Use of Hazelnut Perisperm as an Antioxidant for Production of Sustainable Biodegradable Active Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Active Film Preparation
2.3. Methods
3. Results
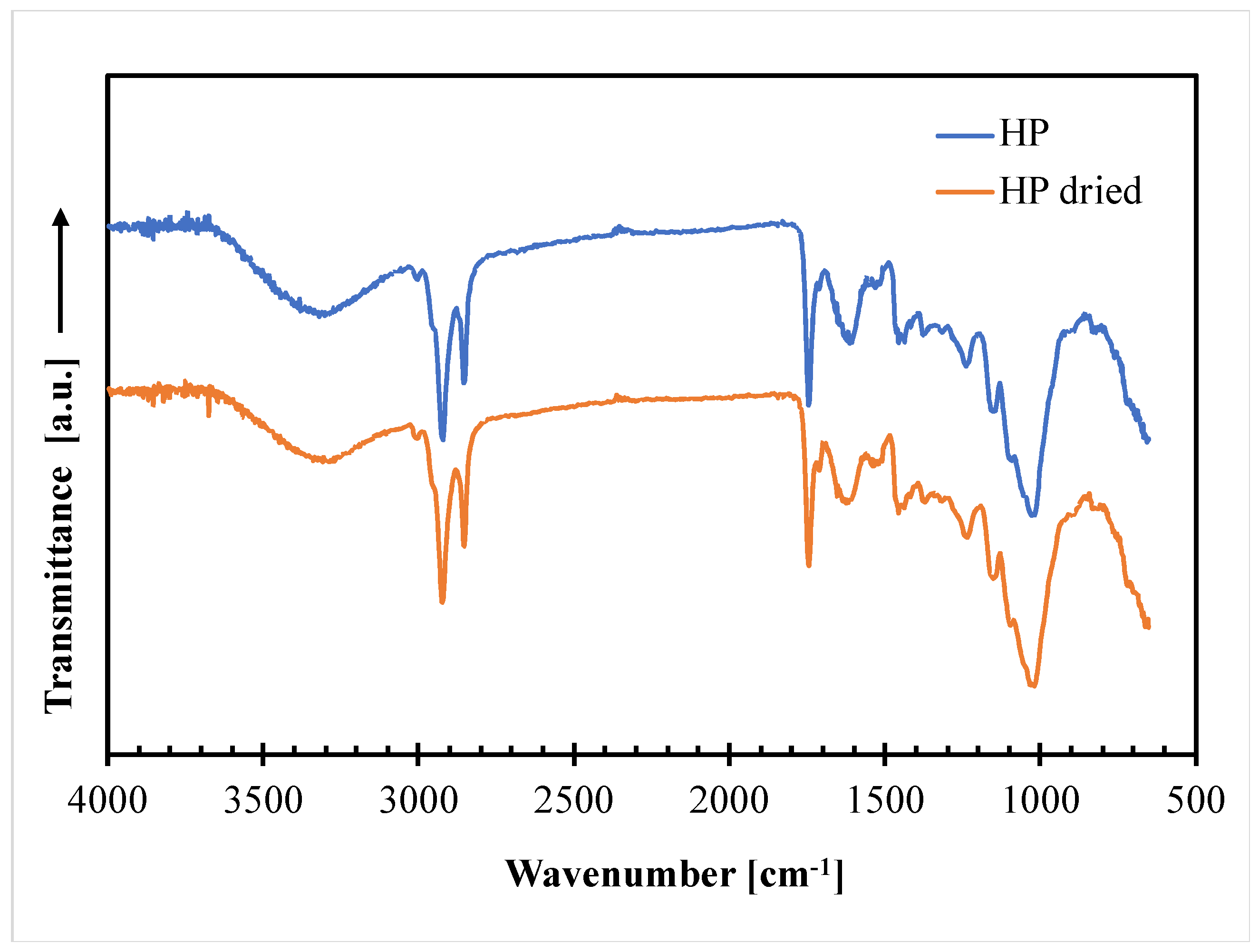
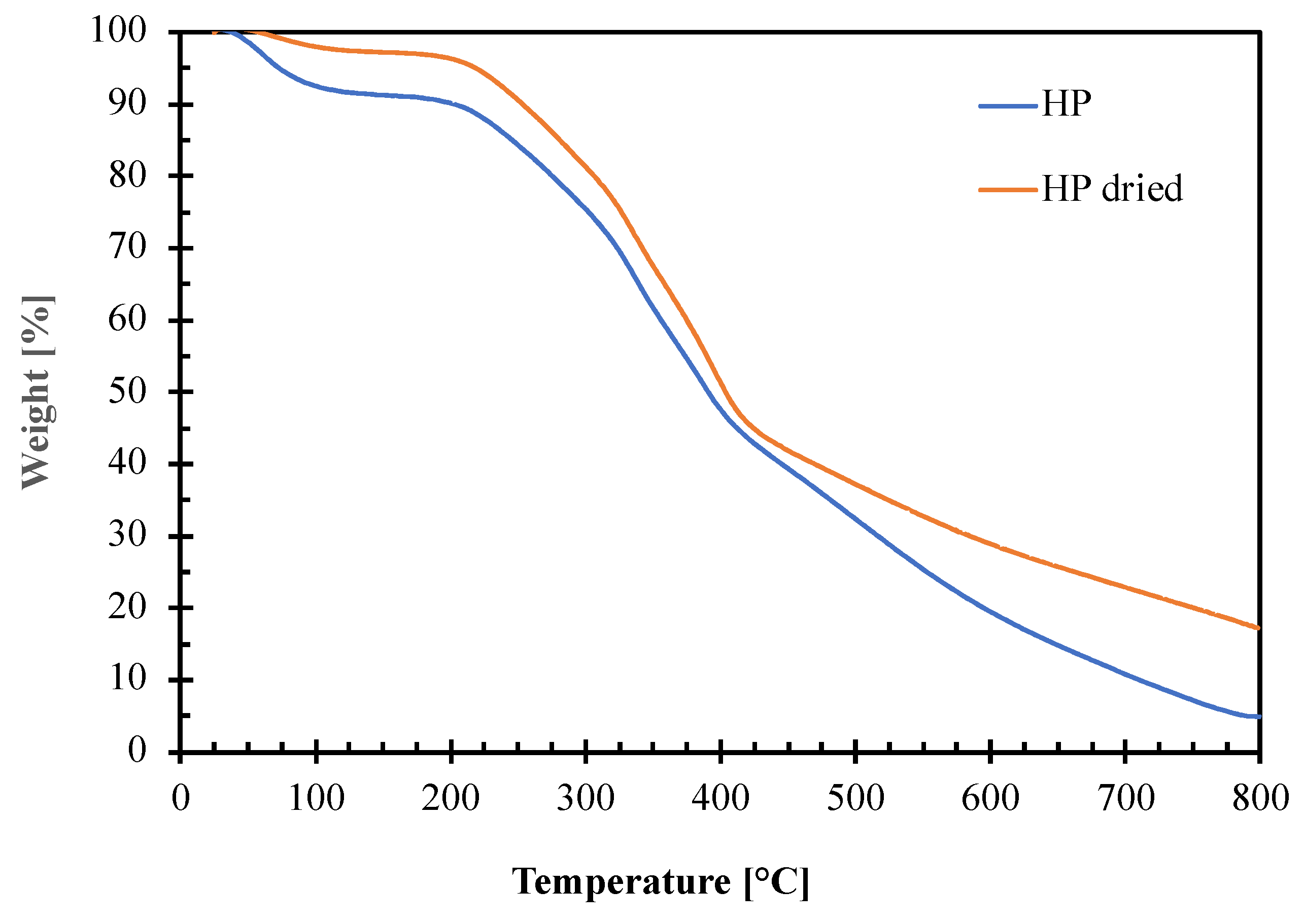
3.1. Hazelnut Perisperm Characterization
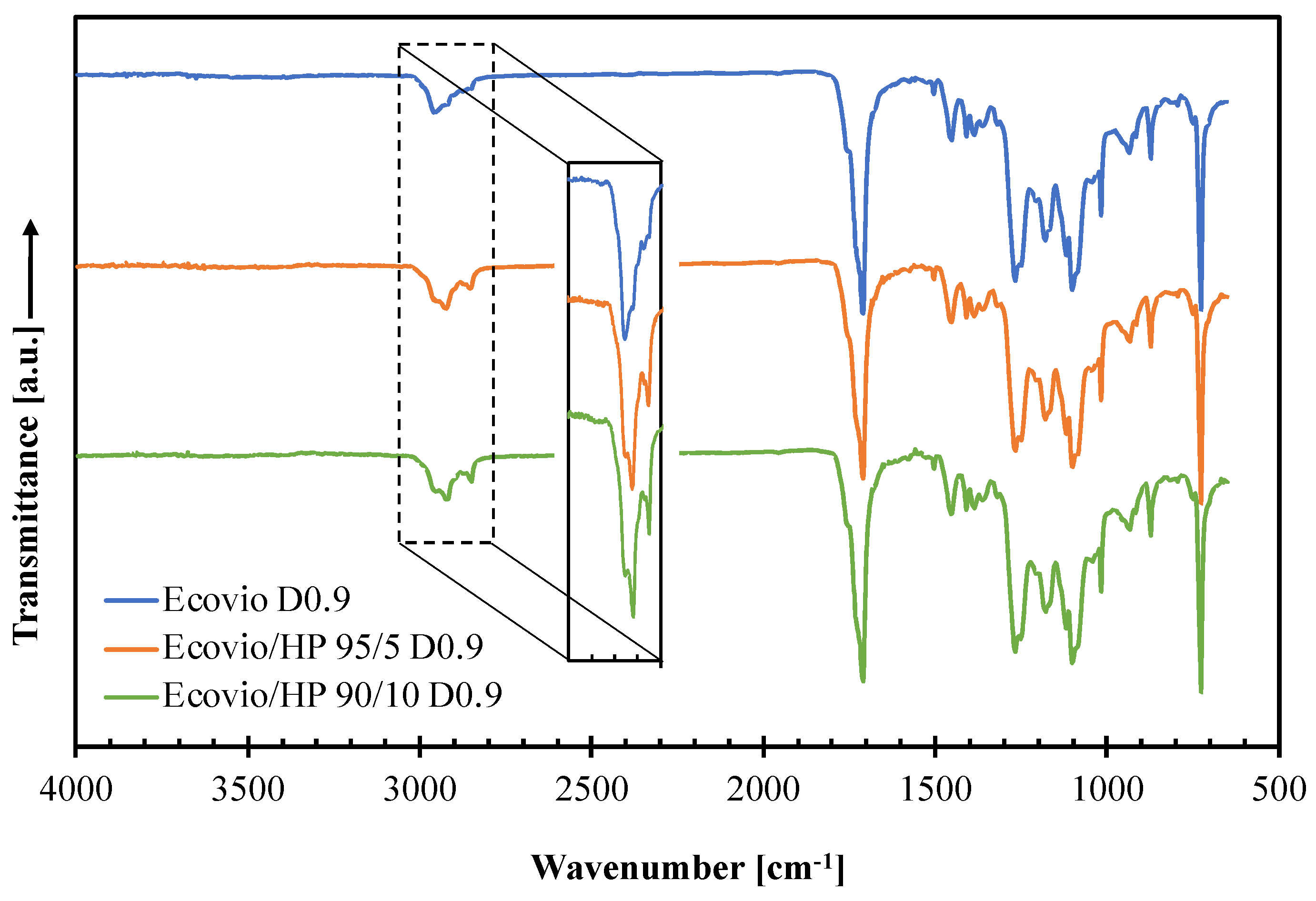
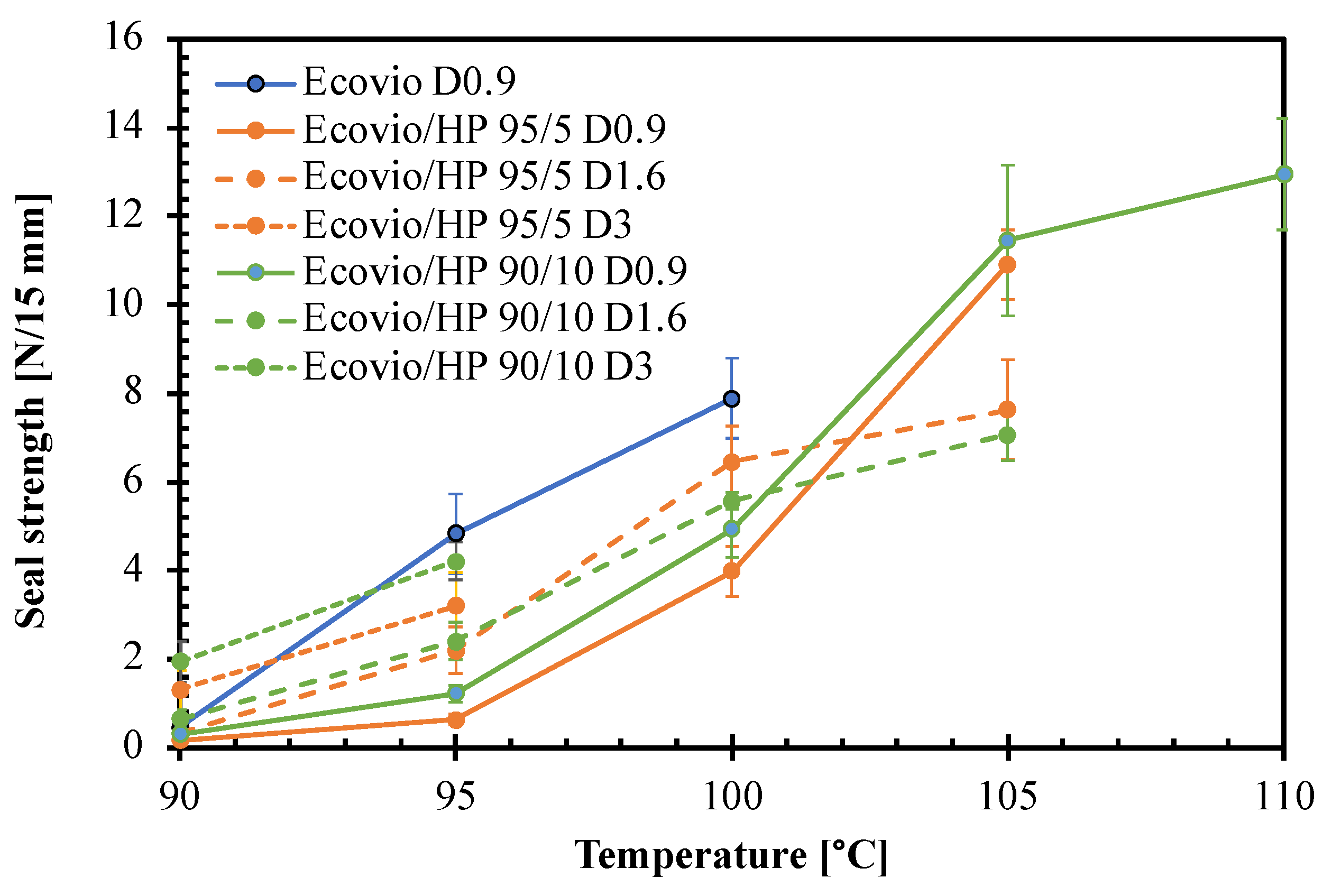
3.2. Active Film Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giwa, A.S.; Ali, N.; Vakili, M.; Guo, X.; Liu, D.; Wang, K. Opportunities for holistic waste stream valorization from food waste treatment facilities: A review. Rev. Chem. Eng. 2022, 38, 35–53. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Hamelin, L.; Thomsen, M. Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy. Sci. Total Environ. 2022, 706, 136033. [Google Scholar] [CrossRef] [PubMed]
- Scarfato, P.; Di Maio, L.; Incarnato, L. Recent advances and migration issues in biodegradable polymers from renewable sources for food packaging. J. Appl. Polym. Sci. 2015, 132, 42597. [Google Scholar] [CrossRef]
- Meherishi, L.; Narayana, S.A.; Ranjani, K.S. Sustainable packaging for supply chain management in the circular economy: A. review. J. Clean. Prod. 2019, 237, 117582. [Google Scholar] [CrossRef]
- Apicella, A.; Barbato, A.; Garofalo, E.; Incarnato, L.; Scarfato, P. Effect of PVOH/PLA + Wax Coatings on Physical and Functional Properties of Biodegradable Food Packaging Films. Polymers 2022, 14, 935. [Google Scholar] [CrossRef] [PubMed]
- Apicella, A.; Scarfato, P.; Di Maio, L.; Incarnato, L. Sustainable Active PET Films by Functionalization with Antimicrobial Bio-Coatings. Front. Mater. 2019, 6, 243. [Google Scholar] [CrossRef]
- Allegrini, A.; Salvaneschi, P.; Schirone, B.; Cianfaglione, K.; Di Michele, A. Multipurpose plant species and circular economy: Corylus avellana L. as a study case. Front. Biosci. 2022, 27, 11. [Google Scholar] [CrossRef]
- Alasalvar, C.; Karmac, M.; Kosinska, A.; Rybarczyk, A.; Shahidi, F.; Amarowicz, R. Antioxidant Activity of Hazelnut Skin Phenolics. J. Agric. Food Chem. 2009, 57, 4645–4650. [Google Scholar] [CrossRef]
- Ivanović, S.; Avramović, N.; Dojčinović, B.; Trifunović, S.; Novaković, M.; Tešević, V.; Mandić, B. Chemical Composition, Total Phenols and Flavonoids Contents and Antioxidant Activity as Nutritive Potential of Roasted Hazelnut Skins (Corylus avellana L.). Foods 2020, 9, 430. [Google Scholar] [CrossRef]
- Taş, N.G.; Gökmen, V. Bioactive compounds in different hazelnut varieties and their skins. J. Food Compos. Anal. 2015, 43, 203–208. [Google Scholar] [CrossRef]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant Phytochemicals in Hazelnut Kernel (Corylus avellana L.) and Hazelnut Byproducts. J. Agric. Food Chem. 2007, 55, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.L.; Pagano, I.; Esposito, T.; Mencherini, T.; Porta, A.; Petrone, A.M.; Gazzerro, P.; Picerno, P.; Sansone, F.; Rastrelli, L.; et al. HRMS Profile of a Hazelnut Skin Proanthocyanidin-rich Fraction with Antioxidant and Anti-Candida albicans Activities. J. Agric. Food Chem. 2016, 64, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, A.; Lauro, G.; Masullo, M.; Cantone, V.; Olas, B.; Kontek, B.; Nazzaro, F.; Bifulco, G.; Piacente, S. Cyclic Diarylheptanoids from Corylus avellana Green Leafy Covers: Determination of Their Absolute Configurations and Evaluation of Their Antioxidant and Antimicrobial Activities. J. Nat. Prod. 2017, 80, 1703–1713. [Google Scholar] [CrossRef]
- Locatelli, M.; Travaglia, F.; Coisson, J.D.; Martelli, A.; Stevigny, C.; Arlorio, M. Total antioxidant activity of hazelnut skin (Nocciola Piemonte PGI): Impact of different roasting conditions. Food Chem. 2010, 119, 1647–1655. [Google Scholar] [CrossRef]
- Odabaş, H.I.; Koca, I. Application of response surface methodology for optimizing the recovery of phenolic compounds from hazelnut skin using different extraction methods. Ind. Crops Prod. 2016, 91, 114–124. [Google Scholar] [CrossRef]
- Yilmaz, C.; Ozdemir, K.S.; Durmaz, G.; Gokmen, V. Hazelnut skin powder: A new brown colored functional ingredient. Int. Food Res. J. 2014, 65, 291–297. [Google Scholar] [CrossRef]
- Anil, M. Using of hazelnut testa as a source of dietary fiber in breadmaking. J. Food Eng. 2007, 80, 61–67. [Google Scholar] [CrossRef]
- Bertolino, M.; Belviso, S.; Bello, B.D.; Ghirardello, D.; Giordano, M.; Rolle, L.; Gerbi, V.; Zeppa, G. Influence of the addition of different hazelnut skins on the physicochemical, antioxidant, polyphenol and sensory properties of yogurt. LWT Food Sci. Technol. 2015, 63, 1145–1154. [Google Scholar] [CrossRef]
- Ceraulo, M.; La Mantia, F.P.; Mistretta, M.C.; Titone, V. The Use of Waste Hazelnut Shells as a Reinforcement in the Development of Green Biocomposites. Polymers 2022, 14, 2151. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Alongi, J.; Frache, A. Plasticizers, antioxidants and reinforcement fillers from hazelnut skin and cocoa by-products: Extraction and use in PLA and PP. Polym. Degrad. Stab. 2014, 108, 297–306. [Google Scholar] [CrossRef]
- Esposito, T.; Silva, N.H.C.S.; Almeida, A.; Silvestre, A.J.D.; Piccinelli, A.; Aquino, R.P.; Sansone, F.; Mencherini, T.; Vilela, C.; Freire, C.S.R. Valorisation of chestnut spiny burs and roasted hazelnut skins extracts as bioactive additives for packaging films. Ind. Crops Prod. 2020, 151, 112491. [Google Scholar] [CrossRef]
- Kirse-Ozolina, A.; Muizniece-Brasava, S.; Veipa, J. Effect of various packaging solutions on the quality of hazelnuts in nut–dried fruit mixes. In Proceeding of FOODBALT 2019—13th Baltic Conference on Food Science and Technology “FOOD. NUTRITION. WELL-BEING.”, Jelgava, Latvia, 2–3 May 2019; Straumite, E., Galoburda, R., Eds.; pp. 216–9817. [Google Scholar] [CrossRef]
- Product Information on Ecovio® F2332 Biodegradable Compound for Compostable Film. Version 1.0, December 2016. Available online: https://download.basf.com/p1/8a8081c57fd4b609017fd63c8fb8061b/en/ecovio%3Csup%3E®%3Csup%3E_F2332_Product_Data_Sheet_English.pdf?view (accessed on 3 October 2022).
- Italian Recommendation Normal 43/93, Misure colorimetriche di superfici opache. In Raccomandazioni Normal. Alterazioni dei Materiali Lapidei e Trattamenti Conservativi: Proposte per L’unificazione dei Metodi Sperimentali di Studio e di Controllo; CNR—ICR: Roma, Italy, 1993; pp. 1–8.
- Mokrzycki, W.; Tatol, M. Color Difference ΔE—A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Liu, Y.; Hu, T.; Wu, Z.; Zeng, G.; Huang, D.; Shen, Y.; He, X.; Lai, M.; He, Y. Study on biodegradation process of lignin by FTIR and DSC. Environ. Sci. Pollut. Res. 2014, 21, 14004–14013. [Google Scholar] [CrossRef] [PubMed]
- Vittadini, E.; Chiavaro, E.; Rodrigues-Estrada, M.T.; Cerretani, L.; Bendini, A. Differential scanning calorimeter application to the detection of refined hazelnut oil in extra virgin olive oil. Food Chem. 2008, 110, 248–256. [Google Scholar] [CrossRef]
- Pietrosanto, A.; Scarfato, P.; Di Maio, L.; Nobile, M.R.; Incarnato, L. Evaluation of the Suitability of Poly(Lactide)/Poly(Butylene-Adipate-co-Terephthalate) Blown Films for Chilled and Frozen Food Packaging Applications. Polymers 2020, 12, 804. [Google Scholar] [CrossRef]
- Pietrosanto, A.; Scarfato, P.; Di Maio, L.; Incarnato, L. Development of Eco-Sustainable PBAT-Based Blown Films and Performance Analysis for Food Packaging Applications. Materials 2020, 13, 5395. [Google Scholar] [CrossRef]
- Saiter, A.; Monnier, X.; Dargent, E. Physical aging in PLA through standard DSC and fast scanning calorimetry investigations. Thermochim. Acta 2017, 648, 13–22. [Google Scholar] [CrossRef]
- Mistretta, M.C.; Botta, L.; Arrigo, R.; Leto, F.; Malucelli, G.; La Mantia, F.P. Bionanocomposite Blown Films: Insights on the Rheological and Mechanical Behavior. Polymers 2021, 13, 1167. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sanchez, A.; Villalonga Santana, R.; Mariniello, L. Effect of Mesoporous Silica Nanoparticles on The Physicochemical Properties of Pectin Packaging Material for Strawberry Wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef]
- Sabbah, M.; Di Pierro, P.; Cammarota, M.; Dell’Olmo, E.; Arciello, A.; Porta, R. Development and properties of new chitosan-based films plasticized with spermidine and/or glycerol. Food Hydrocoll. 2019, 87, 245–252. [Google Scholar] [CrossRef]





| Property | Test Method | Ecovio F2332 |
|---|---|---|
| Mass Density [g/cm3] | ISO 1183 | 1.24–1.26 |
| Bulk Density [kg/m3] | DIN EN ISO 60 | 750 |
| Melt Volume Rate @ 190 °C, 5 kg [mL/10 min] | ISO 1183 | 7.0–11.0 |
| Melting points [°C] | DSC | 110–120140–155 |
| Sample Name | Draw-Up Speed [m/min] | Average Film Thickness [μm] |
|---|---|---|
| Ecovio D0.9 | 0.9 | 55 ± 4 |
| Ecovio/HP 95/5 D0.9 | 0.9 | 120 ± 09 |
| Ecovio/HP 95/5 D1.6 | 1.6 | 90 ± 7 |
| Ecovio/HP 95/5 D3 | 3 | 65 ± 6 |
| Ecovio/HP 90/10 D0.9 | 0.9 | 136 ± 6 |
| Ecovio/HP 90/10 D1.6 | 1.6 | 104 ± 8 |
| Ecovio/HP 90/10 D3 | 3 | 78 ± 5 |
| Sample | 1st Heating | Cooling | 2nd Heating | |||||
|---|---|---|---|---|---|---|---|---|
| Tm I [°C] | ΔHm I [J/g] | Tm II [°C] | ∆Hm II [J/g] | Tc [°C] | ∆Hc [J/g] | Tm I [°C] | ΔHm I [J/g] | |
| HP | −7.6 | 16.8 | 90.4 | 262.2 | −53.3 | 7.9 | −7.7 | 11.9 |
| HP dried | −8.4 | 9.8 | 91.2 | 209.8 | −58.0 | 4.5 | −8.5 | 7.7 |
| Sample | TI [°C] | Weight Loss at TI [%] | Tonset [°C] | TII [°C] | TIII [°C] | TIV [°C] |
|---|---|---|---|---|---|---|
| HP | 63.0 | 8.9 | 214.7 | 275.0 | 340.1 | 388.4 |
| HP dried | 71.6 | 3.0 | 208.5 | 275.9 | 340.0 | 398.0 |
| Sample | RSA [%] |
|---|---|
| HP | 85.7 ± 1.1 |
| HP dried | 83.2 ± 1.3 |
| Film Sample | TgPBAT [°C] | Tmoil [°C] | ΔHmoil [J/g] | TmBA [°C] | ΔHmBA [J/g] | TgPLA [°C] | TmPBAT [°C] | ΔHmPBAT [J/g] | TmPLA [°C] | ΔHmPLA [J/g] |
|---|---|---|---|---|---|---|---|---|---|---|
| Ecovio D0.9 | −29 | - | - | 50 | 1.1 | 64 | 121 | 6.4 | 150 | 0.3 |
| Ecovio/HP 95/5 D0.9 | −30 | - | - | 51 | 0.9 | 63 | 123 | 5.4 | 148 | 2.5 |
| Ecovio/HP 90/10 D0.9 | −31 | −9.4 | 0.8 | 50 | 0.9 | 63 | 122 | 5.5 | 148 | 1.5 |
| Film Sample | E [MPa] | σy [MPa] | σb [MPa] | εb [%] | P O2 [cm3·mm/(m2·d·bar)] |
|---|---|---|---|---|---|
| Ecovio D0.9 | 157 ± 2 | 8.66 ± 0.69 | 9.96 ± 1.18 | 308 ± 25 | 41.0 ± 0.3 |
| Ecovio/HP 95/5 D0.9 | 125 ± 4 | 6.49 ± 0.75 | 7.47 ± 1.48 | 507 ± 42 | 53.2 ± 1.0 |
| Ecovio/HP 95/5 D1.6 | 112 ± 8 | 4.86 ± 0.34 | 6.92 ± 1.39 | 390 ± 56 | 64.5 ± 1.6 |
| Ecovio/HP 95/5 D3 | 76 ± 5 | 4.75 ± 0.41 | 5.15 ± 0.58 | 273 ± 31 | 96.4 ± 0.5 |
| Ecovio/HP 90/10 D0.9 | 118 ± 6 | 6.64 ± 1.01 | 7.94 ± 1.26 | 442 ± 40 | 56.4 ± 0.3 |
| Ecovio/HP 90/10 D1.6 | 96 ± 1 | 5.38 ± 0.57 | 6.27 ± 1.23 | 395 ± 45 | 86.1 ± 2.4 |
| Ecovio/HP 90/10 D3 | 52 ± 6 | 3.91 ± 0.56 | 4.06 ± 0.55 | 270 ± 25 | 135.5 ± 1.0 |
| Film Sample | RSA [%] |
|---|---|
| Ecovio D0.9 | n.d. |
| Ecovio/HP 95/5 D0.9 | 25.4 ± 1.3 |
| Ecovio/HP 95/5 D1.6 | 25.0 ± 1.1 |
| Ecovio/HP 95/5 D3 | 23.0 ± 1.4 |
| Ecovio/HP 90/10 D0.9 | 38.2 ± 1.3 |
| Ecovio/HP 90/10 D1.6 | 28.6 ± 1.4 |
| Ecovio/HP 90/10 D3 | 25.0 ± 1.3 |
| Film Sample | Transparency at 560 nm [%] | CIELAB Coordinates | |||
|---|---|---|---|---|---|
| L* | a* | b* | ∆E | ||
| Ecovio D0.9 | 5.3 ± 1.0 | 96.5 ± 0.1 | −0.8 ± 0.1 | 2.5 ± 0.1 | 0 |
| Ecovio/HP 95/5 D0.9 | 0.9 ± 0.1 | 66.8 ± 2.0 | 9.3 ± 0.6 | 16.9 ± 0.2 | 34.5 |
| Ecovio/HP 95/5 D1.6 | 3.1 ± 0.5 | 76.5 ± 1.6 | 6.0 ± 0.5 | 15.0 ± 0.4 | 24.6 |
| Ecovio/HP 95/5 D3 | 12.7 ± 1.8 | 85.9 ± 1.3 | 2.8 ± 0.4 | 10.5 ± 0.7 | 13.8 |
| Ecovio/HP 90/10 D0.9 | 0.4 ± 0.2 | 49.8 ± 1.2 | 12.9 ± 0.1 | 16.4 ± 0.9 | 50.6 |
| Ecovio/HP 90/10 D1.6 | 3.2 ± 0.7 | 64.8 ± 1.5 | 9.4 ± 0.4 | 18.3 ± 0.1 | 36.9 |
| Ecovio/HP 90/10 D3 | 9.2 ± 2.6 | 77.1 ± 1.9 | 5.6 ± 0.6 | 14.6 ± 0.8 | 23.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarfato, P.; Graziano, M.L.; Pietrosanto, A.; Di Maio, L.; Incarnato, L. Use of Hazelnut Perisperm as an Antioxidant for Production of Sustainable Biodegradable Active Films. Polymers 2022, 14, 4156. https://doi.org/10.3390/polym14194156
Scarfato P, Graziano ML, Pietrosanto A, Di Maio L, Incarnato L. Use of Hazelnut Perisperm as an Antioxidant for Production of Sustainable Biodegradable Active Films. Polymers. 2022; 14(19):4156. https://doi.org/10.3390/polym14194156
Chicago/Turabian StyleScarfato, Paola, Maria Luisa Graziano, Arianna Pietrosanto, Luciano Di Maio, and Loredana Incarnato. 2022. "Use of Hazelnut Perisperm as an Antioxidant for Production of Sustainable Biodegradable Active Films" Polymers 14, no. 19: 4156. https://doi.org/10.3390/polym14194156
APA StyleScarfato, P., Graziano, M. L., Pietrosanto, A., Di Maio, L., & Incarnato, L. (2022). Use of Hazelnut Perisperm as an Antioxidant for Production of Sustainable Biodegradable Active Films. Polymers, 14(19), 4156. https://doi.org/10.3390/polym14194156








