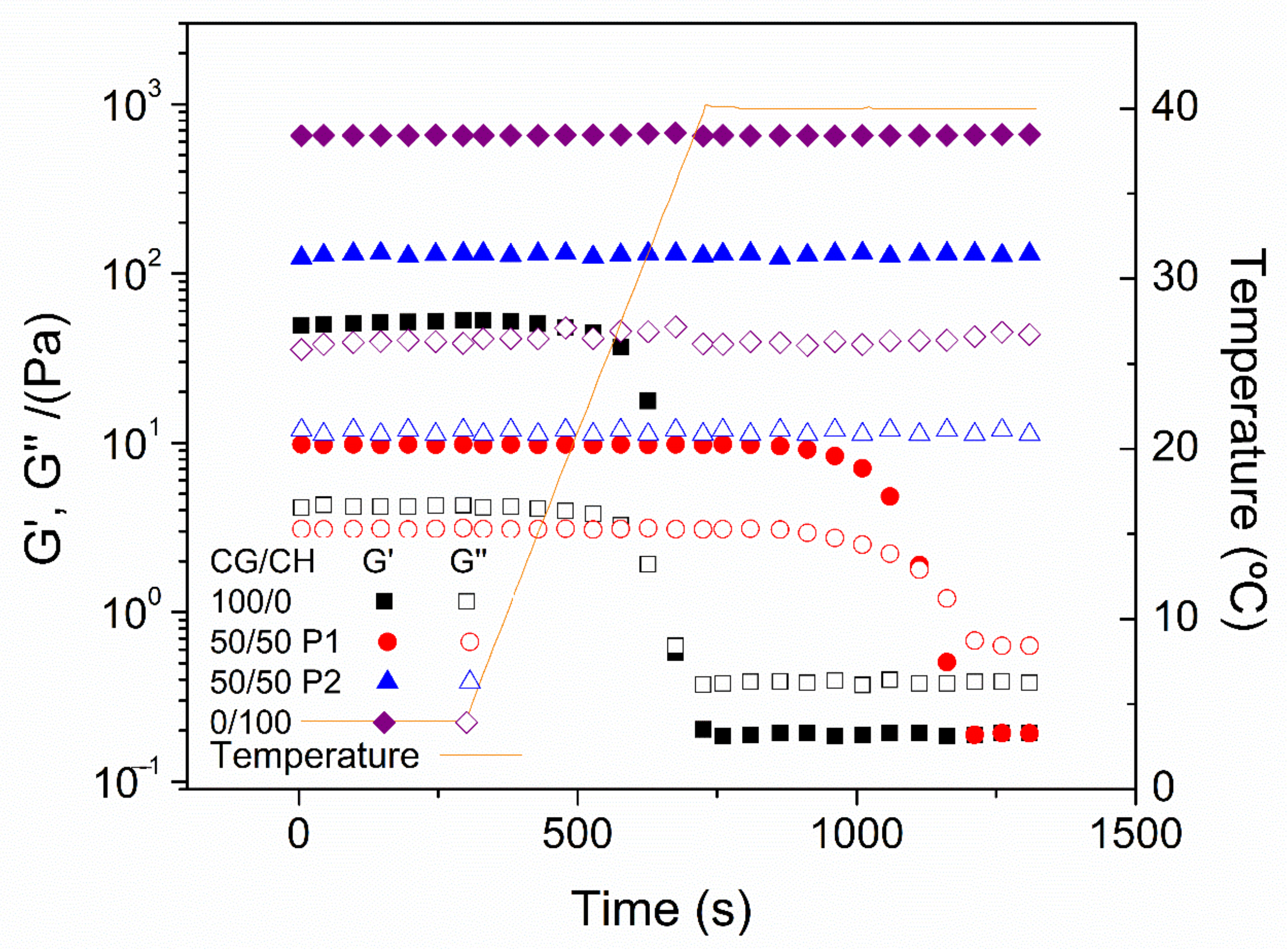
- Temperature ramp tests: The stability of hydrogels with temperature is an important aspect of these systems since they will be incorporated in humans. Thus, temperature ramp tests were performed at 10–40 °C and at a constant strain of 2% and 1 Hz, with a rate of 5 °C/min.
- -
- Time tests: Finally, the hydrogels were evaluated at a temperature of 40 °C during a time interval of 10 min to assess the resistance of the hydrogels at this temperature. These tests were performed with a constant strain of 2% and 1 Hz.
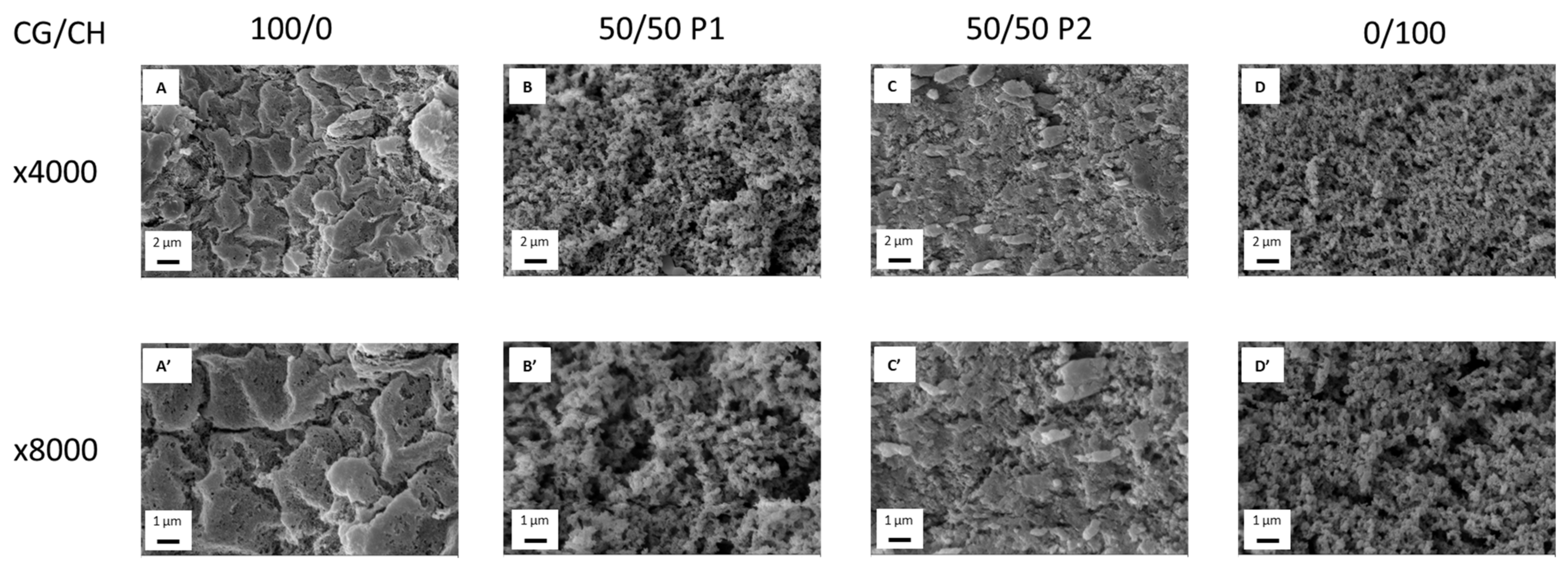
2.4. Microstructural Characterization
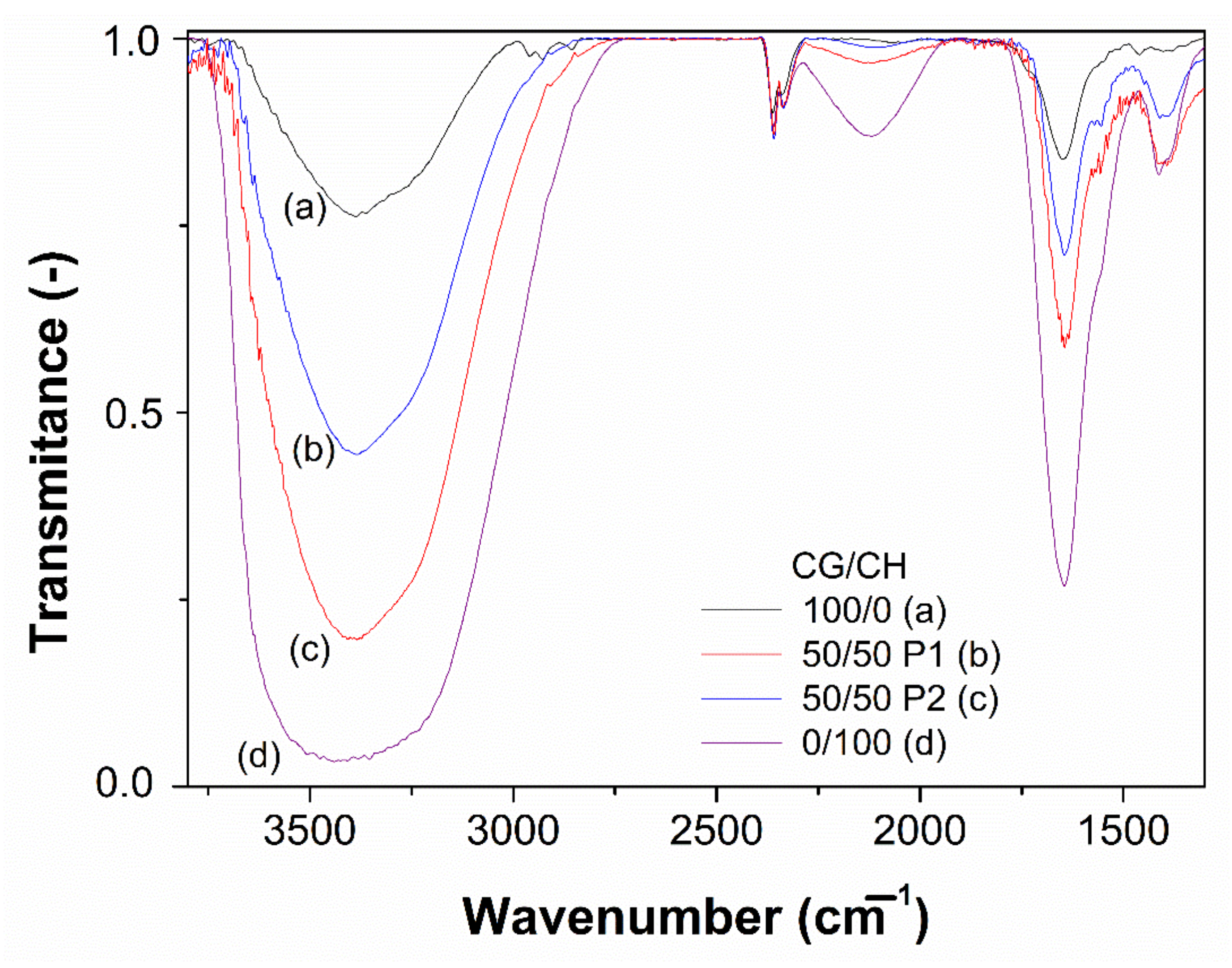
2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Measurements
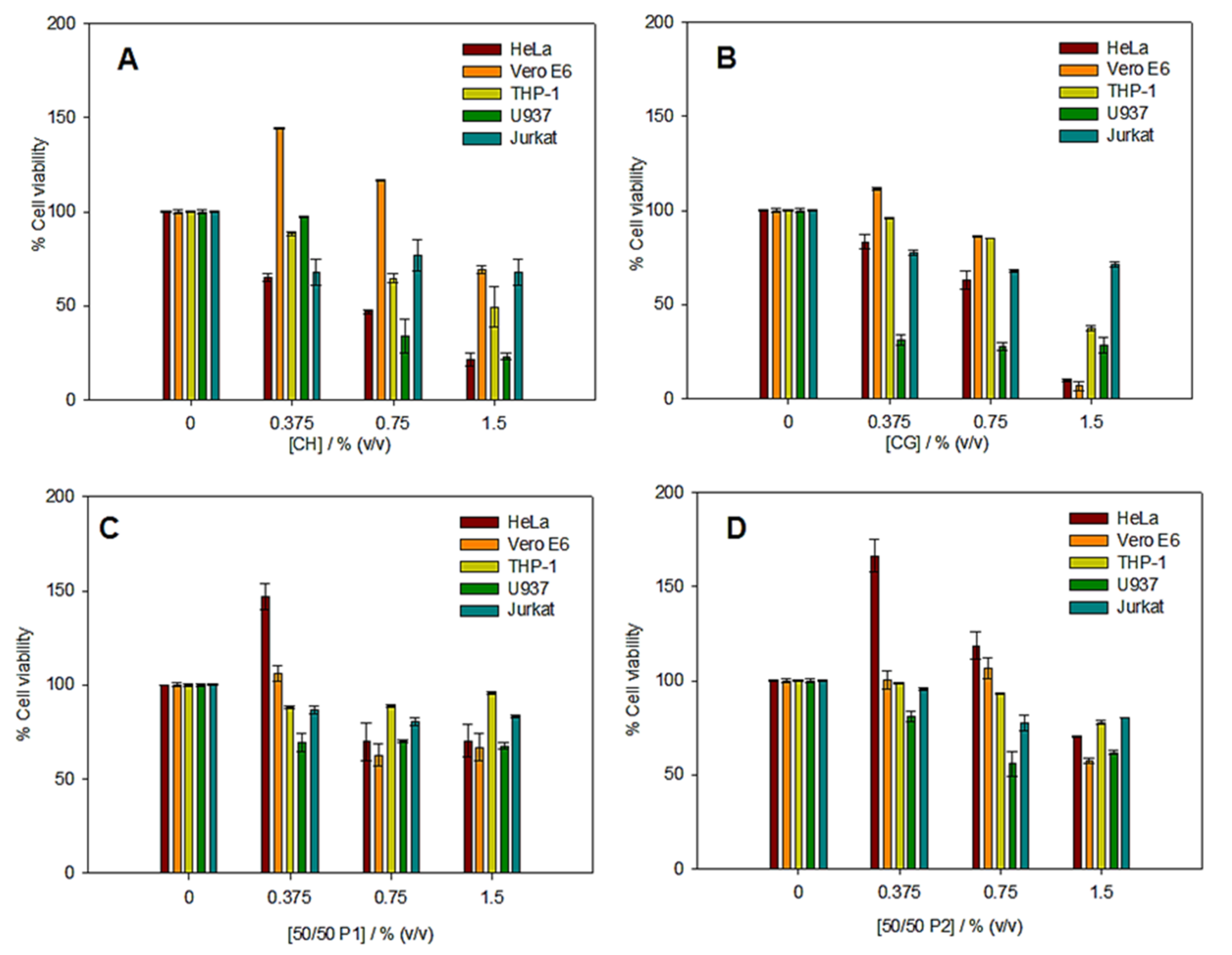
2.6. In Vitro Cytotoxicity Assays
2.7. Statistical Analysis
3. Results and Discussion
3.1. Rheological Characterization
3.2. Microstructural Characterization
3.3. FTIR Measurements
3.4. In Vitro Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]
- Noferini, D.; Faraone, A.; Rossi, M.; Mamontov, E.; Fratini, E.; Baglioni, P. Disentangling Polymer Network and Hydration Water Dynamics in Polyhydroxyethyl Methacrylate Physical and Chemical Hydrogels. J. Phys. Chem. C 2019, 123, 19183–19194. [Google Scholar] [CrossRef]
- Soto, D.; Oliva, H. Métodos para preparar hidrogeles químicos y físicos basados en almidón: Una revisión. Rev. Latinoam. Metal. Y Mater. 2012, 32, 154–175. [Google Scholar]
- Choi, J.H.; Choi, O.K.; Lee, J.; Noh, J.; Lee, S.; Park, A.; Rim, M.A.; Reis, R.L.; Khang, G. Evaluation of double network hydrogel of poloxamer-heparin/gellan gum for bone marrow stem cells delivery carrier. Colloids Surf. B Biointerfaces 2019, 181, 879–889. [Google Scholar] [CrossRef]
- Rubio-Valle, J.F.; Perez-Puyana, V.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Evaluation of smart gelatin matrices for the development of scaffolds via 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2021, 115, 104267. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Fabrication and characterization of hydrogels based on gelatinised collagen with potential application in tissue engineering. Polymers 2020, 12, 1146. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Zahid Butt, M.T.; Khalid, S.; Shafiq, M.; Islam, A.; Asim, S.; Hafeez, S.; Khan, R.U. In vitro study of chitosan-based multi-responsive hydrogels as drug release vehicles: A preclinical study. RSC Adv. 2019, 9, 31078–31091. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Khalid, S.; Zia, S.; Islam, A.; Sabir, A.; Sultan, M.; Hussain, F.; Khan, R.U.; Butt, M.T.Z. Designing of biocompatible and biodegradable chitosan based crosslinked hydrogel for in vitro release of encapsulated povidone-iodine: A clinical translation. Int. J. Biol. Macromol. 2020, 164, 4370–4380. [Google Scholar] [CrossRef]
- Milojević, M.; Harih, G.; Vihar, B.; Vajda, J.; Gradišnik, L.; Zidarič, T.; Stana Kleinschek, K.; Maver, U.; Maver, T. Hybrid 3D Printing of Advanced Hydrogel-Based Wound Dressings with Tailorable Properties. Pharmaceutics 2021, 13, 564. [Google Scholar] [CrossRef]
- Bashari, A.; Rouhani Shirvan, A.; Shakeri, M. Cellulose-based hydrogels for personal care products. Polym. Adv. Technol. 2018, 29, 2853–2867. [Google Scholar] [CrossRef]
- Akbari, E.; Imani, R.; Shokrollahi, P.; Heidari keshel, S. Preparation of Nanoparticle-Containing Ring-Implanted Poly(Vinyl Alcohol) Contact Lens for Sustained Release of Hyaluronic Acid. Macromol. Biosci. 2021, 21, 2100043. [Google Scholar] [CrossRef]
- Zohreband, Z.; Adeli, M.; Zebardasti, A. Self-healable and flexible supramolecular gelatin/MoS2 hydrogels with molecular recognition properties. Int. J. Biol. Macromol. 2021, 182, 2048–2055. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Pei, R. An in situ Gelling BMSC-laden Collagen/Silk Fibroin Double Network Hydrogel for Cartilage Regeneration. Mater. Adv. 2021, 2, 4733–4742. [Google Scholar] [CrossRef]
- Chopra, H.; Singh, I.; Kumar, S.; Bhattacharya, T.; Rahman, M.H.; Akter, R.; Kabir, M.T. Comprehensive Review on Hydrogels. Curr. Drug Deliv. 2021, 18, 1. [Google Scholar] [CrossRef]
- Mozafari, M.; Sefat, F.; Atala, A. Handbook of Tissue Engineering Scaffolds: Volume One; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081025635. [Google Scholar]
- Gorgieva, S.; Kokol, V. Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. In Biomaterials Applications for Nanomedicine; InTech: Ljubljana, Slovenia, 2011. [Google Scholar]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Valencia-Gómez, L.E.; Martel-Estrada, S.A.; Vargas-Requena, C.L.; Rodriguez-González, C.A.; Olivas-Arnendariz, I. Natural polymers aposites for skin regeneration. Rev. Mex. Ing. Bioméd. 2016, 37, 235–249. [Google Scholar] [CrossRef]
- Kanungo, I.; Fathima, N.N.; Rao, J.R.; Nair, B.U. Influence of PCL on the material properties of collagen based biocomposites and in vitro evaluation of drug release. Mater. Sci. Eng. C 2013, 33, 4651–4659. [Google Scholar] [CrossRef]
- Coombes, A.G.; Verderio, E.; Shaw, B.; Li, X.; Griffin, M.; Downes, S. Biocomposites of non-crosslinked natural and synthetic polymers. Biomaterials 2002, 23, 2113–2118. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, K.; Feng, Q.; Cui, F. Fibroin/collagen hybrid hydrogels with crosslinking method: Preparation, properties, and cytocompatibility. J. Biomed. Mater. Res. Part A 2008, 84A, 198–207. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Rubio-Valle, J.F.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Alternative processing methods of hybrid porous scaffolds based on gelatin and chitosan. J. Mech. Behav. Biomed. Mater. 2020, 102, 103472. [Google Scholar] [CrossRef]
- Khan, A.; Alamry, K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: A review. Carbohydr. Res. 2021, 506, 108368. [Google Scholar] [CrossRef] [PubMed]
- Gull, N.; Khan, S.M.; Butt, O.M.; Islam, A.; Shah, A.; Jabeen, S.; Khan, S.U.; Khan, A.; Khan, R.U.; Butt, M.T.Z. Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2020, 162, 175–187. [Google Scholar] [CrossRef]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Alonso-González, M.; Romero, A.; Perez-Puyana, V. Applied Rheology as Tool for the Assessment of Chitosan Hydrogels for Regenerative Medicine. Polymers 2021, 13, 2189. [Google Scholar] [CrossRef]
- Franco, M.K.K.D.; Sepulveda, A.F.; Vigato, A.A.; Oshiro, A.; Machado, I.P.; Kent, B.; Clemens, D.; Yokaichiya, F.; Araujo, D.R. Supramolecular Structure of Temperature-Dependent Polymeric Hydrogels Modulated by Drug Incorporation. Chem. Sel. 2020, 5, 12853–12861. [Google Scholar] [CrossRef]
- Tan, W.; Krishnaraj, R.; Desai, T.A. Evaluation of Nanostructured Composite Collagen–Chitosan Matrices for Tissue Engineering. Tissue Eng. 2001, 7, 203–210. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, P.; Vulesevic, B.; Kuraitis, D.; Li, F.; Yang, A.F.; Griffith, M.; Ruel, M.; Suuronen, E.J. A Collagen–Chitosan Hydrogel for Endothelial Differentiation and Angiogenesis. Tissue Eng. Part A 2010, 16, 3099–3109. [Google Scholar] [CrossRef]
- McBane, J.E.; Vulesevic, B.; Padavan, D.T.; McEwan, K.A.; Korbutt, G.S.; Suuronen, E.J. Evaluation of a Collagen-Chitosan Hydrogel for Potential Use as a Pro-Angiogenic Site for Islet Transplantation. PLoS ONE 2013, 8, e77538. [Google Scholar] [CrossRef]
- Gilarska, A.; Hinz, A.; Bzowska, M.; Dyduch, G.; Kamiński, K.; Nowakowska, M.; Lewandowska-Łańcucka, J. Addressing the Osteoporosis Problem—Multifunctional Injectable Hybrid Materials for Controlling Local Bone Tissue Remodeling. ACS Appl. Mater. Interfaces 2021, 13, 49762–49779. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.; Vårum, K.; Draget, K.; Nordgård, C. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Jones, C.A.R.; Liang, L.; Lin, D.; Jiao, Y.; Sun, B. The spatial-temporal characteristics of type I collagen-based extracellular matrix. Soft Matter 2014, 10, 8855–8863. [Google Scholar] [CrossRef] [PubMed]
- Rebers, L.; Granse, T.; Tovar, G.; Southan, A.; Borchers, K. Physical Interactions Strengthen Chemical Gelatin Methacryloyl Gels. Gels 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Zheng, T.; Tang, P.; Shen, L.; Bu, H.; Li, G. Rheological behavior of collagen/chitosan blended solutions. J. Appl. Polym. Sci. 2021, 138, 50840. [Google Scholar] [CrossRef]
- Coradin, T.; Wang, K.; Law, T.; Trichet, L. Type I Collagen-Fibrin Mixed Hydrogels: Preparation, Properties and Biomedical Applications. Gels 2020, 6, 36. [Google Scholar] [CrossRef]
- Duan, J.; Liang, X.; Cao, Y.; Wang, S.; Zhang, L. High Strength Chitosan Hydrogels with Biocompatibility via New Avenue Based on Constructing Nanofibrous Architecture. Macromolecules 2015, 48, 2706–2714. [Google Scholar] [CrossRef]
- Vulpe, R.; Le Cerf, D.; Dulong, V.; Popa, M.; Peptu, C.; Verestiuc, L.; Picton, L. Rheological study of in-situ crosslinkable hydrogels based on hyaluronanic acid, collagen and sericin. Mater. Sci. Eng. C 2016, 69, 388–397. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Liu, L.; Wen, H.; Rao, Z.; Zhu, C.; Liu, M.; Min, L.; Fan, L.; Tao, S. Preparation and characterization of chitosan—Collagen peptide/oxidized konjac glucomannan hydrogel. Int. J. Biol. Macromol. 2018, 108, 376–382. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Schipper, N.G.M.; Vårum, K.M.; Stenberg, P.; Ocklind, G.; Lennernäs, H.; Artursson, P. Chitosans as absorption enhancers of poorly absorbable drugs. Eur. J. Pharm. Sci. 1999, 8, 335–343. [Google Scholar] [CrossRef]
- Schipper, N.G.; Varum, K.M.; Artursson, P. Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm. Res. 1996, 13, 1686–1692. [Google Scholar] [CrossRef]
- Najafi, M.F.; Vahedi, F.; Ahmadi, S.; Madani, R.; Mehrvarz, M. Effect of Collagen Type I (Rat Tail) on Cell Proliferation and Adhesion of BHK-21. In Proceedings of the 4th Kuala Lumpur International Conference on Biomedical Engineering 2008, Kuala Lumpur, Malaysia, 25–28 June 2008; Springer: Berlin/Heidelberg, Germany, 2008; pp. 806–809. [Google Scholar]
- Koyano, T.; Minoura, N.; Nagura, M.; Kobayashi, K. Attachment and growth of cultured fibroblast cells on PVA/chitosan-blended hydrogels. J. Biomed. Mater. Res. 1998, 39, 486–490. [Google Scholar] [CrossRef]
| Protocol | CG/CH Ratio | Critical Strain (%) | G′1 (Pa) | tan δ1 (-) |
|---|---|---|---|---|
| 1 | 100/0 | 63.5 a | 63 A | 0.065 I |
| 75/25 | >100 b | 49 AC | 0.107 II | |
| 50/50 | >100 b | 7 B | 0.778 III | |
| 25/75 | 15.9 c | 40 C | 0.167 IV | |
| 0/100 | 10.0 d | 2140 D | 0.082 I, I | |
| 2 | 100/0 | 63.5 a | 63 A | 0.065 I |
| 75/25 | >100 b | 115 E | 0.092 II | |
| 50/50 | >100 b | 150 F | 0.082 I, II | |
| 25/75 | 25.3 e | 258 G | 0.082 I, I | |
| 0/100 | 10.0 d | 2140 D | 0.082 I, I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Cid, P.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Romero, A.; Ostos, F.J.; Rafii-El-Idrissi Benhnia, M.; Perez-Puyana, V. Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan. Polymers 2022, 14, 272. https://doi.org/10.3390/polym14020272
Sánchez-Cid P, Jiménez-Rosado M, Rubio-Valle JF, Romero A, Ostos FJ, Rafii-El-Idrissi Benhnia M, Perez-Puyana V. Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan. Polymers. 2022; 14(2):272. https://doi.org/10.3390/polym14020272
Chicago/Turabian StyleSánchez-Cid, Pablo, Mercedes Jiménez-Rosado, José Fernando Rubio-Valle, Alberto Romero, Francisco J. Ostos, Mohammed Rafii-El-Idrissi Benhnia, and Victor Perez-Puyana. 2022. "Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan" Polymers 14, no. 2: 272. https://doi.org/10.3390/polym14020272
APA StyleSánchez-Cid, P., Jiménez-Rosado, M., Rubio-Valle, J. F., Romero, A., Ostos, F. J., Rafii-El-Idrissi Benhnia, M., & Perez-Puyana, V. (2022). Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan. Polymers, 14(2), 272. https://doi.org/10.3390/polym14020272









