Facile Fabrication of Dual Functional Graphene Oxide Microcapsules Carrying Corrosion Inhibitor and Encapsulating Self-Healing Agent
Abstract
:1. Introduction
2. Experimental
2.1. Materials
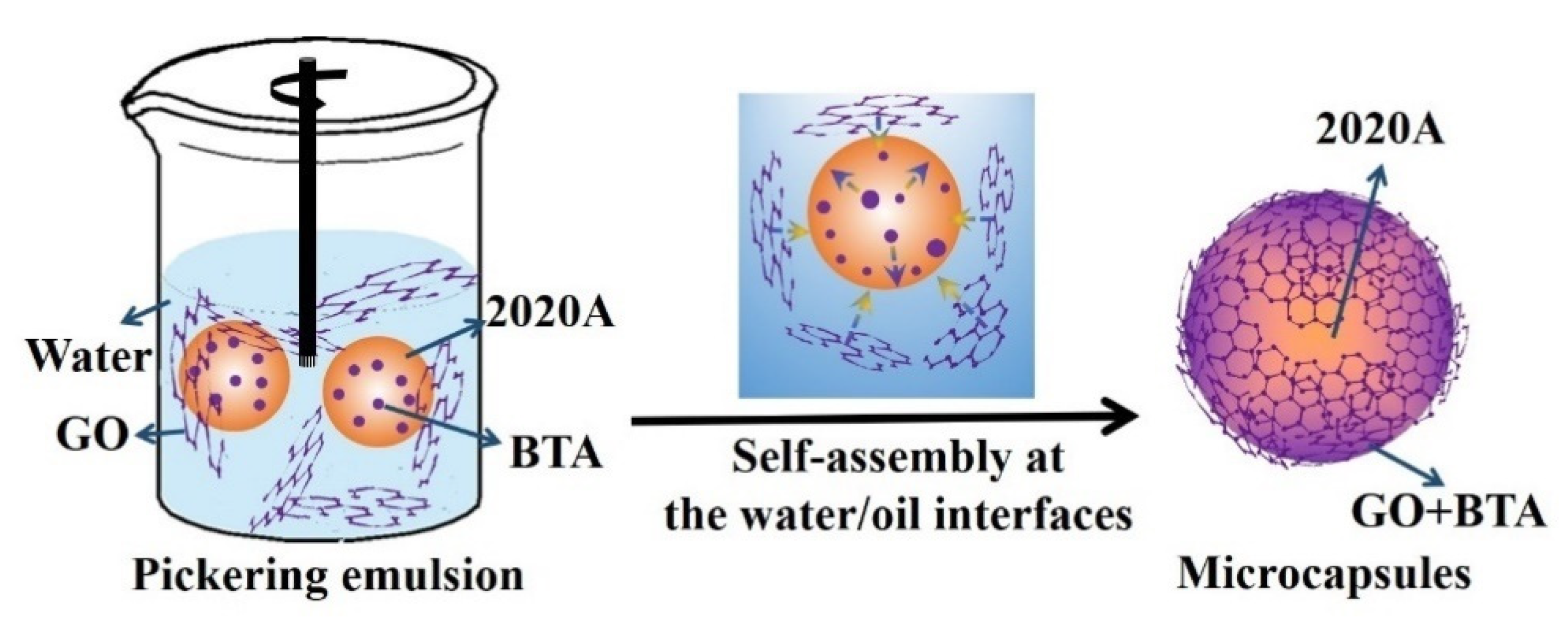
2.2. Fabrication of the GO Microcapsules
2.3. Preparation of the Microcapsules Reinforced WEP Coatings
2.4. Characterization
3. Results and Discussion
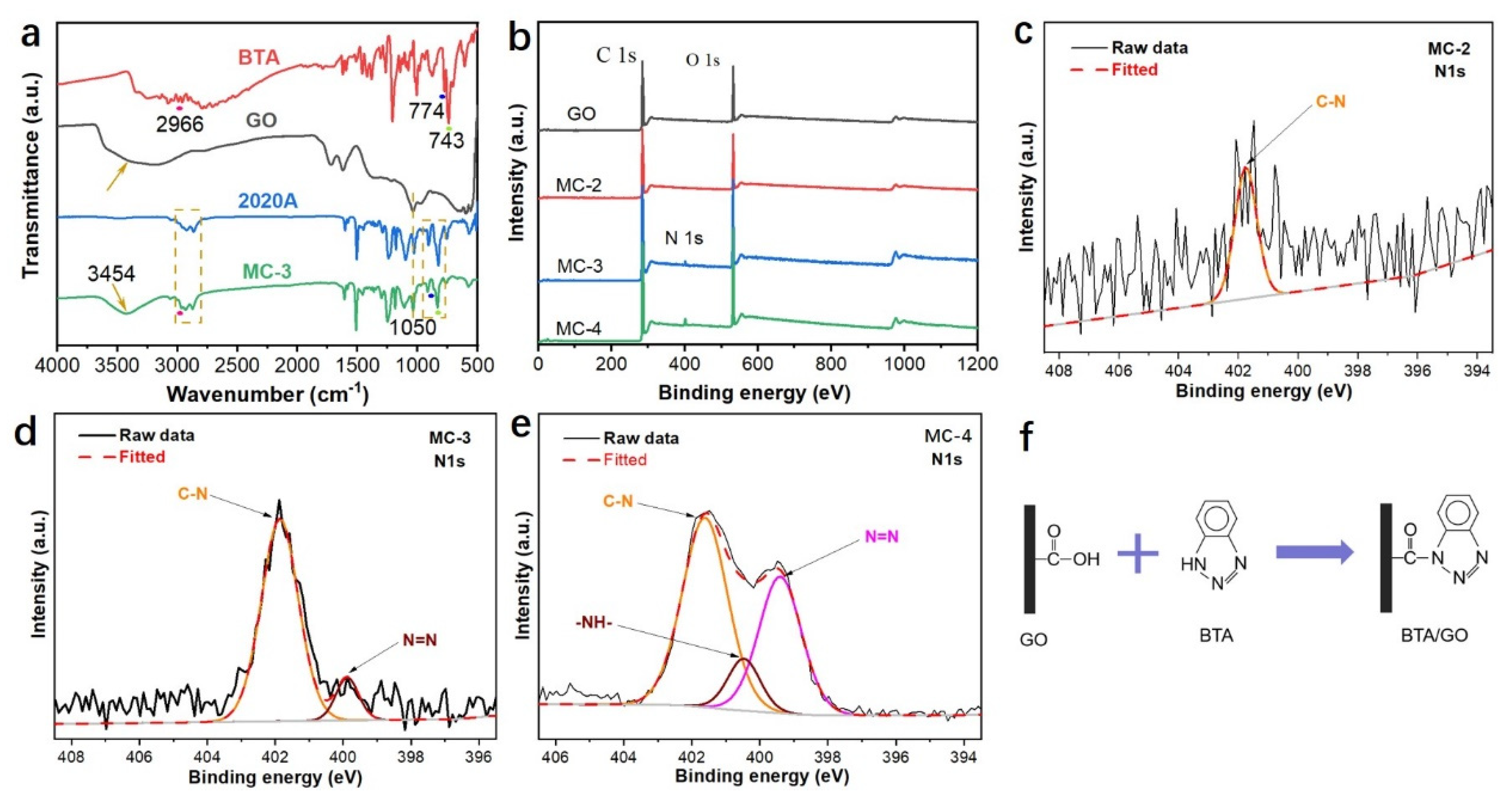
3.1. Formation of the GO Microcapsules with the Assistance of BTA
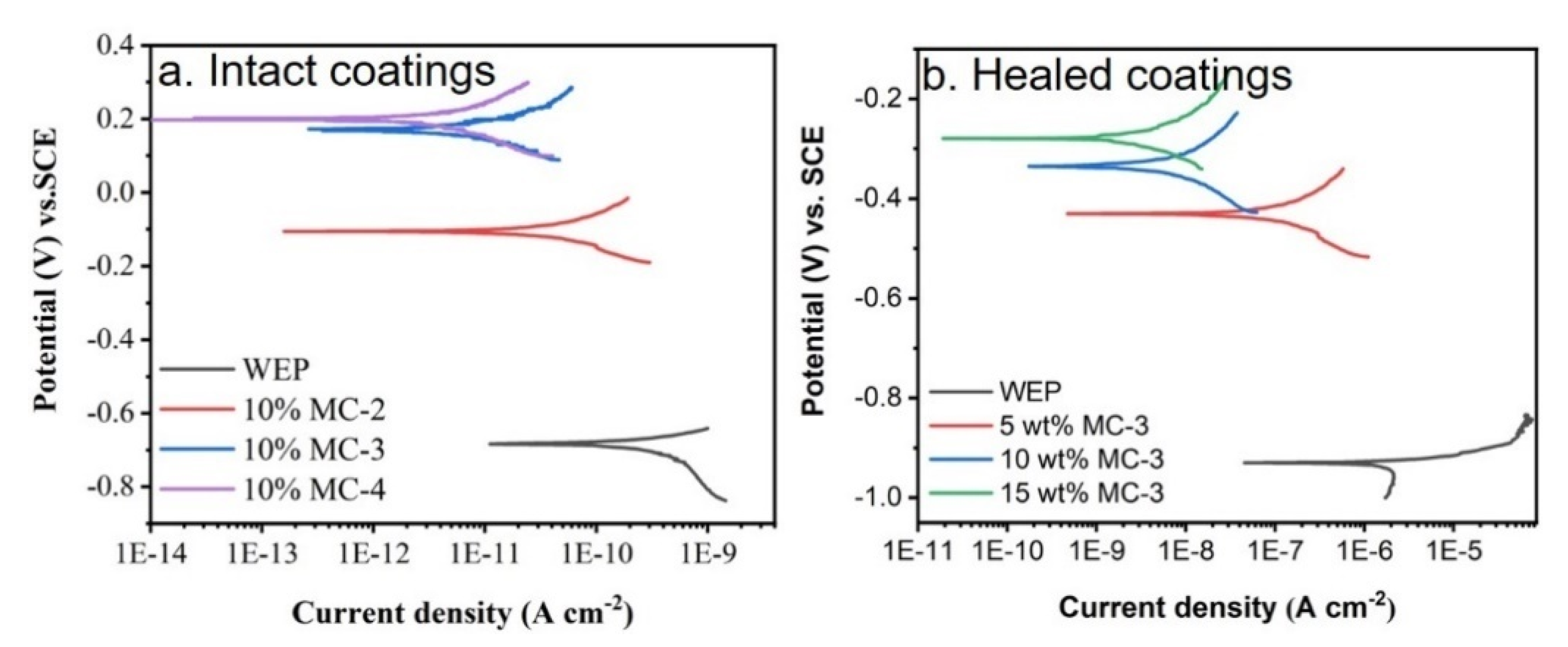
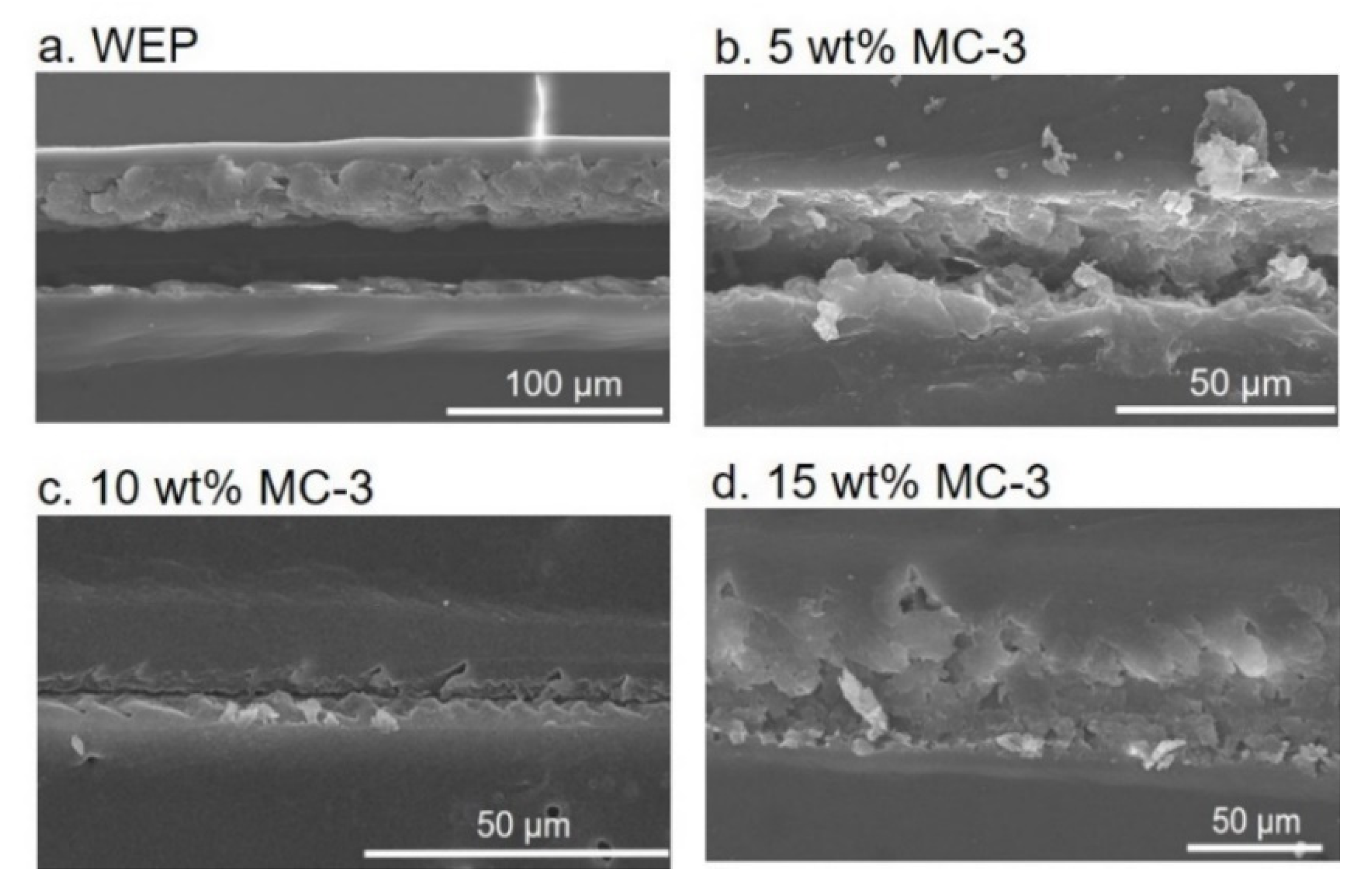
3.2. Corrosion Protection and Self-Healing Properties of the Composite Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, L.; Ren, C.; Wang, J.; Liu, T.; Yang, H.; Wang, Y.; Huang, Y.; Zhang, D. Self-reporting coatings for autonomous detection of coating damage and metal corrosion: A review. Chem. Eng. J. 2021, 421, 127854. [Google Scholar] [CrossRef]
- Li, W.; Matthews, C.C.; Yang, K.; Odarczenko, M.T.; White, S.R.; Scottos, N.R. Autonomous indication of mechanical damage in polymeric coatings. Adv. Mater. 2016, 28, 2189–2194. [Google Scholar] [CrossRef]
- Lv, L.; Guo, P.; Xing, F.; Han, N. Trigger efficiency enhancement of polymeric microcapsules for self-healing cementitious materials. Constr. Build. Mater. 2020, 235, 117443. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Wang, S.; Liu, J.; Xing, X.; Li, Z.; Wang, B. A comprehensive review on smart anti-corrosive coatings. Prog. Org. Coat. 2020, 148, 105821. [Google Scholar] [CrossRef]
- Kosarli, M.; Foteinidis, G.; Tsirka, K.; Bekas, D.G.; Paipetis, A.S. Concurrent recovery of mechanical and electrical properties in nanomodified capsule-based self-healing epoxies. Polymer 2021, 227, 123843. [Google Scholar] [CrossRef]
- Zhang, S.; Kou, K.; Zhang, J.; Jia, Q.; Xu, Y. Compact energetic crystals@ urea-formaldehyde resin micro-composites with evident insensitivity. Compos. Commun. 2019, 15, 103–107. [Google Scholar] [CrossRef]
- Zhao, Y.; Fickert, J.; Landfester, K.; Crespy, D. Encapsulation of self-healing agents in polymer nanocapsules. Small 2012, 8, 2954–2958. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Döhler, D.; Lv, L.P.; Binder, W.H.; Landfester, K.; Crespy, D. Facile phase-separation approach to encapsulate functionalized polymers in core-shell nanoparticles. Macromol. Chem. Phys. 2014, 215, 198–204. [Google Scholar] [CrossRef]
- Zheng, N.; Qiao, L.; Liu, J.; Lu, J.; Li, W.; Li, C.; Liu, Q.; Xue, Y.; Zhang, Q. Microcapsules of multilayered shell structure synthesized via one-part strategy and their application in self-healing coatings. Compos. Commun. 2019, 12, 26–32. [Google Scholar] [CrossRef]
- Yi, H.; Yang, Y.; Gu, X.; Huang, J.; Wang, C. Multilayer composite microcapsules synthesized by Pickering emulsion templates and their application in self-healing coating. J. Mater. Chem. A 2015, 3, 13749–13757. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Yan, X. Effects of adding methods of fluorane microcapsules and shellac resin microcapsules on the preparation and properties of bifunctional waterborne coatings for basswood. Polymers 2022, 14, 3919. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, Y.; Zhang, M.; Li, M.; Li, H. Preparation of tung oil-loaded PU/PANI microcapsules and synergetic anti-corrosion properties of self-healing epoxy coatings. Macromol. Mater. Eng. 2020, 306, 2000581. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Quan, H.; Han, J.; Liu, X.; Zhang, X.; Yang, J.; Xiang, Y. Robust polyurea/poly(urea-formaldehyde) hybrid microcapsules decorated with Al2O3 nano-shell for improved self-healing performance. Appl. Surf. Sci. 2021, 542, 148561. [Google Scholar] [CrossRef]
- Sun, T.; Shen, X.; Peng, C.; Fan, H.; Liu, M.; Wu, Z. A novel strategy for the synthesis of self-healing capsule and its application. Compos. Sci. Technol. 2019, 171, 13–20. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, L.; Zhang, Y.; Yang, Y.; Yu, R.; Wang, J. Microencapsulation of tris(dimethylaminomethyl)phenol using polystyrene shell for self-healing materials. Sci. Rep. 2020, 10, 12315. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, Q.; Cui, J.; Yuan, Q.; Qiu, H.; Gao, S.; Yang, J. Self-assembled graphene oxide microcapsules in Pickering emulsions for self-healing waterborne polyurethane coatings. Compos. Sci. Technol. 2017, 151, 282–290. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Feng, Q.; Qiu, H.; Yang, G.; Zheng, S.; Yang, J. Encapsulation of linseed oil in graphene oxide shells for preparation of self-healing composite coatings. Prog. Org. Coat. 2019, 129, 285–291. [Google Scholar] [CrossRef]
- Pieranski, P. Two-dimensional interfacial colloidal crystals. Phys. Rev. Lett. 1980, 45, 569–572. [Google Scholar] [CrossRef]
- Cunha, A.B.M.; Leal, D.A.; Santos, L.R.L.; Riegel-Vidotti, I.C.; Marino, C.E.B. pH-sensitive microcapsules based on biopolymers for active corrosion protection of carbon steel at different pH. Surf. Coat. Technol. 2020, 402, 126338. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.; Wu, L. Synthesis of UV-responsive dual-functional microspheres for highly efficient self-healing coatings. Chem. Eng. J. 2021, 422, 130034. [Google Scholar] [CrossRef]
- Leal, D.A.; Riegel-Vidotti, I.C.; Ferreira, M.G.S.; Marino, C.E.B. Smart coating based on double stimuli-responsive microcapsules containing linseed oil and benzotriazole for active corrosion protection. Corros. Sci. 2018, 130, 56–63. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Bao, C.; Li, X.; Duan, F.; Friedrich, K.; Yang, J. Skin-inspired, fully autonomous self-warning and self-repairing polymeric material under damaging events. Chem. Mater. 2019, 31, 2611–2618. [Google Scholar] [CrossRef]
- Tao, Z.; Cui, J.; Qiu, H.; Yang, J.; Gao, S.; Li, J. Microcapsule/silica dual-fillers for self-healing, self-reporting and corrosion protection properties of waterborne epoxy coatings. Prog. Org. Coat. 2021, 159, 106394. [Google Scholar] [CrossRef]
- Chen, S.; Han, T.; Zhao, Y.; Luo, W.; Zhang, Z.; Su, H.; Tang, B.Z.; Yang, J. A facile strategy to prepare smart coatings with autonomous self-healing and self-reporting functions. ACS Appl. Mater. Interfaces 2020, 12, 4870–4877. [Google Scholar] [CrossRef]
- Doan, T.Q.; Leslie, L.S.; Kim, S.Y.; Bhargava, R.; White, S.R.; Sottos, N.R. Characterization of core-shell microstructure and self-healing performance of electrospun fiber coatings. Polymer 2016, 107, 263–272. [Google Scholar] [CrossRef]
- Khatir, N.M.; Ahmadi, A.; Taghizade, N.; Khameneh, S.M.; Faghihnasiri, M. Electronic transport properties of nanoribbons of graphene and ψ-graphene -based lactate nanobiosensor. Superlattices Microstruct. 2020, 145, 106603. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Wang, W.; Liu, F.; Hou, B. Application of 1D attapulgite as reservoir with benzotriazole for corrosion protection of carbon steel. Mater. Chem. Phys. 2018, 205, 292–302. [Google Scholar] [CrossRef]
- Gopi, D.; Sherif, E.-S.M.; Manivannan, V.; Rajeswari, D.; Surendiran, M.; Kavitha, L. Corrosion and corrosion inhibition of mild steel in groundwater at different temperatures by newly synthesized benzotriazole and phosphono derivatives. Ind. Eng. Chem. Res. 2014, 53, 4286–4294. [Google Scholar] [CrossRef]
- Prabhu, R.; Roopashree, B.; Jeevananda, T.; Rao, S.; Reddy, K.R.; Raghu, A.V. Synthesis and corrosion resistance properties of novel conjugated polymer-Cu2Cl4L3 composites. Mater. Sci. Energy Technol. 2021, 4, 92–99. [Google Scholar] [CrossRef]



| Specimen | MC-0 | MC-1 | MC-2 | MC-3 | MC-4 | MC-5 |
|---|---|---|---|---|---|---|
| BTA (mg) | 0 | 2 | 9 | 35 | 70 | 140 |
| 2020A (g) | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| GO (mL, 3.75 mg/mL) | 5 | 5 | 5 | 5 | 5 | 5 |
| Average diameter of the microcapsules (μm) | / | / | 12.5 | 14.7 | 17.2 | / |
| Loading capacity of the microcapsules (%) | / | / | 90.5 | 87.7 | 80.9 | / |
| Atomic Content (%) | C | O | N |
|---|---|---|---|
| GO | 68.9 | 31.1 | 0 |
| MC-2 | 80.2 | 19.1 | 0.7 |
| MC-3 | 78.6 | 19.3 | 2.1 |
| MC-4 | 74.7 | 21.7 | 3.6 |
| Coatings | Microcapsules/Content (wt%) | Ecorr (mV) | Icorr (A cm−2) | HE (%) |
|---|---|---|---|---|
| Intact WEP | 0 | −684 | 2.20 × 10−10 | / |
| Intact | MC-2/10 | −104 | 8.62 × 10−11 | / |
| Intact | MC-3/10 | 158 | 4.59 × 10−12 | / |
| Intact | MC-4/10 | 377 | 1.37 × 10−12 | / |
| Scratched WEP | 0 | −930 | 1.68 × 10−6 | 0 |
| Healed | MC-3/5 | −430 | 8.75 × 10−8 | 94.8 |
| Healed | MC-3/10 | −334 | 4.71 × 10−9 | 99.7 |
| Healed | MC-3/15 | −279 | 2.27 × 10−9 | 99.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Tao, Z.; Cui, J.; Shen, S.; Qiu, H. Facile Fabrication of Dual Functional Graphene Oxide Microcapsules Carrying Corrosion Inhibitor and Encapsulating Self-Healing Agent. Polymers 2022, 14, 4067. https://doi.org/10.3390/polym14194067
Li J, Tao Z, Cui J, Shen S, Qiu H. Facile Fabrication of Dual Functional Graphene Oxide Microcapsules Carrying Corrosion Inhibitor and Encapsulating Self-Healing Agent. Polymers. 2022; 14(19):4067. https://doi.org/10.3390/polym14194067
Chicago/Turabian StyleLi, Jing, Zhenglin Tao, Jincan Cui, Shuling Shen, and Hanxun Qiu. 2022. "Facile Fabrication of Dual Functional Graphene Oxide Microcapsules Carrying Corrosion Inhibitor and Encapsulating Self-Healing Agent" Polymers 14, no. 19: 4067. https://doi.org/10.3390/polym14194067
APA StyleLi, J., Tao, Z., Cui, J., Shen, S., & Qiu, H. (2022). Facile Fabrication of Dual Functional Graphene Oxide Microcapsules Carrying Corrosion Inhibitor and Encapsulating Self-Healing Agent. Polymers, 14(19), 4067. https://doi.org/10.3390/polym14194067







