Effect of Different Comonomers Added to Graft Copolymers on the Properties of PLA/PPC/PLA-g-GMA Blends
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of the Graft Copolymers
2.3. Purification of the Graft Copolymers
2.4. Preparation of the Blends
2.5. Characterization
- Fourier transformation infrared spectroscopy (FTIR) analysis: The purified and dried PLA-g-GMA graft copolymers were molded into a film on a plate vulcanizer (400X400X2, Qingdao Yadong Rubber Machinery Co., Ltd., Qingdao, China) and analyzed by FTIR. FTIR spectra were recorded on a NEXUS 470 PC. The scanning range of infrared test was 7400~350 cm−1, the resolution was 0.5 cm−1, the wave number accuracy was 0.01 cm−1, and the number of scans was 75 times/s.
- Nuclear magnetic resonance analysis (1H-NMR) analysis: Weigh about 15 mg of PLA and PLA-g-GMA graft copolymer. Deuterated chloroform (CDCl3) and tetramethylsilane (TMS) were used as solvent and the internal standard, respectively. Bruker Ascend 500 ⅠⅠⅠ magnetic resonance analyzer was used for testing, scanning 128 times.
- Differential Scanning Calorimetry (DSC) analysis: Differential scanning calorimetry analysis (DSC, TA Q200, TA, New Castle, DE, USA) was performed by weighing approximately 5 mg of the PLA/PPC/PLA-g-GMA blends sample into a crucible. The temperature was raised from 0 to 200 °C at a rate of 10 °C/min and kept constant for 5 min to eliminate thermal history, which was further lowered to 20 °C at the same rate. The gas flow was 2.5 L/min.
- Thermogravimetric (TGA) analysis: Thermogravimetric analysis (TGA, STA449C, NETZSCH, Selb, Germany) was performed under flowing nitrogen (100 mL/min) at a heating rate of 10 °C/min. Approximately 15 mg of PLA/PPC/PLA-g-GMA blends were heated from room temperature to 500 °C.
- Rheological analysis: Rheological measurements of the blends were on a Dynamic mechanics analyzer (DHR-2, TA, New Castle, DE, USA). Frequency sweep for the PLA/PPC/PLA-g-GMA blends was under nitrogen using 25 mm plate geometry. The gap distance between the parallel plates was 0.8 mm. The sheet blends were about 1.0 mm in thickness. The angular frequency range used during testing was 0.1–100 rad/s with a shear strain of 1.0%. The temperature was plotted at 180 °C.
- Melt Flow Rate (MFR) analysis: The MFR was determined by a melt flow rate instrument (GT-7100-MH, Gotweil Scientific Instruments Co., Ltd., Qingdao, China) at 190 °C with a load of 2.16 kg (test standard was ASTM D1238-2013), MFR is calculated according to Equation (2):where m was the average mass of the material (g) and t was the interval sampling time (10 s).
- Optical property: The PLA/PPC/PLA-g-GMA blends were pressed into films of approximately 80 μm thickness using a flat vulcanizer and tested for haze and transmittance using a CS-700 haze meter (Hangzhou Caipu Technology Co., Ltd., Hangzhou, China).
- Mechanical property: According to GB/T1843/1-A, Izod notch impact strength was determined with a GT-7045-MD impact tester (Songshu Instrument Co., Ltd., Dongguan, China), the pendulum used was 2.750 J. The tensile properties were measured according to GB/T1040-1BA using a tensile testing machine (Instron 3365, Instron, Boston, MA, USA) at a crosshead speed of 25 mm/min. The test was performed at room temperature, and the average values of at least five tests were reported.
- Scanning electron microscopy (SEM): The fracture surface of the PLA/PPC/PLA-g-GMA blends were sputter-coated with gold and then observed using a scanning electron microscope (JSM-5600LV, JEOL, Tokyo, Japan).
3. Results and Discussion
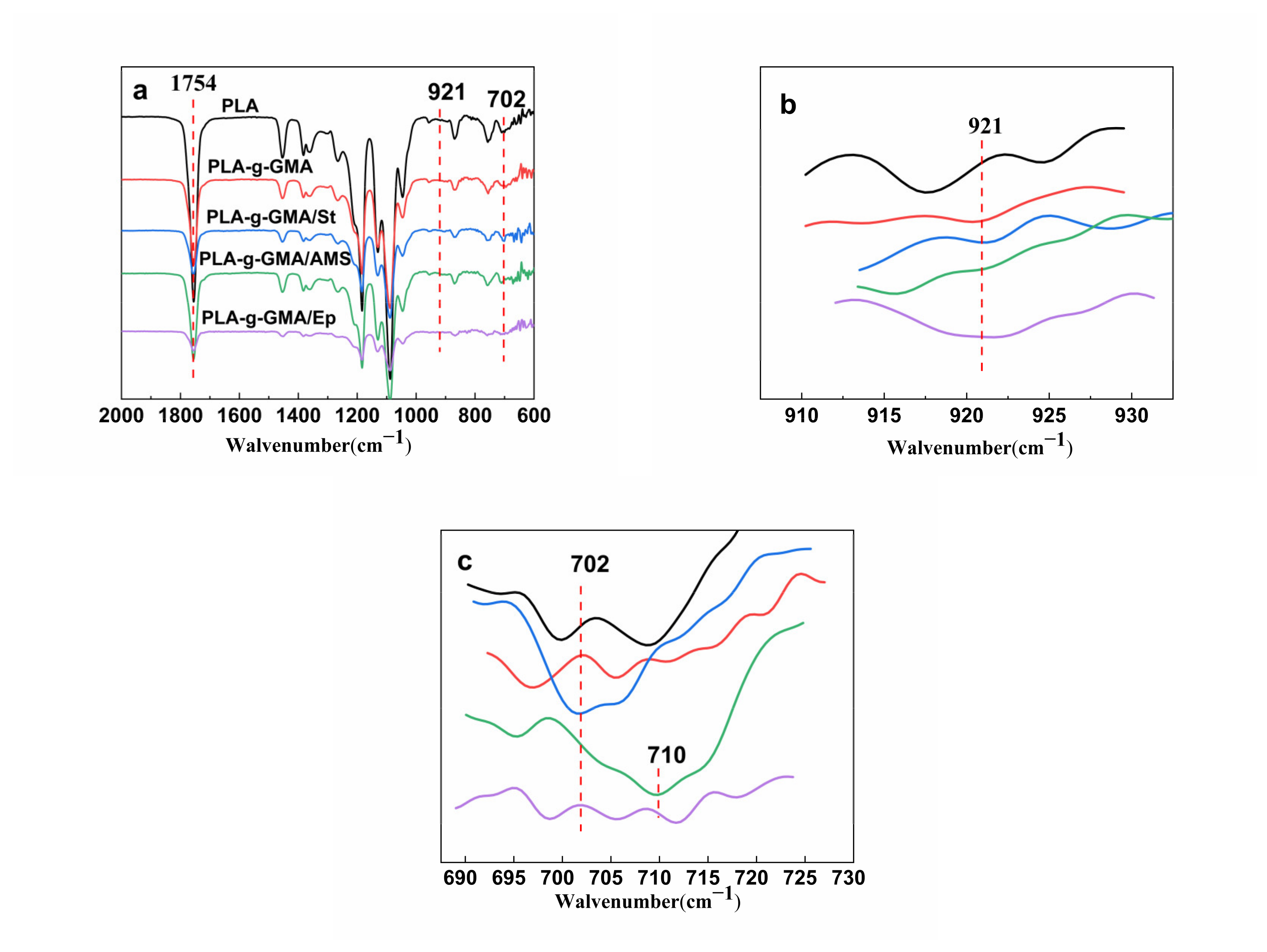
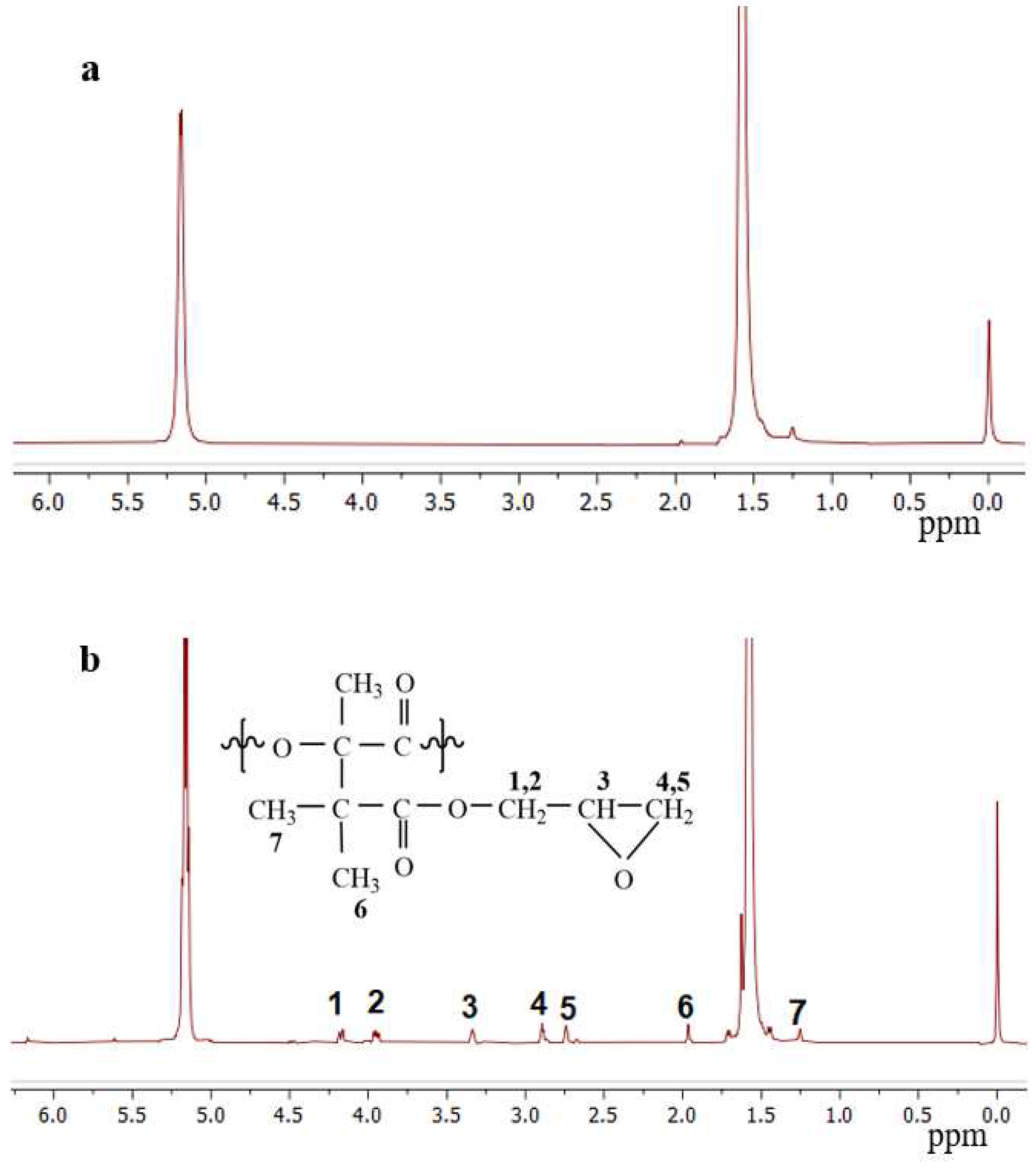
3.1. FTIR and 1H-NMR Analysis
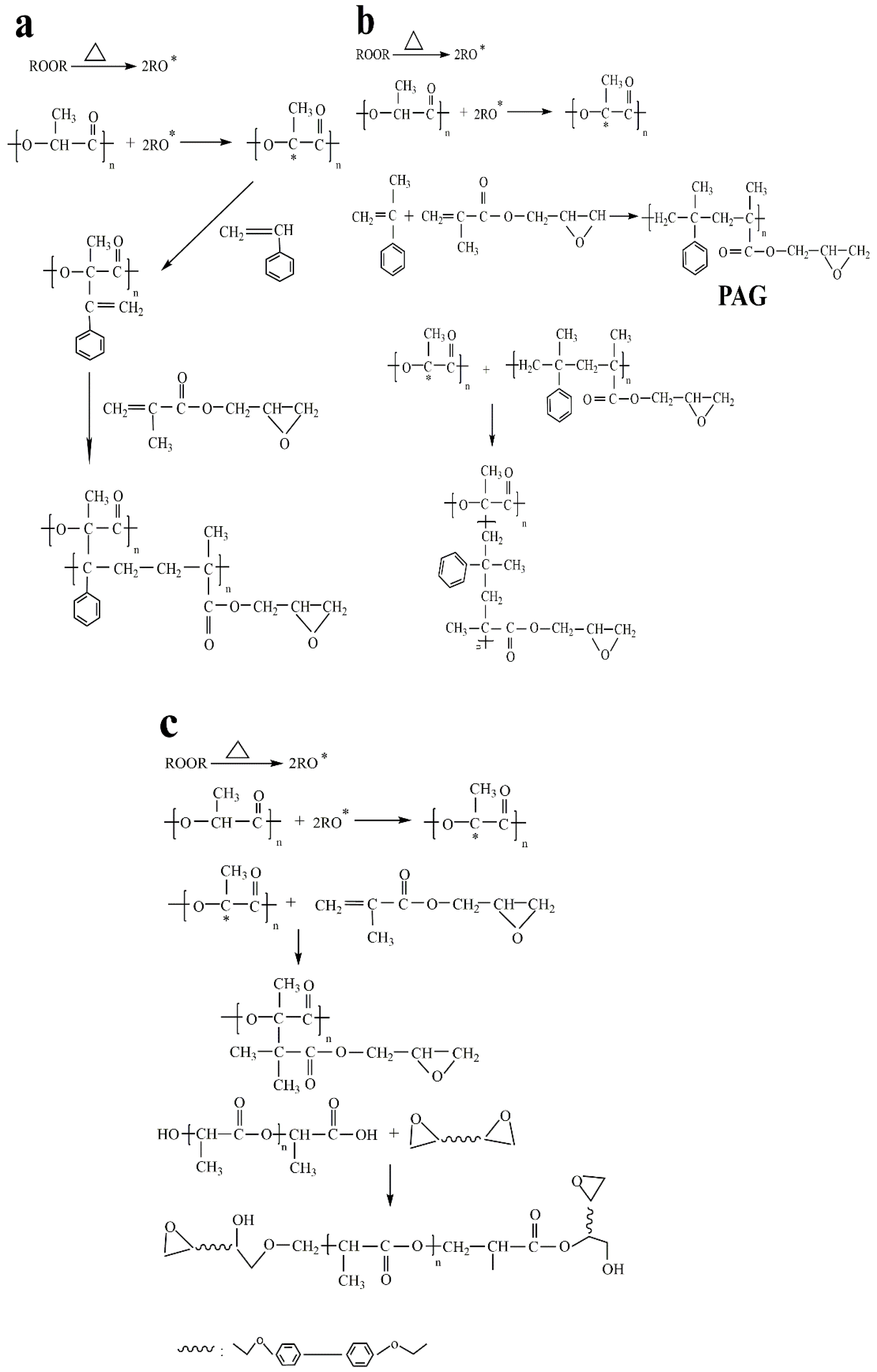
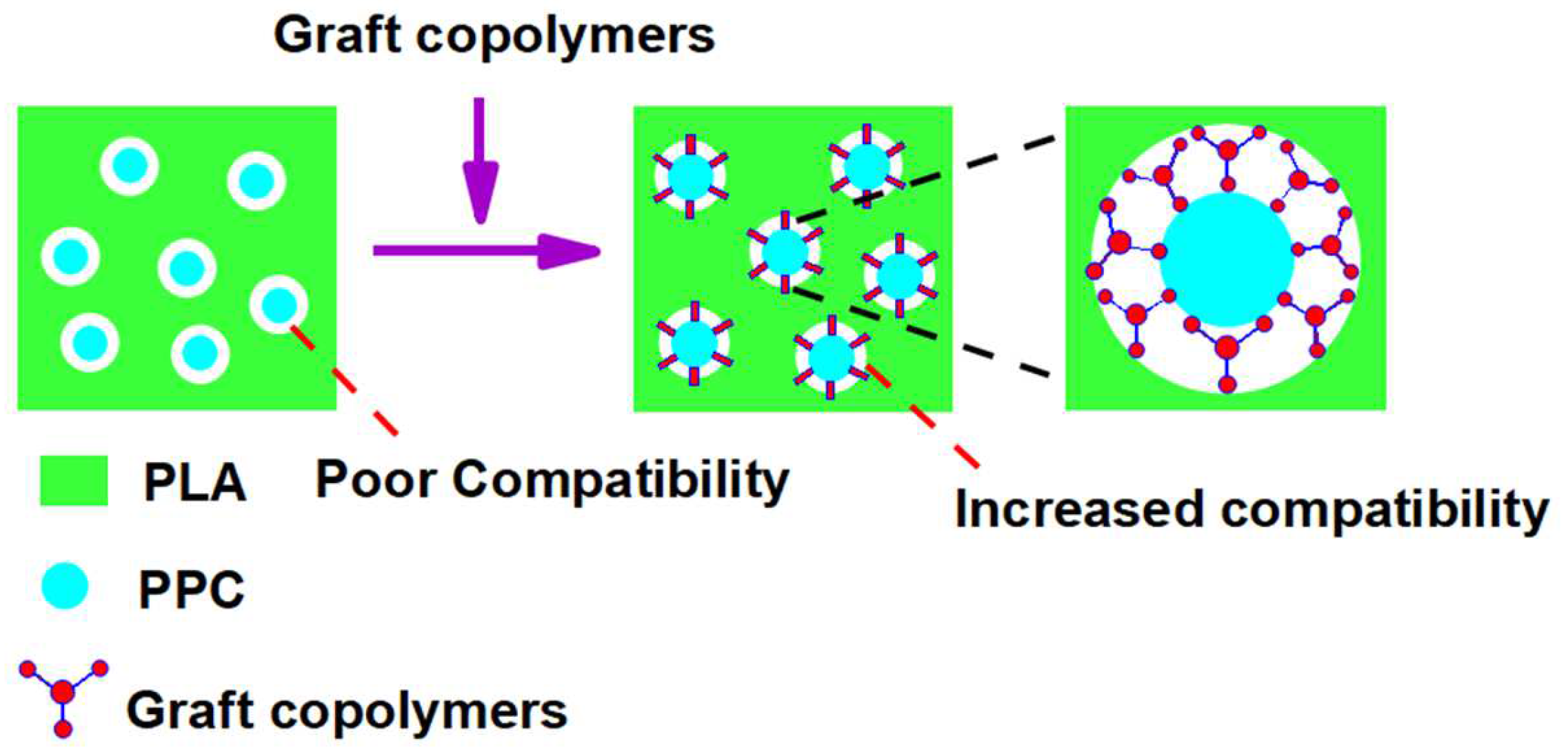
3.2. Characterization of the Graft Copolymers
3.3. Optimum Content of the Graft Copolymer
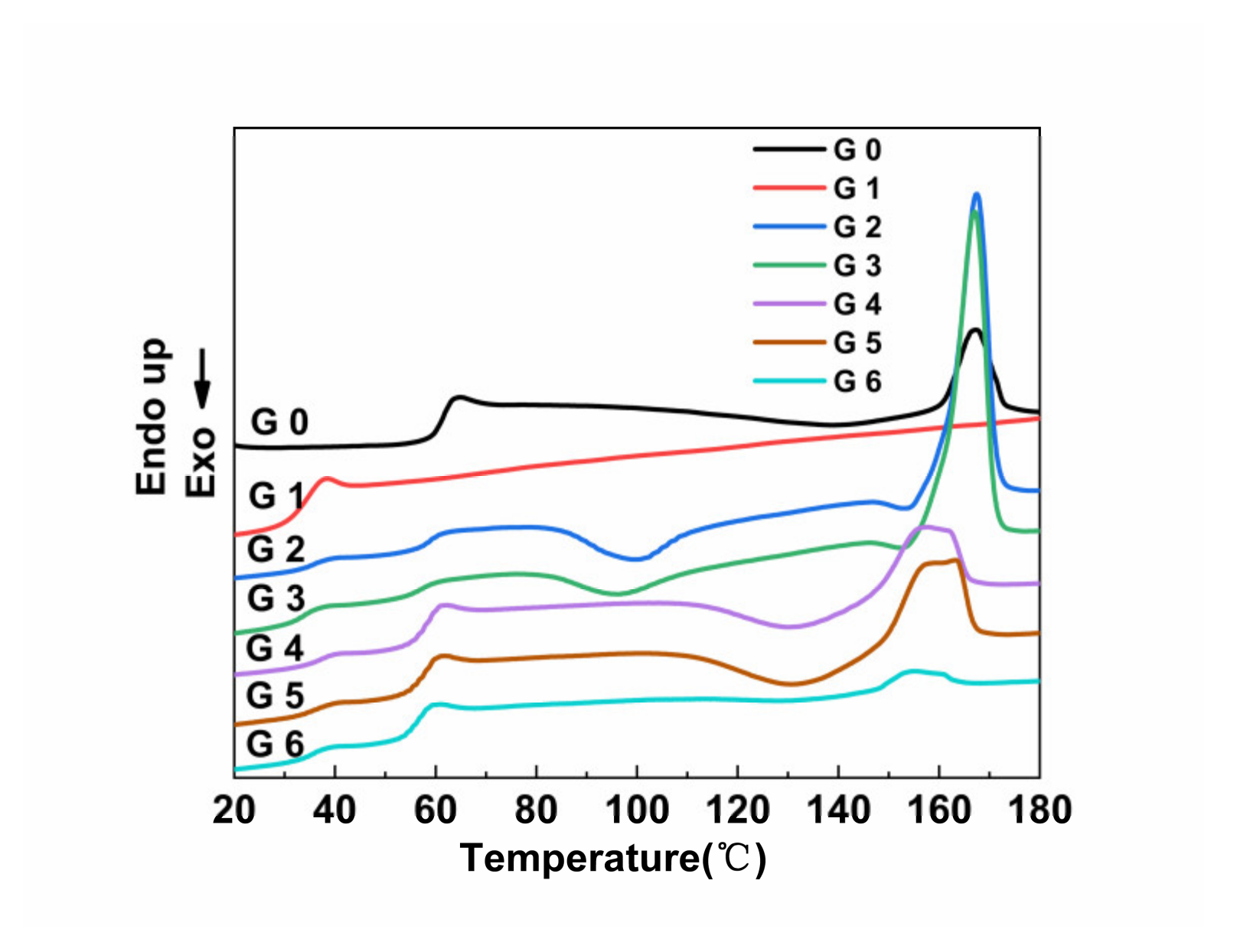
3.4. DSC Analysis
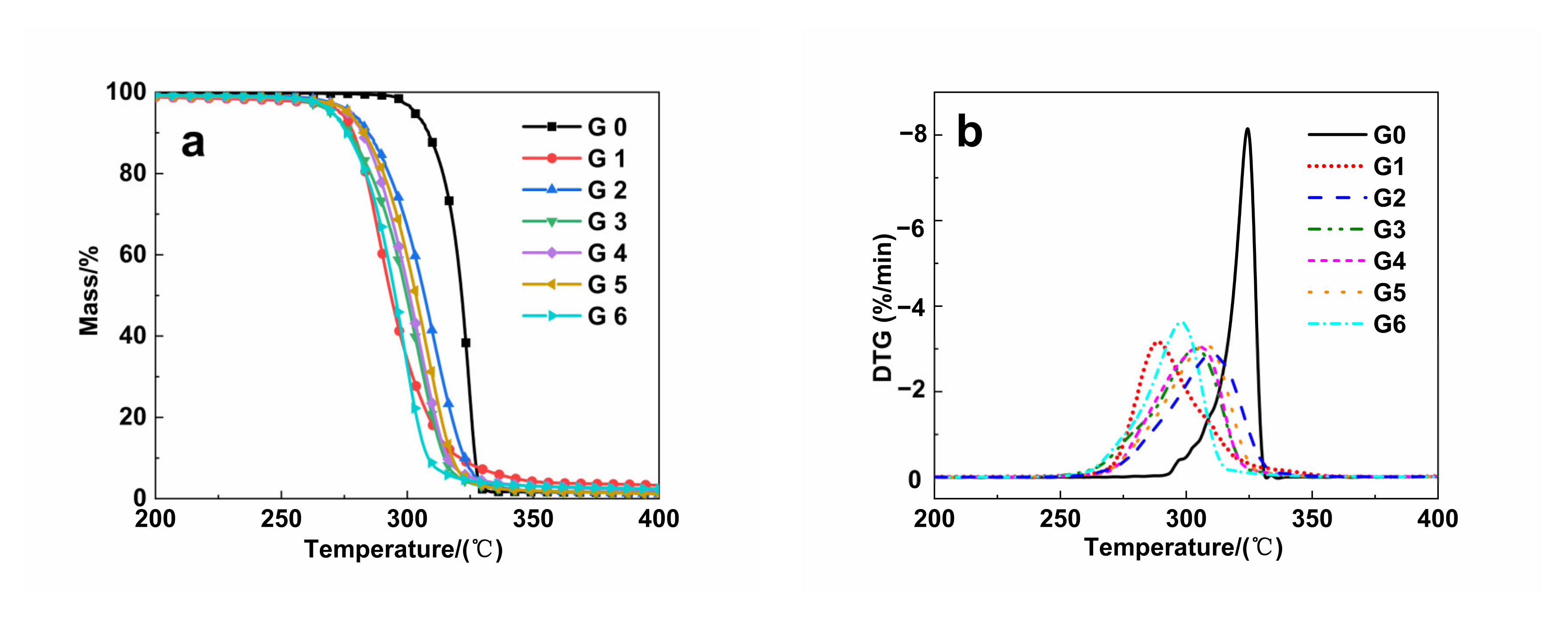
3.5. TGA Analysis
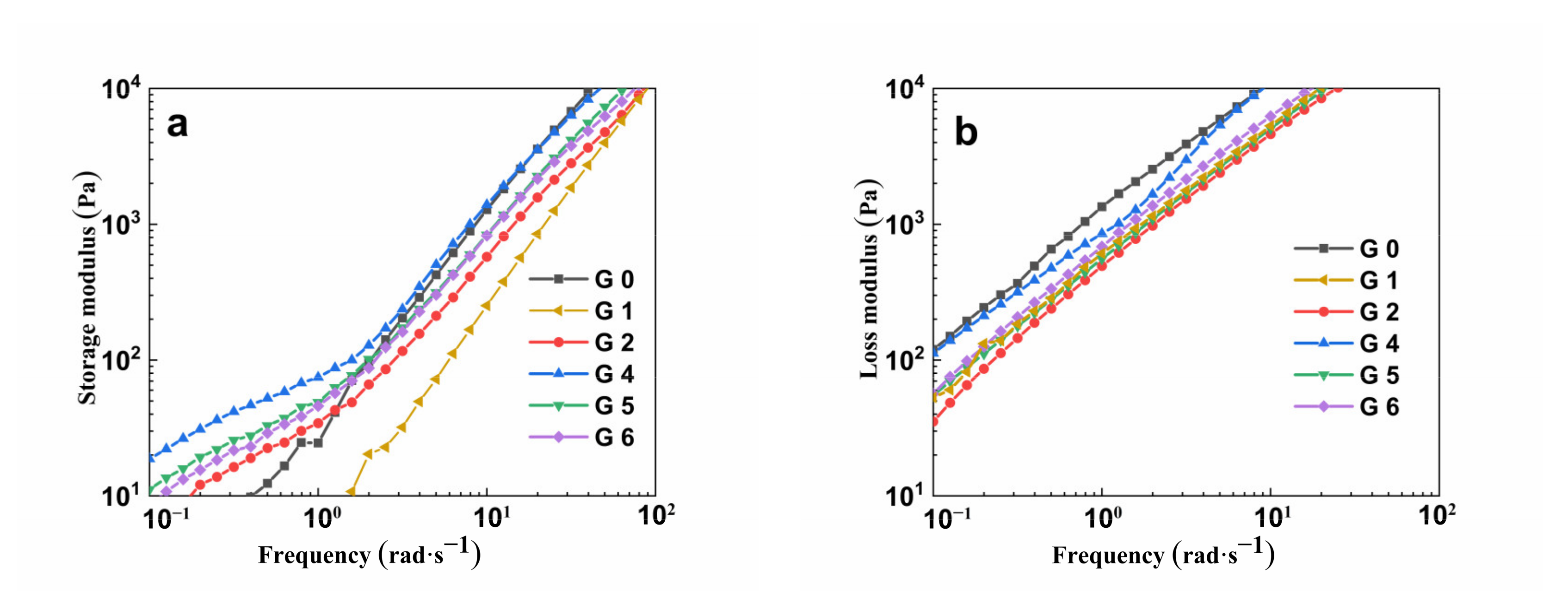
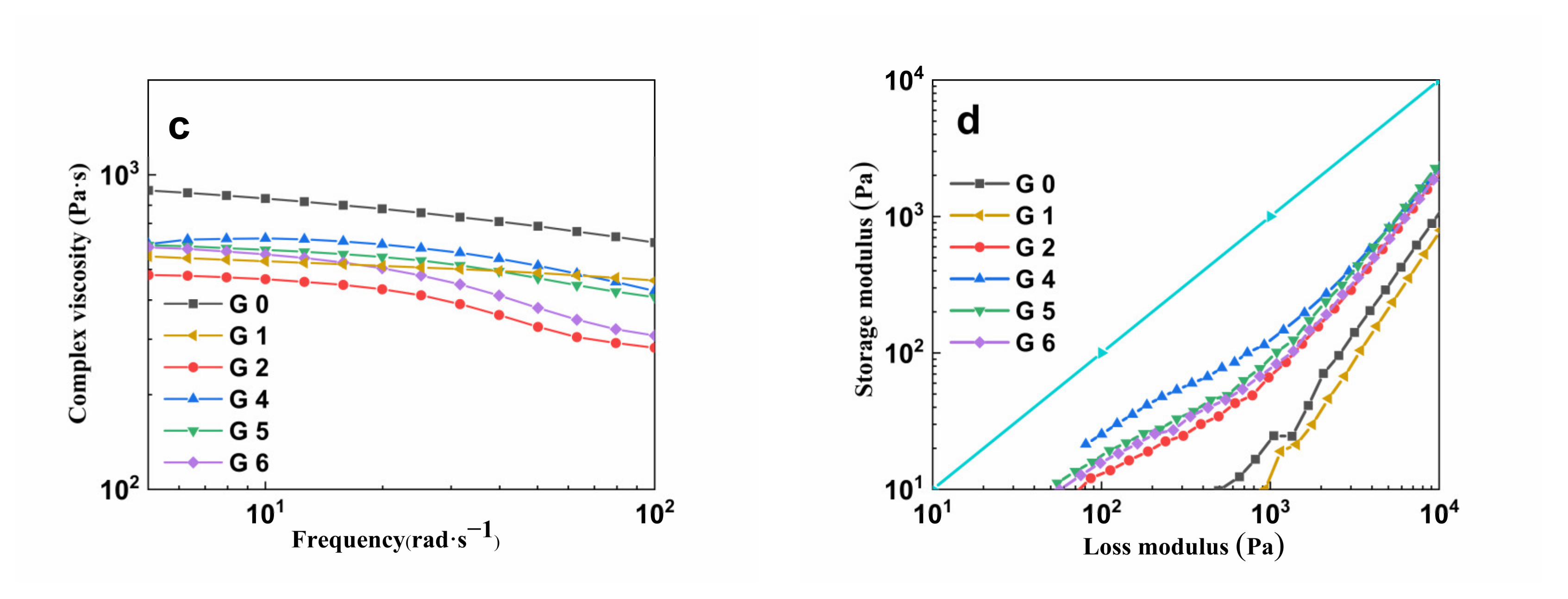
3.6. Dynamic Rheological Analysis
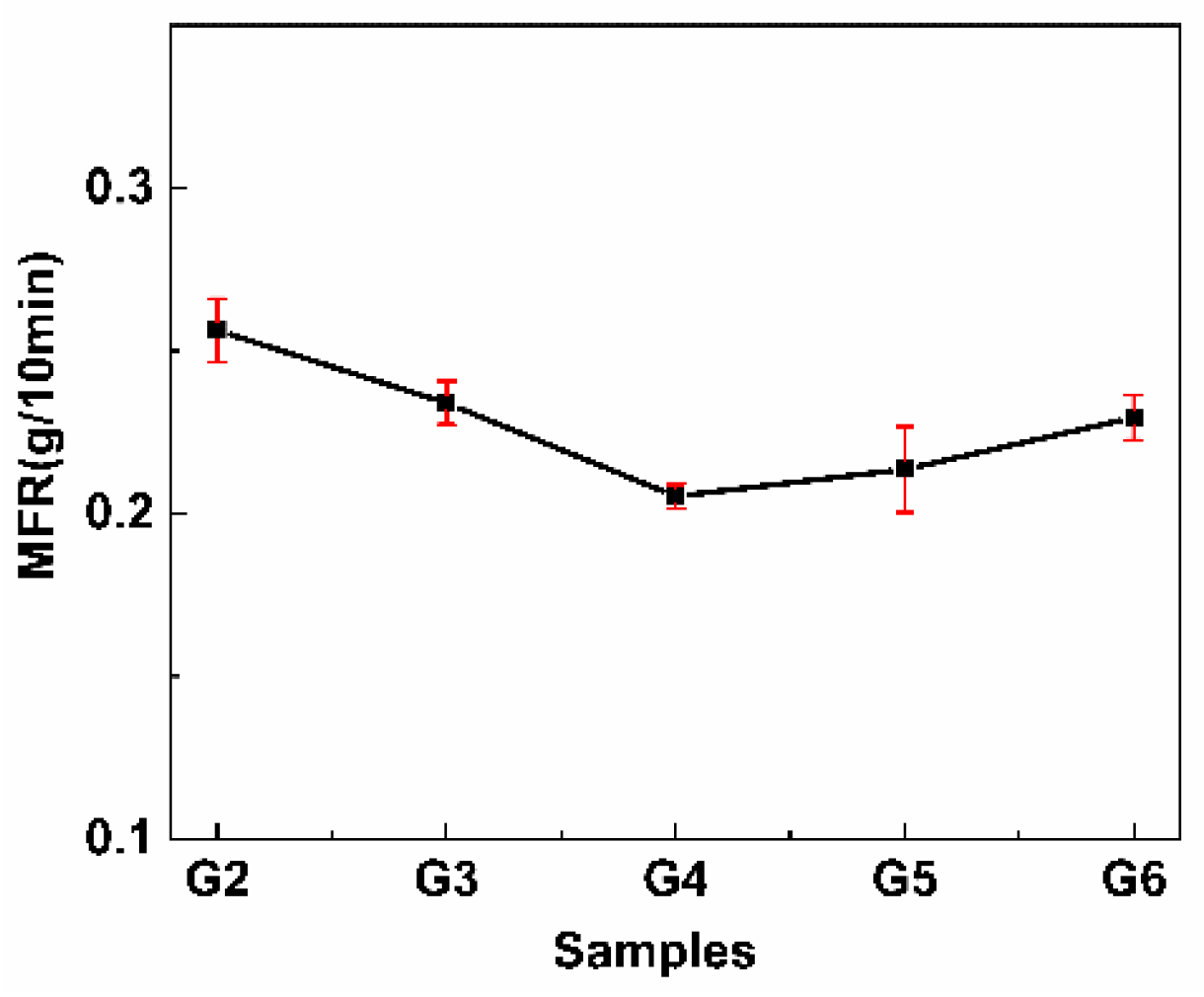
3.7. MFR Analysis
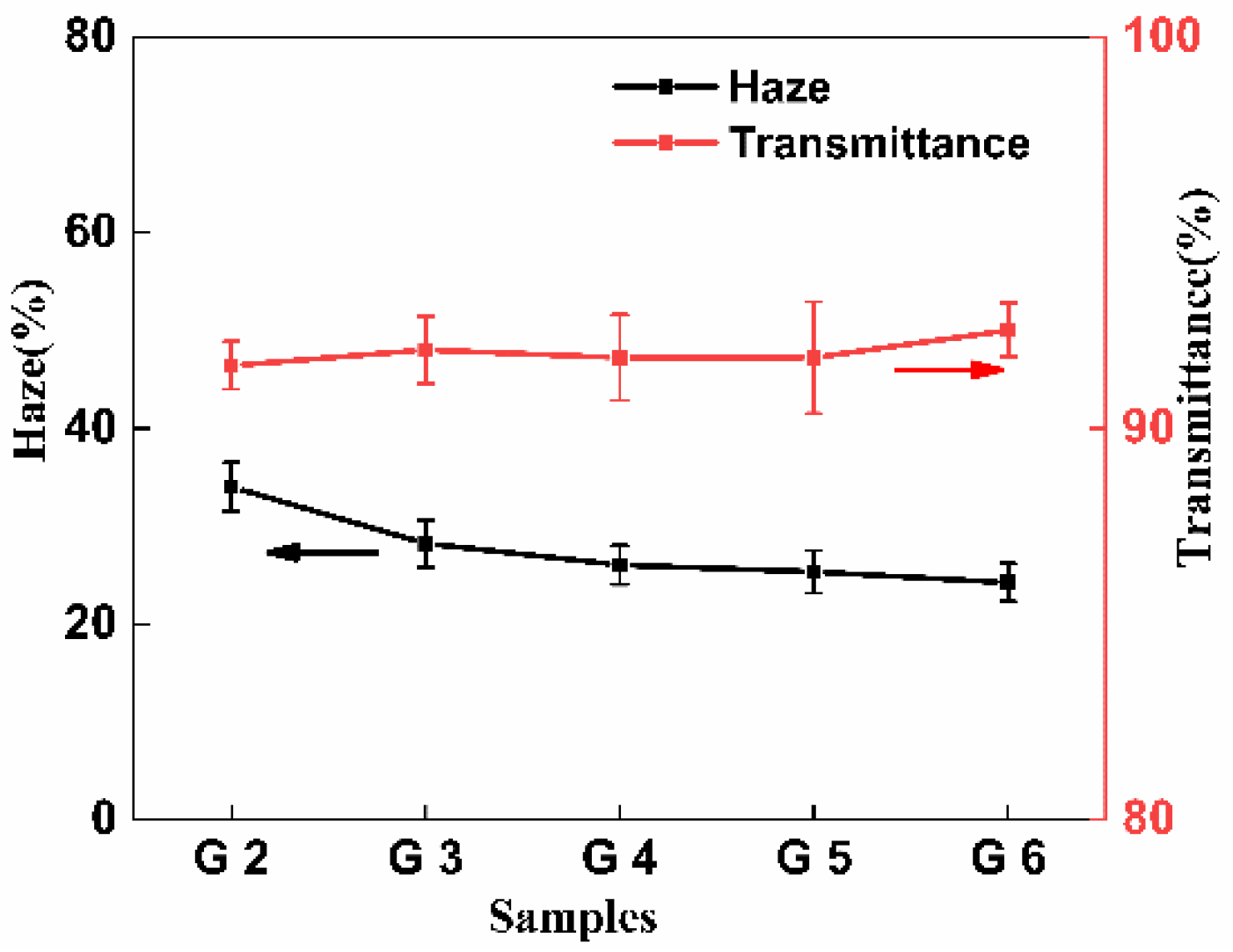
3.8. Optical Properties
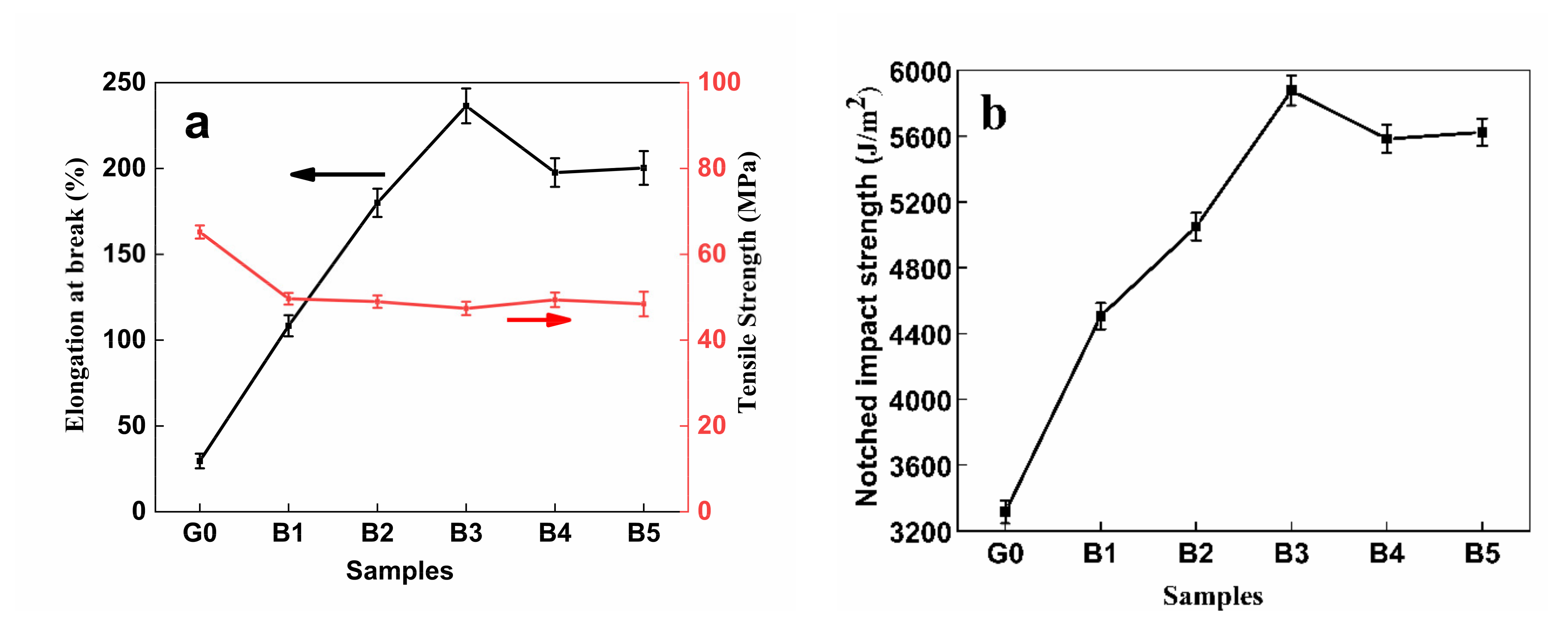
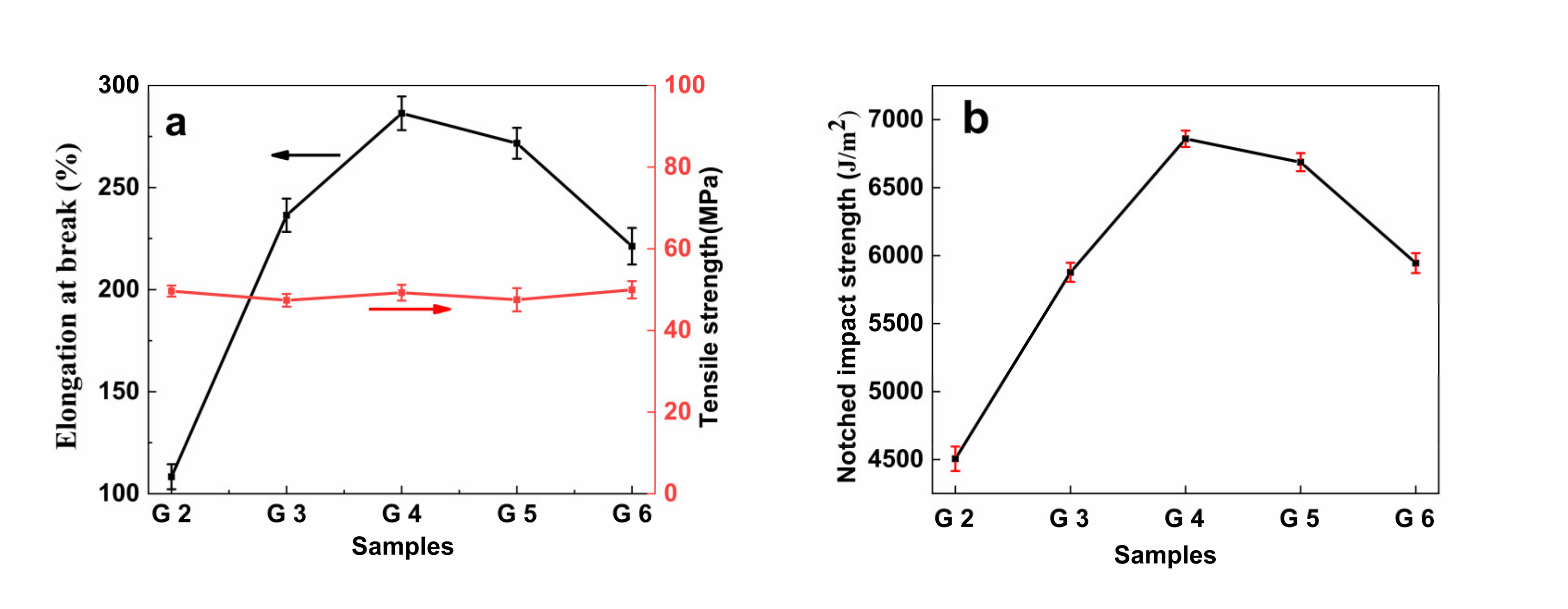
3.9. Mechanical Properties
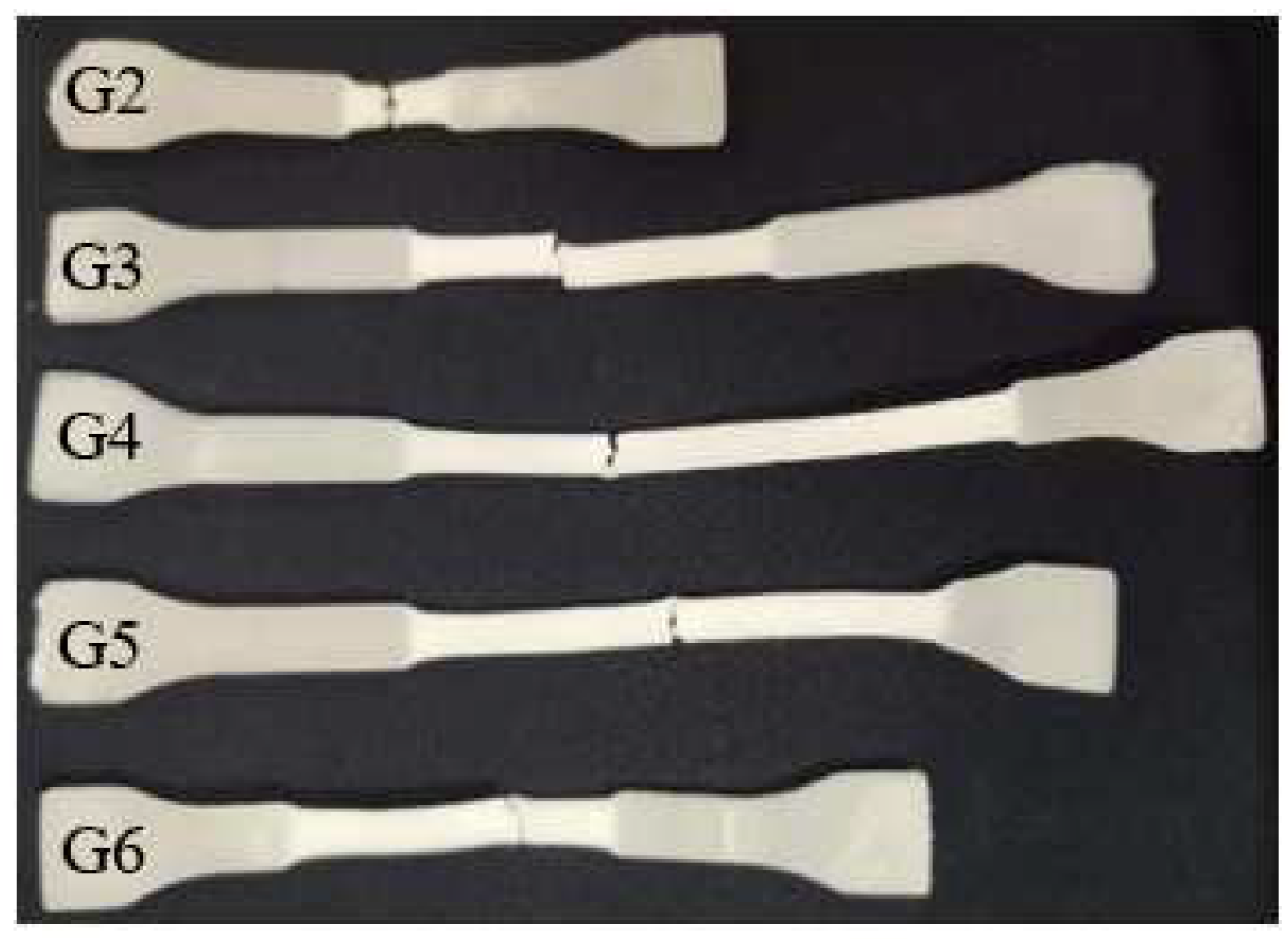
3.10. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwiczak, J.; Frckowiak, S.; Leluk, K. Study of Thermal, Mechanical and Barrier Properties of Biodegradable PLA/PBAT Films with Highly Oriented MMT. Materials 2021, 14, 7189. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhen, W.; Dong, C.; Zhao, L. Performance and multi-scale investigation on the phase miscibility of poly(lactic acid)/amided silica nanocomposites. Int. J. Biol. Macromol. 2021, 177, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.C. From lactic acid to poly(lactic acid) (PLA): Characterization and analysis of PLA and its precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Anderson, K.S.; Lim, S.H.; Hillmyer, M.A. Toughening of polylactide by melt blending with linear low-density polyethylene. J. Appl. Polym. Sci. 2003, 89, 3757–3768. [Google Scholar] [CrossRef]
- Doganci, M.D.; Aynali, F.; Doganci, E.; Ozkoc, G. Mechanical, Thermal and Morphological Properties of Poly(lactic acid) By Using Star-Shaped Poly(ε-caprolactone) with POSS Core. Eur. Polym. J. 2019, 121, 109316. [Google Scholar] [CrossRef]
- Ma, P.; Jiang, L.; Yu, M.; Dong, W.; Chen, M. Green Antibacterial Nanocomposites from Poly (lactide)/Poly (butylene adipate-co-terephthalate)/Nanocrystal Cellulose-Silver Nanohybrids. ACS Sustain. Chem. Eng. 2016, 4, 6417–6426. [Google Scholar] [CrossRef]
- Ding, Y.; Lu, B.; Wang, P.; Wang, G.; Ji, J. PLA-PBAT-PLA tri-block copolymers: Effective compatibilizers for promotion of the mechanical and rheological properties of PLA/PBAT blends. Polym. Degrad. Stab. 2018, 147, 41–48. [Google Scholar] [CrossRef]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT bionanocomposites with antimicrobial natural rosin for green packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Wang, Z.; Sun, N.; Li, H. Enhancement of electrical conductivity by changing phase morphology for composites consisting of polylactide and poly (ε-caprolactone) filled with acid-oxidized multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 4858–4864. [Google Scholar] [CrossRef]
- Chen, H.; Yu, X.; Zhou, W.; Peng, S.; Zhao, X. Highly toughened polylactide (PLA) by reactive blending with novel polycaprolactone-based polyurethane (PCLU) blends. Polym Test. 2018, 70, 275–280. [Google Scholar] [CrossRef]
- Qin, S.; Yu, C.; Chen, X.; Zhou, H.; Zhao, L. Fully biodegradable poly (lactic acid)/poly (propylene carbonate) shape memory materials with low recovery temperature based on in situ compatibilization by dicumyl peroxide. Chin. J. Polym. Sci. 2018, 36, 783–790. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Liu, Z.; Zhang, S.; Cao, Z.; Yang, W.; Yang, M. Compatibilization of the poly (lactic acid)/poly (propylene carbonate) blends through in situ formation of poly (lactic acid)-b-poly (propylene carbonate) copolymer. J. Appl. Polym. Sci. 2018, 135, 46009. [Google Scholar] [CrossRef]
- Xie, L.; Xu, H.; Chen, J.-B.; Zhang, Z.-J.; Hsiao, B.S.; Zhong, G.-J.; Chen, J.; Li, Z.-M. From nanofibrillar to nanolaminar poly (butylene succinate): Paving the way to robust barrier and mechanical properties for full-biodegradable poly (lactic acid) films. ACS Appl. Mater. Interfaces 2015, 7, 8023–8032. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Yu, J.; Fang, J. Partially miscible poly (lactic acid)-blend-poly (propylene carbonate) filled with carbon black as conductive polymer composite. Polym. Int. 2008, 57, 1027–1035. [Google Scholar]
- Tao, J.; Song, C.; Cao, M.; Hu, D.; Liu, L.; Liu, N.; Wang, S. Thermal properties and degradability of poly (propylene carbonate)/poly (β-hydroxybutyrate-co-β-hydroxyvalerate) (PPC/PHBV) blends. Polym. Degrad. Stab. 2009, 94, 575–583. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Pan, H.; Ai, X.; Dong, L. Rheological, thermal and mechanical properties of biodegradable poly (lactic acid)/poly (butylene adipate-co-terephthalate)/poly (propylene carbonate) polyurethane trinary blown films. Polym. Bull. 2020, 77, 4235–4258. [Google Scholar] [CrossRef]
- Dong, X.; Liu, L.; Wang, Y.; Li, T.; Wu, Z.; Yuan, H.; Ma, P.; Shi, D.; Chen, M.; Dong, W. The compatibilization of poly (propylene carbonate)/poly (lactic acid) blends in presence of core-shell starch nanoparticles. Carbohydr. Polym. 2021, 254, 117321. [Google Scholar] [CrossRef]
- Song, L.; Li, Y.; Meng, X.; Wang, T.; Shi, Y.; Wang, Y.; Liu, L. Crystallization, Structure and Significantly Improved Mechanical Properties of PLA/PPC Blends Compatibilized with PLA-PPC Copolymers Produced by Reactions Initiated with TBT or TDI. Polymers 2021, 13, 3245. [Google Scholar] [CrossRef]
- Phetwarotai, W.; Maneechot, H.; Kalkornsurapranee, E.; Phusunti, N. Thermal behaviors and characteristics of polylactide/poly (butylene succinate) blend films via reactive compatibilization and plasticization. Polym. Adv. Technol. 2018, 29, 2121–2133. [Google Scholar] [CrossRef]
- Teamsinsungvon, A.; Jarapanyacheep, R.; Ruksakulpiwat, Y.; Jarukumjorn, K. Melt processing of maleic anhydride grafted poly (lactic acid) and its compatibilizing effect on poly (lactic acid)/poly (butylene adipate-co-terephthalate) blend and their composite. Polym. Sci. 2017, 59, 384–396. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, C.; Kim, Y.; Lee, K.; Lee, M. Compatibilization of immiscible poly (l-lactide) and low density polyethylene blends. Fiber Polym. 2004, 5, 270–274. [Google Scholar] [CrossRef]
- Wei, Q.; Chionna, D.; Galoppini, E.; Pracell, M. Functionalization of LDPE by melt grafting with glycidyl methacrylate and reactive blending with polyamide-6. Macromol. Chem. Phys. 2003, 204, 1123–1133. [Google Scholar] [CrossRef]
- Chen, L.F.; Wong, B.; Baker, W.E. Melt grafting of glycidyl methacrylate onto polypropylene and reactive compatibilization of rubber toughened polypropylene. Polym. Eng. Sci. 1996, 36, 1594–1607. [Google Scholar] [CrossRef]
- Huang, H.; Liu, N.C. Nondegradative melt functionalization of polypropylene with glycidyl methacrylate. J. Appl. Polym. Sci. 1998, 67, 1957–1963. [Google Scholar] [CrossRef]
- Cartier, H.; Hu, G.H. Styrene-assisted melt free radical grafting of glycidyl methacrylate onto polypropylene. J. Polym. Sci. Pol. Chem. 1998, 36, 1053–1063. [Google Scholar] [CrossRef]
- Xie, X.; Chen, N.; Guo, B.; Li, S. Study of multi-monomer melt-grafting onto polypropylene in an extruder. Polym. Int. 2000, 49, 1677–1683. [Google Scholar] [CrossRef]
- Saeb, M.R.; Garmabi, H. Investigation of styrene-assisted free-radical grafting of glycidyl methacrylate onto high-density polyethylene using response surface method. J. Appl. Polym. Sci. 2009, 111, 1600–1605. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Li, Z.; Pan, H.; Dong, Q.; Han, L.; Zhang, H.; Dong, L. Rheology, mechanical properties and crystallization behavior of glycidyl methacrylate grafted poly (ethylene octene) toughened poly (lactic acid) blends. Korean J. Chem. Eng. 2016, 33, 1104–1114. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Wei, Y.; Huang, B. Styrene-assisted melt-free radical grafting of glycidyl methacrylate onto isotactic poly (1-butene). Polym. Eng. Sci. 2011, 51, 1669–1674. [Google Scholar] [CrossRef]
- Luo, W.; Liu, X.; Fu, Y. Melt grafting of maleic anhydride onto polypropylene with assistance of α-methylstyrene. Polym. Eng. Sci. 2012, 52, 814–819. [Google Scholar] [CrossRef]
- Gong, K.; Tian, H.; Liu, H.; Liu, X.; Hu, G.; Ning, N.; Tian, M.; Zhang, L. Grafting of Isobutylene–Isoprene Rubber with Glycidyl Methacrylate and Its Reactive Compatibilization Effect on Isobutylene–Isoprene Rubber/Polyamides 12 Blends. Ind. Eng. Chem. Res. 2021, 60, 16258–16266. [Google Scholar] [CrossRef]
- Lou, J.; Luo, Z.; Li, Y. The effect of epoxy and tetramethyl thiuram disulfide on melt-grafting of maleic anhydride onto polypropylene by reactive extrusion. Appl. Polym. Sci. 2016, 133, 43422. [Google Scholar] [CrossRef]
- Meng, X.Y. Study on the Structure and Properties of PLA/PPC Blends. Master’s Thesis, Shenyang University of Chemical Technology, Shenyang, China, 2020. [Google Scholar]
- Wang, F.; Dai, L.; Ge, T. α-methylstyrene-assisted maleic anhydride grafted poly (lactic acid) as an effective compatibilizer affecting properties of microcrystalline cellulose/poly (lactic acid) composites. Express Polym. Lett. 2020, 14, 530–541. [Google Scholar] [CrossRef]
- Nakano, K.; Hashimoto, S.; Nakamura, M.; Kamada, T. Stereocomplex of Poly (propylene carbonate): Synthesis of Stereogradient Poly (propylene carbonate) by Regio- and Enantioselective Copolymerization of Propylene Oxide with Carbon Dioxide. Angew. Chem. Int. Ed. 2011, 50, 4868–4871. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, G.; Jiang, W. Effects of Catalytic Transesterification and Composition on the Toughness of Poly(lactic acid)/Poly(propylene carbonate) Blends. Ind. Eng. Chem. Res. 2016, 55, 5565–5573. [Google Scholar] [CrossRef]
- Ma, P.; Jiang, L.; Ye, T.; Dong, W.; Chen, M. Melt free-radical grafting of maleic anhydride onto biodegradable poly (lactic acid) by using styrene as a comonomer. Polymer 2014, 6, 1528–1543. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, H.; Chen, L. Grafting of glycidyl methacrylate onto poly (lactide) and properties of PLA/starch blends compatibilized by the grafted copolymer. J. Polym. Environ. 2012, 20, 810–816. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, L.; Li, D.; Xu, W. The mechanism of grafting of maleic anhydride onto isotactic polybutene-1 assisted by comonomers. Colloid Polym. Sci. 2017, 295, 463–469. [Google Scholar] [CrossRef]
- Xie, L.; Huang, H.; Yang, L.; Zhang, F.; Luo, Z. Effect of Melt Blending Preparation of PLA-g-MAH/EP on the Properties of PLA/PBAT Composites. Polym. Mater. Sci. Eng. 2019, 35, 7. [Google Scholar]
- Legros, A.; Carreau, P.J.; Favis, B.D. Reactive compatibilization of polyester/vinyl acetate copolymer blends: Rheological, morphological and mechanical properties. Polymer 1994, 35, 758–764. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Xie, X.; Xu, M.; Song, W. Styrene-assisted maleic anhydride grafted poly (lactic acid) as an effective compatibilizer for wood flour/poly (lactic acid) bio-composites. Polymer 2017, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Deng, J.; Yu, Q.; Yang, W. Degradation and initiation polymerization mechanism of α-methylstyrene-containing macroinitiators. J. Appl. Polym. Sci. 2011, 120, 466–473. [Google Scholar] [CrossRef]
- Liang, S.; Deng, J.; Yang, W. Monomer reactivity ratio and thermal performance of α-methyl styrene and glycidyl methacrylate copolymers. Chin. J. Polym. Sci. 2010, 28, 323–330. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Ren, M.; Chen, Q.; Song, J.; Zhang, H.; Mo, Z. Thermal and mechanical properties of poly (butylene terephthalate)/epoxy blends. J. Appl. Polym. Sci. 2008, 109, 4082–4088. [Google Scholar] [CrossRef]
- Utracki, L.A. Viscoelastic behavior of polymer blends. Polym. Eng. Sci. 1988, 28, 1401–1404. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Q.; Ren, J.; Wang, L. Preparation and properties of biodegradable poly (lactic acid)/poly (butylene adipate-co-terephthalate) blend with glycidyl methacrylate as reactive processing agent. J. Mater. Sci. 2009, 44, 250–256. [Google Scholar] [CrossRef]
- Chen, J.; Wei, W.; Qian, Q.; Xiao, L.; Liu, X.; Xu, J.; Huang, B.; Chen, Q. The structure and properties of long-chain branching poly (trimethylene terephthalate). Rheol. Acta 2014, 53, 67–74. [Google Scholar] [CrossRef]
- Zheng, C.; Yao, X.R.; Shi, Y.; Ren, Y.; Liu, L. Internal and surface structures and optical properties of crystalline polymer films. Polym. Mater. Sci. Eng. 2018, 34, 7. [Google Scholar]
- Ojijo, V.; Sinha Ray, S.; Sadiku, R. Toughening of biodegradable polylactide/poly (butylene succinate-co-adipate) blends via in situ reactive compatibilization. ACS Appl. Mater. Interfaces 2013, 5, 4266–4276. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, C.; Wang, L. Preparation and properties of biodegradable poly (lactic acid)/poly (butylene adipate-co-terephthalate) blend with epoxy-functional styrene acrylic copolymer as reactive agent. J. Polym. Environ. 2013, 21, 286–292. [Google Scholar] [CrossRef]

| Samples | PLA (phr) | GMA (phr) | St (phr) | AMS (phr) | EP (phr) | DCP (phr) |
|---|---|---|---|---|---|---|
| A0 | 100 | 3 | 0 | 0 | 0 | 0.15 |
| A1 | 100 | 3 | 6 | 0 | 0 | 0.15 |
| A2 | 100 | 3 | 0 | 6 | 0 | 0.15 |
| A3 | 100 | 3 | 0 | 0 | 6 | 0.15 |
| Samples | PLA (phr) | PPC (phr) | PLA-g-GMA (phr) |
|---|---|---|---|
| B1 | 70 | 30 | 0 |
| B2 | 65 | 30 | 5 |
| B3 | 60 | 30 | 10 |
| B4 | 55 | 30 | 15 |
| B5 | 50 | 30 | 20 |
| Samples | PLA (phr) | PPC (phr) | PLA-g-GMA (phr) | PLA-g-GMA/St (phr) | PLA-g-GMA/AMS (phr) | PLA-g-GMA/EP (phr) |
|---|---|---|---|---|---|---|
| G0 | 100 | 0 | 0 | 0 | 0 | 0 |
| G1 | 0 | 100 | 0 | 0 | 0 | 0 |
| G2 | 70 | 30 | 0 | 0 | 0 | 0 |
| G3 | 60 | 30 | 10 | 0 | 0 | 0 |
| G4 | 60 | 30 | 0 | 10 | 0 | 0 |
| G5 | 60 | 30 | 0 | 0 | 10 | 0 |
| G6 | 60 | 30 | 0 | 0 | 0 | 10 |
| Sample | PLA-g-GMA | PLA-g-GMA/St | PLA-g-GMA/AMS | PLA-g-GMA/EP |
|---|---|---|---|---|
| Gd (phr) | 0.8 | 1.6 | 1.3 | 0.9 |
| Ge (%) | 26 | 53.3 | 43 | 30 |
| Samples | Elongation at Break (%) | Tensile Strength (MPa) | Notched Impact Strength (J/m2) |
|---|---|---|---|
| G0 | 29.58 ± 4.25 | 65.19 ± 1.53 | 3315.4 ± 69.15 |
| B1 | 108.33 ± 6.14 | 49.64 ± 1.36 | 4505.8 ± 80.10 |
| B2 | 180.00 ± 8.25 | 48.96 ± 1.44 | 5050.0 ± 84.14 |
| B3 | 236.46 ± 10.15 | 47.38 ± 1.55 | 5876.9 ± 90.25 |
| B4 | 197.60 ± 8.34 | 49.40 ± 1.69 | 5583.1 ± 85.36 |
| B5 | 200.31 ± 9.81 | 48.42 ± 2.88 | 5623.8 ± 81.91 |
| Samples | Tg (PLA)/°C | Tg (PPC)/°C | ΔTg)/°C | Tm)/°C | Xc/% |
|---|---|---|---|---|---|
| G0 | 61.01 ± 0.30 | - | - | 167.30± 0.20 | 7.31 ± 0.21 |
| G1 | - | 30.51± 0.21 | - | - | - |
| G2 | 59.01 ± 0.25 | 36.03 ± 0.23 | 22.98 ± 0.05 | 167.75 ± 0.11 | 7.49 ± 0.22 |
| G3 | 58.80 ± 0.28 | 36.75 ± 0.27 | 22.05 ± 0.07 | 167.59 ± 0.15 | 9.17 ± 0.37 |
| G4 | 57.67 ± 0.19 | 37.25 ± 0.15 | 20.42 ± 0.05 | 157.48 ± 0.21 | 3.94 ± 0.42 |
| G5 | 57.69 ± 0.31 | 36.73 ± 0.23 | 20.96 ± 0.03 | 162.95 ± 0.20 | 1.69 ± 0.15 |
| G6 | 56.51 ± 0.23 | 35.46 ± 0.42 | 21.05 ± 0.02 | 155.19 ± 0.13 | 0.26 ± 0.21 |
| Samples | T95% (℃) | Tmax (℃) | T5% (℃) |
|---|---|---|---|
| G0 | 303.19 ± 0.12 | 325.10 ± 0.21 | 328.08 ± 0.24 |
| G1 | 273.32 ± 0.23 | 286.48 ± 0.19 | 342.94 ± 0.19 |
| G2 | 277.75 ± 0.14 | 310.25 ± 0.23 | 327.60 ± 0.28 |
| G3 | 269.86 ± 0.21 | 293.88 ± 0.19 | 320.85 ± 0.17 |
| G4 | 276.29 ± 0.20 | 304.42 ± 0.15 | 326.53 ± 0.22 |
| G5 | 276.45 ± 0.17 | 307.75 ± 0.18 | 323.02 ± 0.45 |
| G6 | 270.31 ± 0.09 | 298.45 ± 0.26 | 320.38 ± 0.36 |
| Samples | Elongation at Break (%) | Tensile Strength (MPa) | Notched Impact Strength (J/m2) |
|---|---|---|---|
| G0 | 29.58 ± 4.25 | 65.19 ± 1.53 | 3315.4 ± 69.15 |
| G1 | 761.32 ± 8.69 | 12.14 ± 1.62 | 5950.0 ± 85.27 |
| G2 | 108.33 ± 6.14 | 49.64 ± 1.36 | 4505.8 ± 80.10 |
| G3 | 236.46 ± 10.15 | 47.38 ± 1.55 | 5876.9 ± 90.25 |
| G4 | 286.35 ± 9.25 | 49.24 ± 1.93 | 6858.6 ± 98.05 |
| G5 | 271.67 ± 11.58 | 47.52 ± 2.84 | 6686.5 ± 96.47 |
| G6 | 221.25 ± 8.98 | 49.96 ± 2.11 | 5944.3 ± 82.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Zhang, Q.; Hao, Y.; Li, Y.; Chi, W.; Cong, F.; Shi, Y.; Liu, L.-Z. Effect of Different Comonomers Added to Graft Copolymers on the Properties of PLA/PPC/PLA-g-GMA Blends. Polymers 2022, 14, 4088. https://doi.org/10.3390/polym14194088
Song L, Zhang Q, Hao Y, Li Y, Chi W, Cong F, Shi Y, Liu L-Z. Effect of Different Comonomers Added to Graft Copolymers on the Properties of PLA/PPC/PLA-g-GMA Blends. Polymers. 2022; 14(19):4088. https://doi.org/10.3390/polym14194088
Chicago/Turabian StyleSong, Lixin, Qian Zhang, Yongsheng Hao, Yongchao Li, Weihan Chi, Fei Cong, Ying Shi, and Li-Zhi Liu. 2022. "Effect of Different Comonomers Added to Graft Copolymers on the Properties of PLA/PPC/PLA-g-GMA Blends" Polymers 14, no. 19: 4088. https://doi.org/10.3390/polym14194088
APA StyleSong, L., Zhang, Q., Hao, Y., Li, Y., Chi, W., Cong, F., Shi, Y., & Liu, L.-Z. (2022). Effect of Different Comonomers Added to Graft Copolymers on the Properties of PLA/PPC/PLA-g-GMA Blends. Polymers, 14(19), 4088. https://doi.org/10.3390/polym14194088







