1. Introduction
Throughout human life, the cornea is constantly in contact with its surrounding environment in a way that is harmful to the delicate tissue. The immediate environment includes high and low temperatures, air pollution and toxic substances, ultraviolet radiation, and other types of radiation. However, owing to its complex structure, which includes the unique nature of trophism, innervation, and a type of cellular regeneration, it remains transparent and is the main refractive lens of the eye [
1].
Any damage or disease of the cornea can lead to a complete or partial loss of its properties; in particular, the loss of transparency leads to a significant decrease in visual acuity. The most common diseases include keratoconus, dystrophies of various origins, ulcers, and corneal injury [
2].
Donor cornea transplantation is the most common method of surgical treatment for most diseases of the cornea; however, the global shortage of donor tissue significantly complicates its use. Thus, the development of alternative approaches based on tissue engineering and regenerative medicine is necessary to solve this problem [
3].
Thus far, advances in the field of tissue engineering have shifted the therapeutic focus to creating an artificial cornea that produces the conditions for restoring specific layers through cellular regeneration [
3]. This shift has become possible owing to the development of new, less invasive surgical techniques, including anterior and posterior lamellar keratoplasty, in which only the damaged area of the cornea is replaced, while leaving the surrounding healthy tissue intact [
4]. These techniques can improve outcomes in terms of graft survival and the number of postoperative complications compared with penetrating keratoplasty, in which the entire cornea is replaced.
In our previous work, we demonstrated the safety and biocompatibility of a Viscoll collagen membrane based on medical-grade Viscoll collagen [
5]. Implantation of this Viscoll collagen membrane resulted in an increased overall thickness of the cornea and its strength characteristics, as well as the maintenance of transparency for up to six months postoperatively. Importantly, the cornea is highly innervated, and nerve regeneration after injury plays a critical role in the restoration of normal function [
6,
7]. Therefore, it is essential to investigate the regeneration of the replaced corneal tissue and the innervation of the restored area.
The primary aim of this study is to conduct a comprehensive assessment of the suitability of the Viscoll collagen membrane as an artificial analogue of the corneal stroma for the regeneration of its defects. A feature of our manufacturing process of the collagen membrane was increasing the concentration of collagen instead of the standard chemical crosslinking protocols to improve the biomechanical characteristics of the final product. The specific objectives of the current work include the study of the biomechanical characteristics and transparency of the Viscoll collagen membrane in comparison with the stroma from the human cornea; toxicological studies of the Viscoll collagen membrane; and evaluation of the effectiveness of the Viscoll collagen membrane for corneal regeneration during its implantation in the cornea of rabbits, in which a section of the stroma was surgically removed.
2. Materials and Methods
2.1. Animal Experiments
All experiments were carried out in compliance with Directive 2010/63/EU and the Research Institute of Eye Diseases Animal Care and Use Committee guidelines, and the study was approved by the aforementioned institution’s review board (№763, date of approval 11 May 2021). The experimental studies involved 10 male Chinchilla rabbits, aged 12–16 weeks and weighing 3.0–3.5 kg. All animals were allowed to adapt to their environment for 2 weeks before surgery. The rabbits received general and local anesthesia before and during surgery. General anesthesia was administered via intramuscular injection of ketamine (50 mg/kg) and xylazine (15 mg/kg). After the surgery, the animals were maintained under controlled conditions: temperature, 22 ± 1 °C; relative humidity, 45%; 10 air changes per hour; and a 12 h light/dark cycle. The rabbits had free access to water and standard compound feed. The surgeries were performed under general anesthesia. The rabbits were euthanized under deep anesthesia (100 mg/kg ketamine and 4 mg/kg xylazine) with 80 mg/kg pentobarbital.
2.2. Collagen Membrane Preparation
The production of Viscoll collagen membrane was certified according to ISO 13485 (Quality System for Medical Devices). Viscoll Collagen Membrane was obtained sterile from initially sterile components as a result of an aseptic manufacturing process (according to ISO 13408) in ISO 5 and ISO 7 class cleanrooms (according to ISO 14644-1) and did not require final sterilization.
The Viscoll collagen membrane was prepared using a previously published method [
5] consisting of two stages: gelation and vitrification at constant pressure.
2.2.1. Gelation
Briefly, 0.5 mL of a sterile 30 mg/mL solution of medical grade native porcine collagen type I (Viscoll®; Imtek Ltd., Moscow, Russia) was added to each well of a 24-well plate. To remove air bubbles from the collagen solution, the plates were centrifuged for 30 min using an Avanti J-26XP centrifuge (oscillating bucket rotor JS-5.2; Beckman Coulter, Inc., Brea, CA, USA) at 3200 rpm and 4 °C. To induce collagen gelation, the plates were incubated in an atmosphere of ammonia vapor for 12 h at 20 °C. After incubation, collagen hydrogels were collected, immersed in water for injection (Solopharm, Saint-Petersburg, Russia), and incubated for 24 h at 20 °C.
2.2.2. Plastic Compression and Vitrification
At this stage, collagen hydrogels were placed between two teflon plates, one of which was subjected to a constant load of 3 kg until complete vitrification.
2.2.3. Packaging
For long-term storage, the resulting material was rehydrated in water for injection (Solopharm) for 5 min. Then, the collagen membrane was placed on a square Teflon plate and hermetically packed in sterile primary packaging that met the requirements of ISO 11607-1 (Clinipak
®, Klinipak LLC, Moscow, Russia). The material in the primary package was hermetically packed under vacuum into a secondary vacuum package (
Figure 1). The obtained material was stored at 4–10 °C for 10 months before use.
2.3. Human Corneal Stroma Samples Preparation
Sections of the human corneal stroma were obtained using a Moria Evolution 3E microkeratome (Moria, Antony, France). The human corneoscleral disc from healthy donors was placed in a chamber with connected irrigation and securely fixed. First, the surface layer of the cornea was removed, including the Bowman’s membrane, and then a stromal section of a given thickness was performed directly using a 150 μm microkeratome head. An ultrasonic pachymeter was used to measure the thickness of the central part of the cornea before and after the incision. As a result, stromal samples with an average thickness of 300 ± 20 µm were obtained.
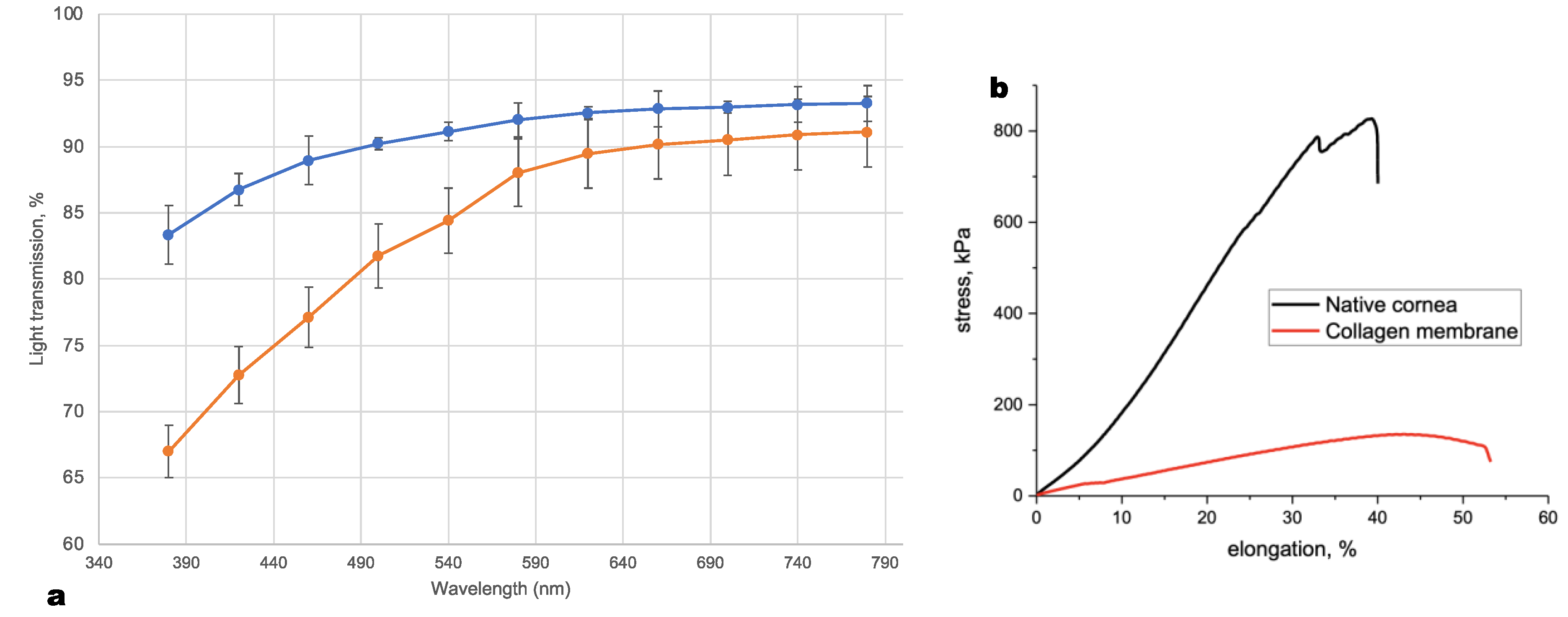
2.4. Optical Properties of Collagen Membranes and Human Cornea
Light transmission through five Viscoll collagen membranes and five human corneal stroma samples were measured in the 380–780 nm wavelength range using a UV-VIS spectrophotometer (PE-5400UV, Ecroskhim, Saint-Petersburg, Russia). All samples were about 300 µm thick. Samples were immersed in 150 mM NaCl before measurement. During the measurement, samples were placed on one side of an empty transparent cuvette. An identical empty cuvette served as a reference.
2.5. Toxicology
Toxicological tests of the Viscoll collagen membrane were performed according to ISO 10993 standards by an independently certified testing laboratory center (IMBIIT, Moscow, Russia). Viscoll collagen membranes were tested according to the following protocols:
ISO 10993-5: In vitro cytotoxicity. Direct effect of extracts from membranes on L929 mouse fibroblasts.
ISO 10993-6: Implantation. The study is intended to evaluate the biocompatibility of the test material and tissue within the eye by surgical implantation of the material into the eye of a rabbit for an appropriate period of time. The reverse tolerance of the test material and eye tissues after implantation is evaluated.
ISO 10993-10: Irritating effect. In vivo. Ocular irritation in rabbits.
ISO 10993-10: Irritating effect. In vivo. Skin sensitization in guinea pigs.
ISO 10993-11: Acute systemic toxicity test in rabbits.
GPM.1.2.4.0005.15: Pyrogenicity. The test is based on the measurement of body temperature in rabbits before and after the injection of membrane extracts.
2.6. Ex Vivo Sewing Test
The cadaveric eye was fixed in a special holder. Non-penetrating trepanation of the cornea was performed at 2/3 of its depth, after which the upper layers of the stroma were removed, and a collagen graft was placed in the formed trepanation bed and fixed with a continuous 10-0 nylon suture. The detailed technique is presented in
Supplementary Information (Video S1).
2.7. Surgery
The rabbits received general and local anesthesia before and during surgery. General anesthesia was administered via intramuscular injection of ketamine (50 mg/kg) and xylazine (15 mg/kg). The local anesthesia (2% (
w/
v) lidocaine eye drops) was administered to the right eye of each rabbit. A mark was made on the cornea using an 8 mm-diameter trephine, and an approximately 3 mm-long incision was made along the marking line at 1/3 of the depth, with a disposable blade. An intrastromal pocket was formed through the formed access. To do this, a tunnel was formed along the notch line using a spatula, which was cut with scissors concentrically to the markup. Thus, intraoperative access was expanded by ½ of the circumference, and then, using a stratifier, we formed an intracorneal pocket. Repeated stratification was performed in the formed pocket, thereby highlighting the layer of the underlying stroma that was then removed. In one group of animals, a collagen membrane was implanted into the area where the stroma was excised (first group—surgery and implantation of membranes). In another group (second group—only surgery), consisting of two rabbits, implantation of the collagen membrane was not performed after the removal of the stroma, and the wound was sutured using 10-0 nylon sutures (MANI Inc., Utsunomiya, Japan). Eye drops containing 0.3% (
w/
v) gentamicin were applied daily for the first 3 days. The detailed technique is presented in
Supplementary Information (Video S2).
2.8. Postoperative Observation
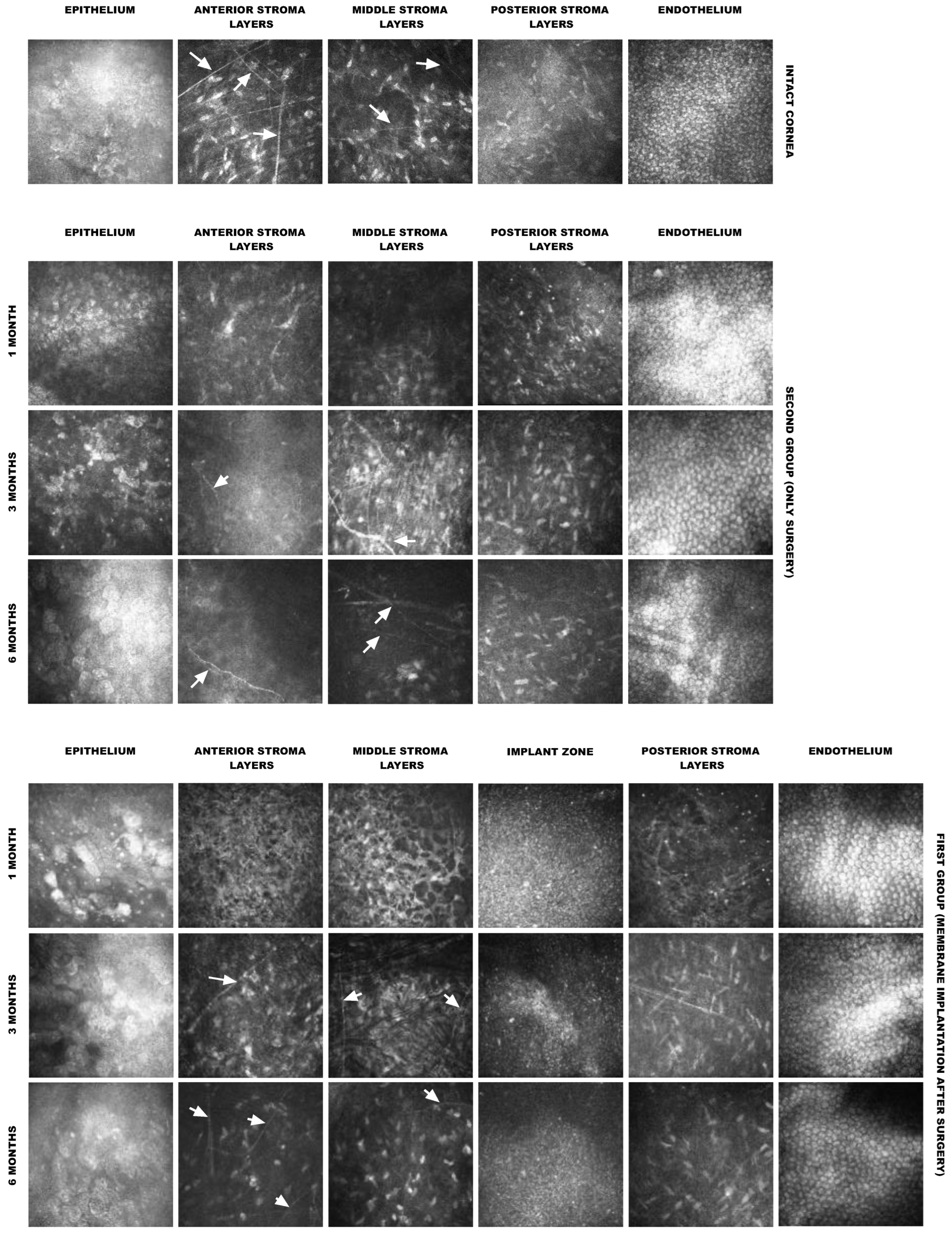
Clinical evaluations of the corneas were performed on days 30, 90, and 180 post surgery using slit-lamp microscopy. On days 30, 90, and 180, anterior segment optical coherence tomography (OCT) and in vivo confocal microscopy (IVCM) were performed on the eight rabbits from the first group. The contralateral intact eyes served as the reference.
2.9. Histological and Immunohistological Study
The rabbits were sacrificed on the 180th day after surgery. Corneal samples were excised from four rabbits, fixed in 10% (v/v) neutral buffered formalin, dehydrated, and embedded in paraffin wax. Sections of dehydrated, paraffin-embedded samples (5 μm thick) were stained with hematoxylin and eosin using standard techniques and observed under an optical microscope.
For the immunohistochemistry analysis, fixed corneal samples from four rabbits in the first group and two rabbits in the second group and the contralateral intact eyes from both groups were washed in PBS and transferred to 30% (w/v) sucrose in PBS. Sections (14 μm thick) were obtained using a cryostat (CM1900, Leica, Weltzar, Germany). The sections were incubated for 1 h at 20–25 °C in blocking solution: 5% (v/v) normal goat serum (Sigma-Aldrich, St. Louis, MO, USA), 0.3% (v/v) Triton X-100, and 0.01 M PBS (pH 7.4). This was followed by incubation with mouse anti-alpha SMA (1:200; BioLegend, San Diego, CA, USA) in the blocking solution at 4 °C overnight. Thereafter, the sections were washed and incubated for 2 h with goat anti-mouse IgG (AlexaFluor488, 1:600; Abcam, Cambridge, UK) in 0.3% (v/v) Triton X-100 and 0.01 M PBS (pH 7.4). Sections were then washed in PBS, and nuclei were stained with DAPI solution (2 μg/mL; Sigma, St. Louis, MI, USA). Histological images were obtained using a BZ-9000E fluorescence microscope (Keyence, Osaka, Japan).
2.10. Mechanical Testing
The tensile strengths of the five collagen membranes were measured using an Instron Universal Testing Instrument (model 5982; Illinois Tool Works, Inc., Glenview, IL, USA) and a 2530-series load cell (model 2530-50N; Illinois Tool Works, Inc.). Mechanical trials were conducted in compliance with the ASTM D5748 standard. This testing method was designed to provide the conditions for biaxial deformation with a constant crosshead speed (1 mm/min). The specimen holder has a diameter of 5 mm. The loading element tip radius was 1.25 mm. During mechanical testing, all the samples were kept in Ringer’s solution at 20 °C. During the test, we considered the test samples as conical surfaces of variable height. The obtained experimental dependences in the load-displacement coordinates were recalculated into stress–strain curves using the following formulas.
Sample deformation:
where S
0 is the initial area of the sample and S
tek is the current area of the sample, calculated by the following formula:
where R is the sample radius and Δl is the displacement of the loading element.
The stress was calculated by the following formula:
where P is the current load on the sample.
Based on the stress–strain curves (
Figure 2b), the fracture strain (at the maximum load) and the elastic modulus (in the section of the curve with the maximum slope) were calculated. All calculations were carried out using Instron Bluehill 2 Universal software (Illinois Tool Works, Inc.).
2.11. Statistical Analysis
The basic summary statistics are presented as the mean ± standard deviation. All statistical analyses were performed using Excel 16.4 (Microsoft, Inc., Redmond, DC, USA).
4. Discussion
Given that more than 90% of the human cornea is in the stroma, the solution of creating a fully functional and biocompatible stromal replacement is key to both artificial cornea technology and the ability to restore a healthy stroma in patients with corneal blindness [
3]. Therefore, active practical research is being conducted in this direction, among which corneal stroma tissue engineering with cell-free collagen hydrogels [
10,
11] is a promising approach with potential clinical application. The pioneers in this field are the Griffith et al. group, who were the first to conduct clinical trials of collagen hydrogels, which were prepared from recombinant type III collagen chemically cross-linked with EDC/N-hydroxysuccinimide to impart mechanical strength to the material [
12]. Although this work demonstrated the safety of this approach and even the restoration of innervation, in subsequent studies from the same group, the absence of host cell migration into such material was noted four years after surgery in humans [
13]; therefore, complete regeneration of the cornea has not been achieved thus far. There may be several reasons for this, but it is most likely that the chemical cross-linking of the collagen material significantly impedes the migration of cells into the implant and, as a result, complete regeneration of the corneal stroma is unattainable.
Many years of experience with collagen experimentation have shown that native highly purified collagen has extremely low antigenic properties and low inflammatory potential [
14], leading to the adoption of collagen biomaterials in clinical practice. Therefore, when developing new collagen biomaterials, it is necessary to be guided by the following principle: the fewer structural and property changes to collagen during the process, the more likely it is to obtain an ideal collagen biomaterial. This principle is especially important if the goal of this study is to achieve the clinical application of the biomaterial.
Our approach to the creation of an artificial cornea is to reject the use of any chemical cross-links, as they can impair the biocompatibility of the entire implant [
15] and hinder the process of host cell migration into the implant [
16], or, in some cases, completely block it [
13]. Therefore, to enhance the biomechanical characteristics of the implant, a solution of concentrated medical-grade Viscoll collagen I was used. In contrast to our previous work [
5], we used a higher concentration of collagen to manufacture the membrane. This made it possible to increase the mechanical properties of the membrane compared to our previous work [
5], but this was not enough to achieve the mechanical properties of the human cornea. It should be noted that this is not a critical disadvantage since the properties of the Viscoll collagen membrane are sufficient for its fixation with a classic surgical suture. At the same time, we had previously shown that its mechanical properties after implantation into the stroma of the cornea will inevitably increase due to the natural process of adaptation of the collagen membrane with the surrounding tissues [
5]. Moreover, cells can also enhance the mechanical strength of collagen hydrogels [
17]. Thus, the success of the regeneration of the damaged cornea will depend on how effectively the corneal cells will interact with the collagen membrane in vivo.
An important result obtained in this study was that active cell migration was observed in all implanted collagen membranes after 6 months. This is consistent with our previous in vitro results, which demonstrated that collagen hydrogels prepared from a concentrated collagen solution maintained a high survival rate of encapsulated cells, maintained their morphology and functional activity for at least 28 days, and did not interfere with cellular movement [
17]. Our cellular migration results were also consistent with earlier in vivo work by our group [
5]. These results are in stark contrast with those from the cross-linked recombinant human collagen membrane, where host cell migration into the hydrogel was not observed up to 4 years post operation [
13], as well as with the data obtained for cross-linked porcine collagen in a rabbit study, in which only sparse cell migration into the hydrogel was observed at 6 months post surgery [
10]. This may be evidence that the chemical cross-linking of collagen hydrogels impairs cell permeability in vivo.
In the study of corneal tissue regeneration, it is extremely important to investigate the issue of innervation of the restored area because nerve regeneration after corneal injury plays a decisive role in restoring normal function.
A study of the micromorphology of the cornea using laser confocal microscopy revealed that the removal of a portion of the stroma during anterior lamellar keratoplasty leads to the initial loss of nerves; however, in all experimental animals, nerve regeneration was confirmed at 6 months after the operation. Similar results were obtained during the implantation of chemically cross-linked collagen membranes into the corneal stroma of animals [
10,
11,
18] and humans [
13]. It is worth noting here that the process of reinnervation of implanted donor corneas in humans is very slow, lasting from one to several years [
6,
19]. The results obtained in this study may indicate that the Viscoll collagen membrane supports, or at least does not interfere with, the process of nerve repair in the cornea after layered keratoplasty.
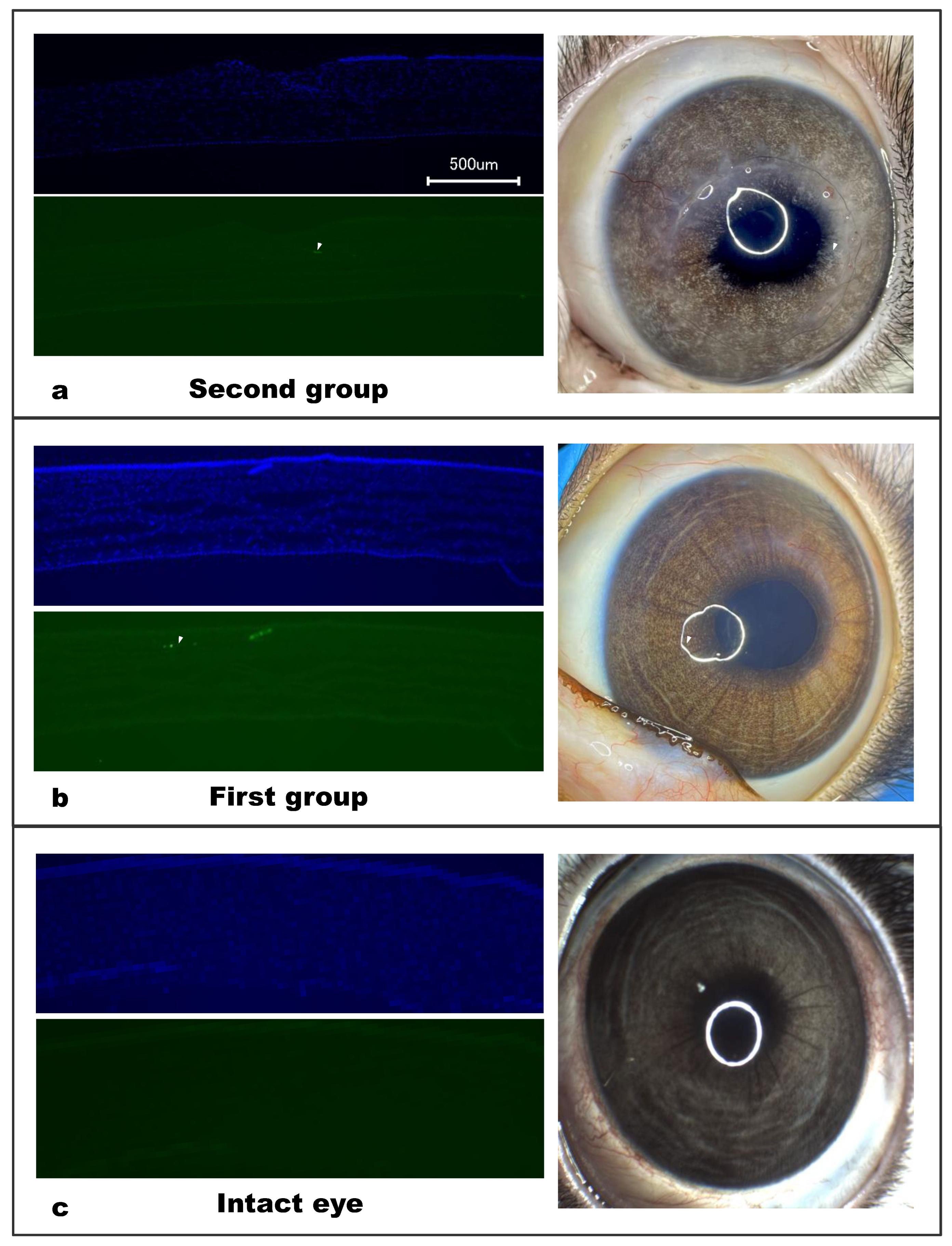
According to our OCT data, after implantation, there was no migration or displacement of the collagen membrane implant; it took its position and fixed itself within the surrounding tissues. This behavior was also confirmed by histological examination, which showed that the collagen fibers of the stroma were closely intertwined with those of the implant, indicating a good survival rate.
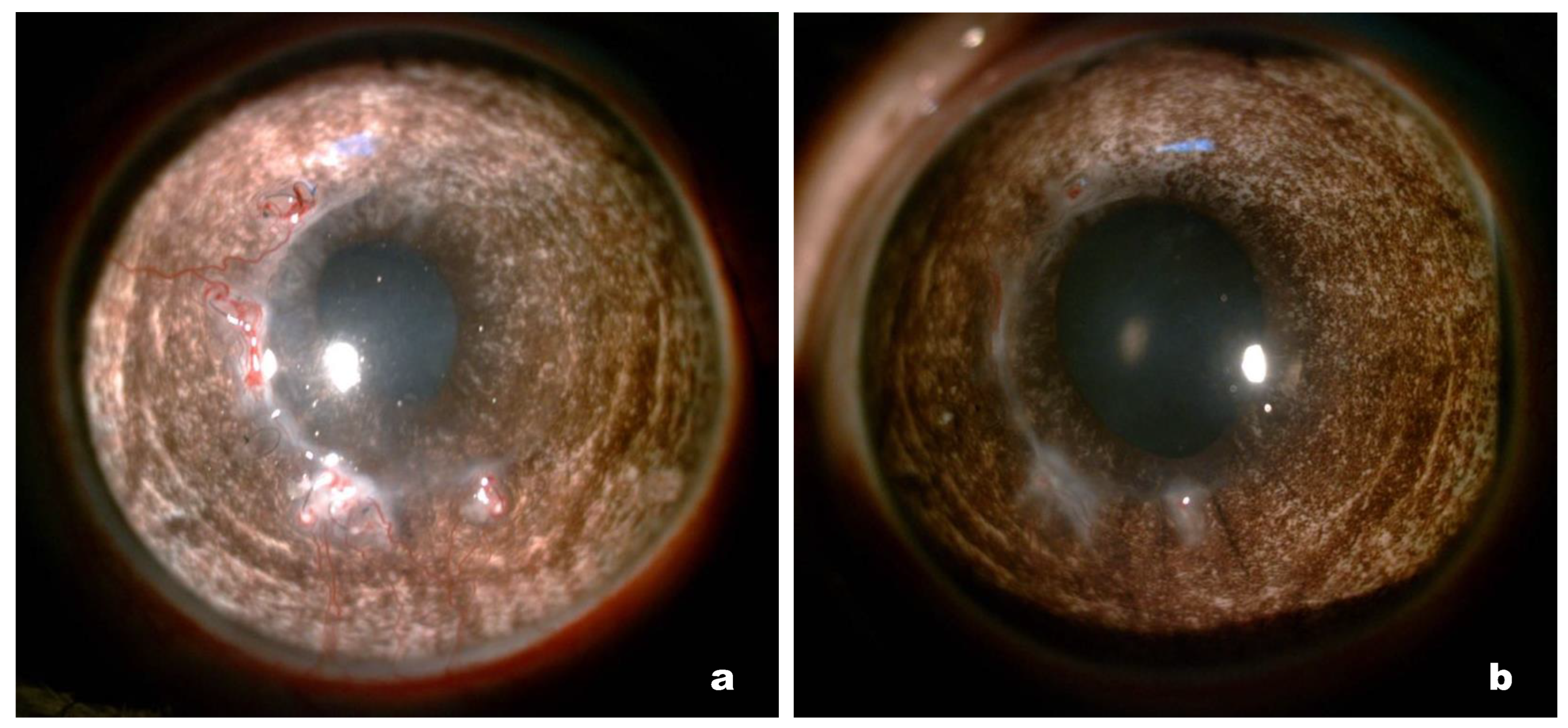
Corneal neovascularization in the peripheral implanted zone was noted in four rabbits in the first group and two rabbits in the second group. Neovascularization is associated with sagging of the suture material due to the gradual integration of collagen with the corneal stroma; this involves natural cross-linking and strengthening of the biomechanics, which significantly weakens the sutures and causes sagging [
20]. In such cases, the timely removal of the sutures completely resolves the problem and, according to the results of our study, this should be done 4–5 months after the operation.
Immunohistochemical staining of cryosectioned cornea samples revealed the presence of single α-SMA-positive stromal cells in both groups, whereas none were found in the intact cornea samples. Notably, the presence of these cells did not affect the transparency of the corneas. The presence of α-SMA-positive cells in the corneal stroma was likely induced by the operation itself, during which a part of the stroma was removed. The results provide evidence that the Viscoll collagen membrane does not have an irritating effect on the corneal tissue, which is also consistent with the results of the toxicological tests performed.
Our work has several limitations. (1) The experiments were carried out on a healthy cornea, in which a section of healthy stroma was surgically removed and a collagen membrane was implanted in its place. There is no doubt that the processes of tissue regeneration in various pathologies of the cornea, especially in case of chemical burns, will proceed differently. However, our results strongly suggest that the Viscoll collagen membrane may also be effective in the treatment of pathological conditions of the cornea. It should be noted that in this case, it may be necessary to develop new approaches for the treatment of various corneal pathologies using the Viscoll collagen membrane. (2) Short-term observation: Ideally, for a clear understanding of the reparative processes occurring in the damaged cornea during implantation of the Viscoll collagen membrane, a four-year follow-up is required. At the same time, it is extremely difficult to monitor rabbits that have undergone a full course of anesthesia for more than 6 months. At the same time, the processes of regeneration of the cornea in rabbits proceed faster than in humans. Therefore, to a certain extent, the results could be extrapolated to the regeneration of the human cornea.
Considering the proven safety of the Viscoll collagen membrane and its possible potential for the treatment of various corneal pathologies, especially under the conditions of a global shortage of donor tissue, our results can serve as the basis for future clinical trials.