3.1. PHBV Extraction and Purification
PHBV containing biomass was produced at pilot-scale by MMC fed fermented dairy manure as described by Guho et al. [
6]. The PHBV amount per dry cell of biomass, the 3HV content in the PHBV rich biomass, and the purity of PHBVs was determined by GC-MS and the results are given in
Table 1. The 3HV content in purified PHBV was determined by
1H-NMR and data was from Guho et al. [
6]. The lipid extracted from lyophilized biomass in the pre-treatment step (acetone wash) was 5–10% of the biomass weight which improves PHBV extraction [
30]. The result showed that the 3HV molar fraction of the purified PHBV was less than that of PHBV rich biomass. The lower 3HV molar fraction in all the extracted polymers compared PHBV-rich biomass shows that CHCl
3 selectively extracted PHBV with lower 3HV content. Wei et al. [
8] reported the 3HV ratio of PHBV rich biomass produced using MMC was about 0.39 and decreased to 0.34 on purified PHBV. All the purified PHBVs showed lower HV content than PHBV rich biomass. This can be attributed to the effect of solvent type, extraction time and temperature on the HV content of the extracted PHBV [
31]. There is an optimum time and temperature in extraction with every solvent and method. It has been seen that the HV content and
Mw of the extracted PHBVs decreased when extraction time exceeded the optimum level [
31], and a longer extraction time can result in hydrolyses of polymer chains [
32,
33]. Furthermore, depending on the solvent type the atomic interactions between solvent and solute vary which affects the composition of the extracted polymer [
19,
34].
3.2. PHBV Molar Mass Determination
A variety of parameters in both upstream and downstream processing of PHA production can influence the molar mass of the PHA produced. The upstream parameters include the microorganisms present in the MMC, the medium composition, the state of inoculum, and the fermentation conditions. The downstream processing parameters includes pre-treatment of biomass, extraction method, and subsequent polymer purification procedures [
35]. The molar mass (
Mw and
Mn) and polydispersity index (
PDI) of purified PHBV were determined by SEC-MALS and the results were from Guho et al. [
6] (
Table 2). The
PDI varied between 1.06 and 1.43. The higher
PDI indicates a broader range of distribution of molar mass. The
PDI values of the PHBV in this study were lower than those reported for PHBV (
PDI of 2.1–2.2) [
8]. In an study PHBV extracted with CHCl
3 showed a high
PDI of 3.06 [
36]. PHBV having low
PDI can be used for applications such as controlled drug delivery [
37]. Lower
PDI values of the PHBV in this study can be attributed to both upstream and downstream parameters. Upstream processing parameters which mentioned earlier will determine the initial HV content and
Mw of the cultured PHBV. Downstream processing parameters include some polymer removal in the pretreatment step from lyophilized biomass, solvent type and its atomic interactions with solute, heating energy, and time applied in extraction and purification steps. The combination of these upstream and downstream parameters affects HV content,
Mw,
Mn, and as a result
PDI of the purified polymer [
6,
8,
19,
31,
32,
33,
34].
It was found that there was a negative linear correlation between Mw (= −152.0 × HV + 42.68) and Mn (= −134.27 × HV + 37.939) with the HV content of the extracted PHBVs (R2 ≥ 0.83). Pearson tests were performed between Mw and HV content (p = 0.031), and Mn and HV content (p = 0.014). Furthermore, similar correlation was seen between Mw (= −33.261× HV + 17.475) with the HV content of the PHBV in lyophilized biomass (R2 = 0.91). Pearson test was performed between Mw and HV content of the PHBV in lyophilized biomass (p = 0.012). There was no significant relation between Mn with HV content, and PDI with HV content of the PHBVs in lyophilized biomass.
3.3. FTIR Spectral Analysis
Purified PHBV samples were analyzed by FTIR spectroscopy to gain insight into its chemical structure; results are given in
Table 3. The C–C stretching band occurs at 977 cm
−1 [
38,
39]. The C–O stretching bands are observed between 1054 and 1129 cm
−1 and from 1226 to 1275 cm
−1 [
38,
39,
40,
41]. The band at 1179 cm
−1 was assigned to the C–O–C stretching of the amorphous PHBV [
42]. The band at 1720 cm
−1 (highest intensity) was assigned to a C=O stretching of an ester structure in PHBV [
6,
38,
40]. The C–H stretching bands of methyl (–CH
3) and methylene (–CH
2) groups appeared at 2975 cm
−1 and 2933 cm
−1, respectively [
39,
40,
43]. Analysis of the FTIR spectra can be used to gain insight into the relative amounts of amorphous versus crystalline material in PHBV by determining the carbonyl index (
IC=O). Curve fitting of the crystalline band at 1720 cm
−1 to amorphous band at 1740 cm
−1 was used to determine band areas and
IC=O (
Figure 1) [
25,
26,
31]. The
IC=O for purified PHBV ranged from 2.01 to 2.59 (
Table 4). A poor correlation (
R2 = 0.53) between
IC=O and
Xc by DSC (17–29%) was observed. These results are lower than reported values for PHB (
IC=O 6.94 and
Xc 57.6%) [
38], and higher than that reported for PHBV with a 25% 3HV content (
IC=O 1.6 and
Xc 53.7%) [
44]. It has been shown that as the crystallinity index decreased from 0.99 to 0.81 as HV content increased from 0% to 47% [
45].
3.4. Thermal Analysis of PHBV
The purified PHBV samples and commercial standards were analyzed by DSC to determine their thermal properties (glass transition temperature,
Tg; melting temperature,
Tm; crystallization temperature (
Tc) and degree of crystallization,
Xc); the results obtained from Guho et al. [
6] and the reanalyzed data are given in
Table 4 and (
Supplementary Material Figure S1). The result of
Tg of the PHBV samples was similar to the results obtained from Guho et al. [
6] (−0.7 °C to −7.4 °C vs. −2.8 °C to −10 °C) consistent with commercial PHBV (22% HV, −1.5 °C). The result of
Tm1 and
Tm2 of the PHBV samples was similar to the results obtained from Guho et al. [
6], except PHBV-61 and -71 which showed one melting peak. Isomorphism phenomena can lead to the presence of two melting peaks [
6,
31]. As PHBV presents a semi-crystalline structure the crystals with higher HV content will have a higher amorphous phase ratio and thus during heating will be melted (
Tm1) first. In crystals with lower HV content, the crystallinity ratio is higher, so the crystals will melt at a higher temperature (
Tm2) [
31,
46]. Other factors that contribute to two melting points include the different crystalline morphologies (thickness, perfection, lamellar stability, or distribution), crystallites of different molar masses, physical aging, and amorphous phase relaxation [
31,
46].
There was no correlation between the melting temperature of the polymers and 3HV content. The
Xc of the purified PHBV obtained from first cooling cycle which was slightly higher than that obtained from Guho et al. [
6] (16.6–29% vs. 13.9–25%). The
IC=O of all the extracted PHBV samples ranged between 2.01 to 2.59 which was higher than the previously reported value of 0.949 for PHB [
47]. PHB was shown to have a higher
Xc (61%), by X-ray diffraction, than PHBV (36.2%) and also showed higher value of
IC=O by FTIR (3.8 vs. 2.7) [
26]. The values of
IC=O by FTIR and
Xc by DSC of the extracted PHBVs gave a poor correlation (
R2 = 0.53).
The
Tc for the extracted PHBVs ranged from 56 to 107 °C (
Table 4). Sample PHBV-32 showed the highest
Tc (107.1 °C) with a 3HV content of 0.18 while sample PHBV-71 had the lowest
Tc (56.5 °C) and a higher 3HV content of 0.23. There was a negative linear correlation between
Tc and 3HV content (
R2 = 0.54), and also between
Tc and
Xc of the extracted PHBVs (
R2 = 0.54). An Increase in 3HV content results in an increase in the amorphous phase and thus leads to a reduction in
Xc and
Tc [
48]. A lower
Tc indicates that the complete crystallization takes a longer time [
49]. PHBV-15 has the lowest 3HV content (0.16), high
Xc (29%), and low
Tc of 62 °C. This can be attributed to the higher
PDI of 1.43 in PHBV-15. Higher
PDI indicates a larger size distribution of polymer chains. Shorter chains need less time for crystallization compared to longer chains which results in reduction of
Tc [
49]. A
Tc value ranging from 41 to 82 °C for CHCl
3 extracted PHBV with 3HV contents of (20–60%) have been reported [
50]. Another study had shown that PHBV (16.6% HV) extracted with NaClO and CHCl
3 had a
Tc value of 109 °C [
51].
TGA analysis was performed to determine the thermal degradation behavior of the purified PHBV samples (
Table 4, and
Supplementary Material Figure S2). All of the PHBV samples showed a 1-step degradation behavior. Based on the derivative thermogravimetric (DTG) curves the temperature at which the maximum weight loss occurred (
Td) was determined. The
Td values of the PHBV biopolymers was between 318 °C and 329 °C, which was higher than those reported in the literature for PHBV (
Td = 286 °C) [
52] and PHB (280 °C) [
53]. For PHB thermal degradation occurs just above its
Tm which limits its processability [
7]. The presence of 3HV in PHBV lowers its
Tm relative to
Td and thus improves its processing window for molding and extrusion [
21,
31,
49,
54]. The thermal degradation of PHBV occurs in a single step due to a nonradical random chain scission process involving a six-membered ring transition state [
24,
31,
52,
55]. The onset temperature of degradation was from 286.4 °C to 303.7 °C, and the end set temperature of degradation was from 325.3 °C to 336.1 °C. These temperatures are higher than those reported in the other literatures [
9,
20]. The
Tonset of 177–238 °C,
Td 276–296 °C, and end set temperature of 291–309 °C is reported for PHBV (5–20% HV) [
9]. Furthermore, the
Td1% (temperature at which 1% weight loss occurs) of 258 °C and
Td 284 °C for PHB is reported [
20] indicating an increase in thermal stability of PHBV samples studied here compared to pure PHB. While
Td was found to decrease with increasing 3HV content (
R2 = 0.49), the difference between
Tonset and
Tendset was found to increase with increasing 3HV content further reinforcing that the thermal stability of PHBV improved [
7].
3.5. Comonomer and Composition Sequence Distribution Analysis
The molar HV content in PHBV was determined by
1H-NMR spectroscopy (
Table 1,
Supplementary Material Figure S3). The molar HV content ranged between 0.16 and 0.24 which was in good agreement with those obtained by GC-MS (HV molar content 0.17 to 0.25,
Table 1).
To determine whether the PHBV samples were random or co-block-polymer a sequence distribution (CSD) was determined by
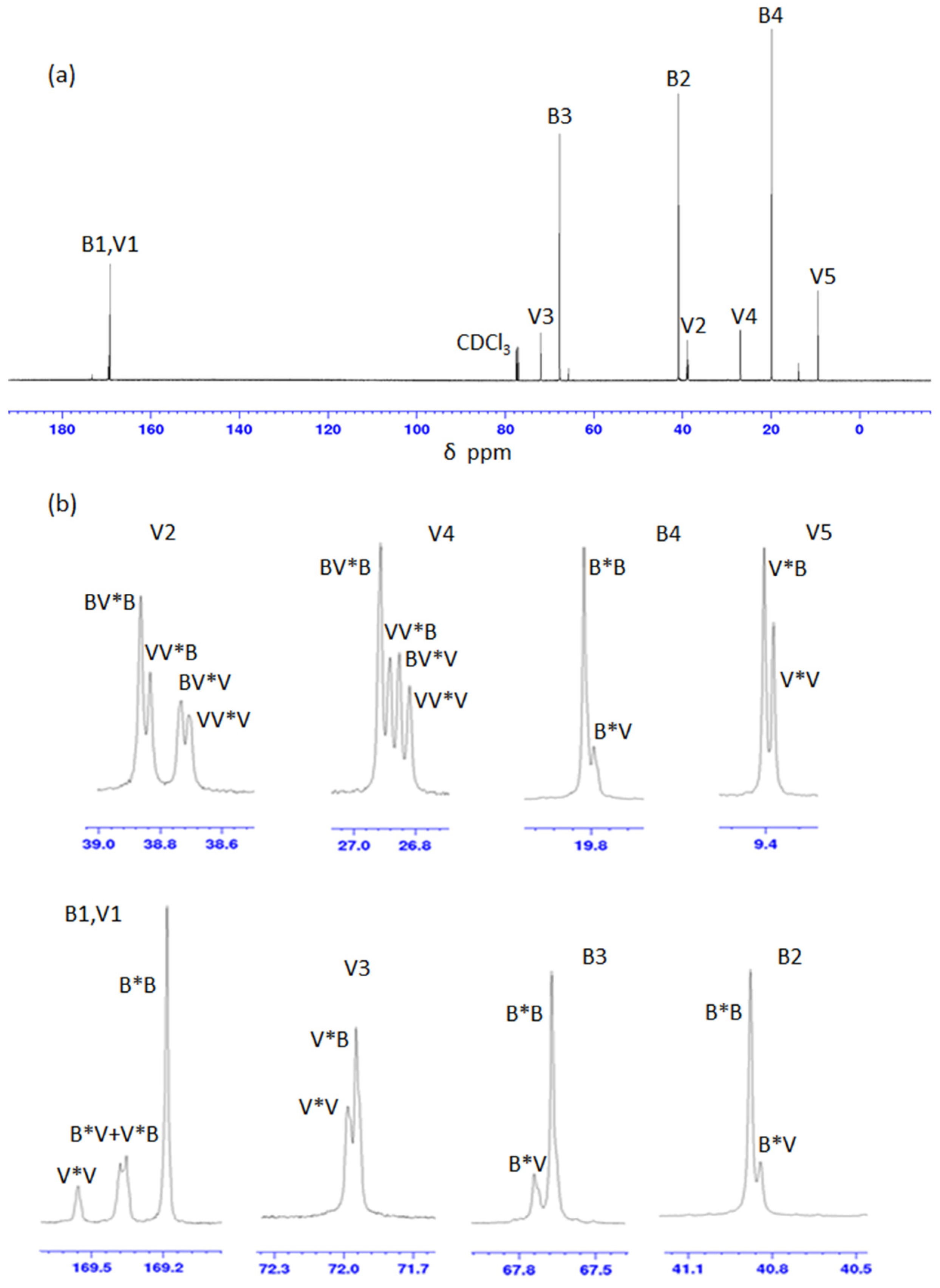
13C-NMR spectroscopy (
Figure 2) based on diad and triad sequence analysis [
8]. The carbon resonances of HB and HV were split into multiplets due to diad and/or HV-centered triad comonomer sequences and assignments [
8,
29] (
Figure 2b, and
Supplementary Material Table S1). The carbonyl area was composed of four peaks at δ = 169.56, 169.38, 169.35, and 169.18 ppm attributed to different diad sequences of 3HV*3HV, 3HB*3HV, 3HV*3HB, and 3HB*3HB, respectively. The resonance of the 3HV side-chain methylene C (V4 and V2) was split into four triad sequences peaks attributed to 3HV-centered triads of 3HB-3HV*3HB, 3HV-3HV*3HB, 3HB-3HV*3HV, and 3HV-3HV*3HV. The chemical shifts observed (
Supplementary Material Table S1) were in agreement with those reported previously [
8,
29].
The relative peak intensities obtained for the carbonyl (V1 and B1) resonances and the methylene (V2, B2, and V4) resonances of PHBV samples were used to determine the mole, diad, and HV-centered triad sequence distributions (
Table 5). In the PHBV-15, -32, and -39 samples some signals were assigned to carbonyl and methylene C (B2, V2, V4) and in the PHBV-71 a signal assigned to methylene C (B2) were not observed. Therefore, the calculation of moles for diad and HV-centered triad fractions of HB and HV monomers on these samples were not performed.
The degree of randomness of copolymer based on experimental diad sequence distribution of the PHBV samples was determined by parameter
D (
Table 6), which is defined as
FXY represents the mole fraction of
XY sequence. The subscripts VV, BB, VB, and BV represent valerate-valerate, butyrate-butyrate, valerate-butyrate and butyrate-valerate of diad sequences, respectively. The statistically random copolymers have
D value of 1.0. The
D value for blocky and alternating copolymers are >1 and very close to 0, respectively. The term “blocky” means that the copolymer is a mixture of copolymers with different compositions or a mixture of PHB and PHV homopolymers. The parameter
D is sensitive to the bimodal or multimodal (very large
D) CSD, whereas it is not sensitive to the broadness of CSD [
28].
A higher-order sequence distribution analysis such as the triad sequence distribution provides more accurate information on CSD. The degree of randomness in the PHBV copolymers based on the experimental triad sequence distribution was determined by the R parameter (
Supplementary Material Equations (S1)–(S3). In the PHBV copolymers with a completely randomized distribution of HV and HB monomers,
R has a value of 1 (
Table 6) [
28].
The diad and triad sequence distribution of PHBV biopolymer samples were calculated using three models including (i) Bernoullian, (ii) first-order Markovian, and (iii) a mixture of two Bernoullian random copolymers (explanation of the models is given in
Supplementary Material Equations (S4)–(S6) [
8,
29]. The result of molar fractions of diad, and 3HV-centered triads of 3HB and 3HV for PHBV-46, -61, -78, and -84 is listed in
Table 7.
The PHBV samples sequence distributions followed the Bernoullian statistics model. The results confirm that the PHBV samples were all random copolymers and these results are in agreement with those reported previously [
8,
29].
3.6. Rheology
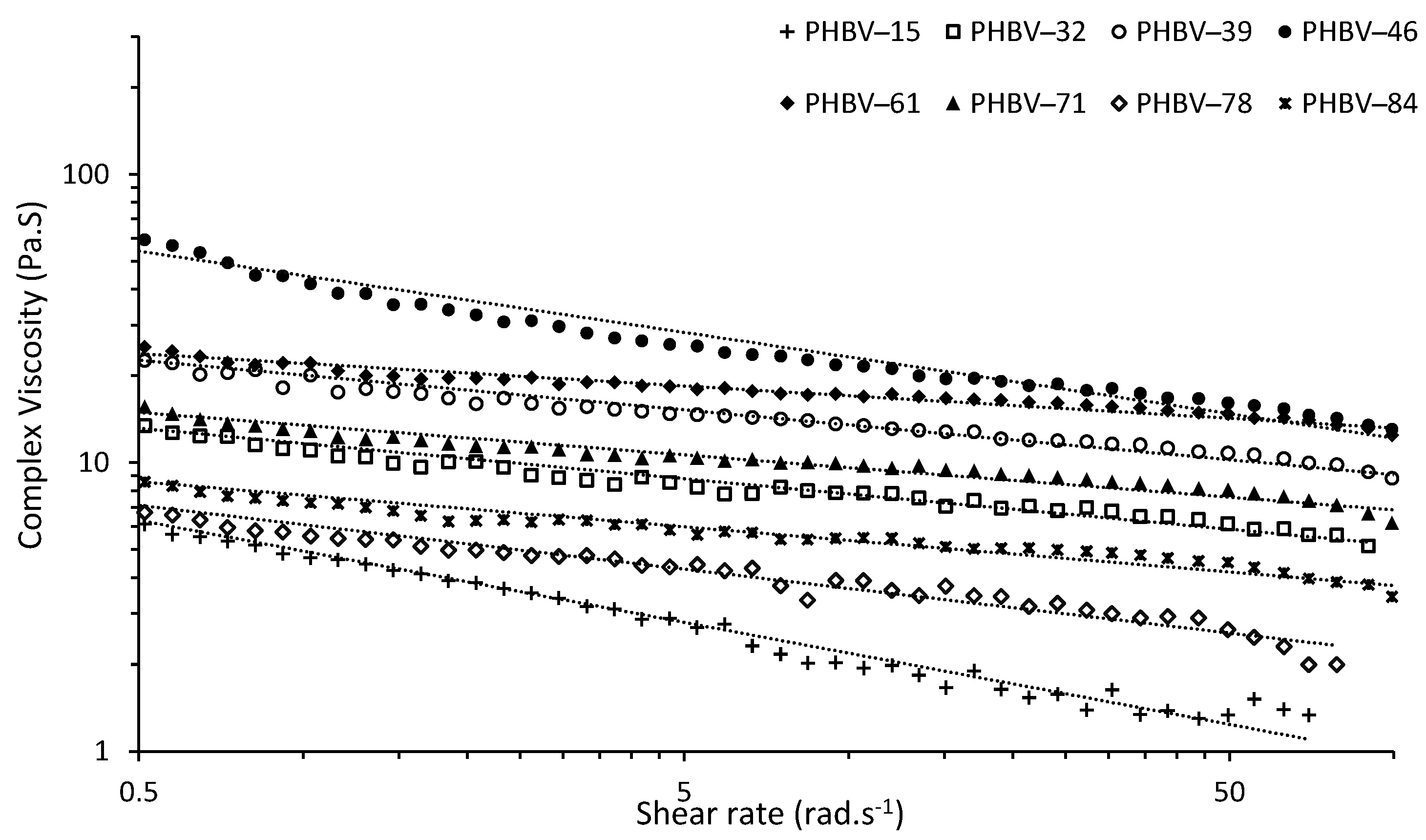
Rheology presents information about of the behavior of polymer melts through measuring the flow (viscosity) as a function of shear rate [
56]. From dynamic rheological measurements the complex viscosity (
η*) was determined for PHBV at 180 °C against shear rate (
Ꞷ) as frequency sweeps (also known as flow curves); results are shown in
Figure 3. The PHBV flow curves show a reduction in
η* with
Ꞷ showing shear thinning behavior typical for non-Newtonian fluid [
31,
57]. At high shear rates the anisotropic polymer chains with high molar mass will disentangle and aligned along the shear direction [
57,
58]. Thus, less intermolecular interaction happens among the polymer chains resulting in higher free volume and a lower viscosity [
31,
57,
58]. The
η* of PHBV-15 decreased more rapidly than the other PHBV samples (
Figure 3 and
Table 8). This might be attributed to the lower purity (80%) of the polymer and partial plasticizing effect of impurities present and high
PDI of 1.47 in the polymer [
31,
59,
60]. Smaller chains act as a plasticizer for longer chains, increasing the free volume space between polymer chains leading to improvement in the mobility of the polymer chains. The viscosity of the polymer therefore decreases with higher slope comparing the polymers without plasticizer [
60].
To quantify the shear thinning behavior, the rheological data were fitted to the Power–law (Ostwald–de Waele) model, which represents viscosity as a function of some power of the shear rate (
Ꞷ) [
31,
61]. The Cox–Merz rule is used to establish the relationship between steady shear and dynamic shear viscosity, which is valid for PHA, PHB, and PHBV [
31,
61]. Exchanging the steady shear terms with the dynamic viscosity terms yields a modified Power-law model, expressed as:
The modified model has two parameters of
K and
n that must be fitted to experimental data. These parameters are determined from the plot double log
η*-
Ꞷ. The parameter
K is known as the consistency coefficient, is the y-intercept of the log
η*-log
Ꞷ fitted plot. The parameter
n is the non-Newtonian or flow behavior index which is the slope of the log
η*-log
Ꞷ fitted curve. For
n < 1 the material is pseudo-plastic, when
n = 1 the material is Newtonian, and
n > 1 the material is dilatant [
31,
61,
62,
63]. However, a drawback to this model is that the power-law model does not accurately represent experimental data in the low and high shear ranges, so it should not be extended to these regions [
62,
63]. The goodness of fit for the models
R2 was used. All the PHBV samples had
R2 ≥ 0.92. The
K and
n parameters, and complex viscosity at the frequency of
Ꞷ = 6.28 rad·s
−1 (1 Hz) for PHBV-15 to -84 are summarized in
Table 8. The Power-law model was well fitted to the data, with
K ranging from 3.27 to 28.07 Pa·s. The
n ranged from 0.645 to 0.888 showing pseudo-plastic behavior. Reported values for PHBV (5% HV) at 160 °C at were
K = 1.35 × 10
6 Pa·s and
n = 0.0823 [
58,
61]. The
η* of the PHBV samples at 1 Hz ranged from 1.7 Pa·s to 14.57 Pa·s. The PHBV samples with higher HV content showed higher
η* at 1 Hz. It is expected an increase in the HV content leads to a reduction in
η* [
31,
64,
65]. This difference can be attributed to the simultaneous effect of higher crystallinity which increases the
η* and lower
PDI which decreases the
η* in polymers [
60].
3.7. Mechanical Properties
The mechanical (flexural and tensile) properties of materials provide information on how they will behave under load.
Table 9 shows the tensile and flexural properties of the PHBV samples and a commercial PHBV (8% HV) sample. The PHBV tensile strength ranged between 10.1 and 13.7 MPa and Young’s moduli ranged between 0.57 and 1.12 GPa. The commercial PHBV had a strength of 25.9 MPa and modulus 0.75 GPa. Tensile strength values from the literature were 27.1 MPa (injection molded 8% HV) [
66], 29.14 MPa (solution cast 5% HV) [
67], and 14.1 MPa (solution cast 12% HV) [
68]. While the Young’s modulus values were 3.5 GPa for injection molded (8% HV) [
66], 2.19 GPa (solution cast 5% HV) [
67], and 0.82 GPa (solution cast 12% HV) [
68]. In a separate study, the tensile strength and Young’s modulus of 20 MPa and 0.8 GPa for PHBV-20% HV were reported [
69]. An inverse relationship was between strength and modulus values and HV content.
The PHBV samples were shown to have a flexural strength of 18.1 to 27.5 MPa and moduli of 0.80 to 1.54 GPa. Literature values for flexural strength for PHBV(8% HV) were 34.5 MPa [
66] and 28.2 MPa [
70] and the flexural moduli for PHBV(8%HV) were 3.2 GPa [
66] and 1.3 GPa [
70]. The low mechanical properties for the pilot plant PHBV samples were due to their high HV content. A negative linear relationship between flexural strength and HV% (
R2 = 0.68), flexural modulus and HV% (
R2 = 0.78), tensile strength and HV% (
R2 = 0.69), and Young’s modulus and HV% (
R2 = 0.83) were observed. The strength and flexibility of these polymers was comparable to LDPE which makes them applicable for the packaging industry [
5,
8]. It has been reported that an increase in HV content (0 to 30%) reduces the melting point, crystallization rate, tensile strength, and stiffness of the PHBV [
71]. PHBV shows a comparable modulus to polyethylene terephthalate (PET), higher than to that for low density polyethylene (LDPE) and polypropylene (PP) [
71]. For comparative purposes, the tensile strength and Young’s moduli for PHB were 40 MPa and 3.5 GPa, for LDPE were 10 MPa and 0.2 GPa, and PP were 38 MPa and 1.7 GPa PP [
69,
72]. Low content HV PHBV has applications where rigidity is required such as bottle caps, while high content HV PHBV would be suitable as flexible packaging and bottles [
71].
The molar mass distribution of polymers, PDI, is known to influence their properties. Polymers with higher PDI contain some shorter chain polymers that can act as a plasticizer and therefore reduce their mechanical properties [
60]. A negative correlation between flexural strength and PDI (
R2 = 0.68), flexural modulus and PDI (
R2 = 0.78), tensile strength and PDI (
R2 = 0.69), and Young’s modulus and PDI (
R2 = 0.83) were observed.