Novel Functional Glass–Ceramic Coatings on Titanium Substrates from Glass Powders and Reactive Silicone Binders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glass Synthesis and Preliminary Studies on Glass–Ceramic Pellets
2.2. Airbrush Coating
2.3. Surface Finish Assessment
2.4. Mechanical Characterization: Scratch Test
2.5. Infrared Heating Test
3. Results
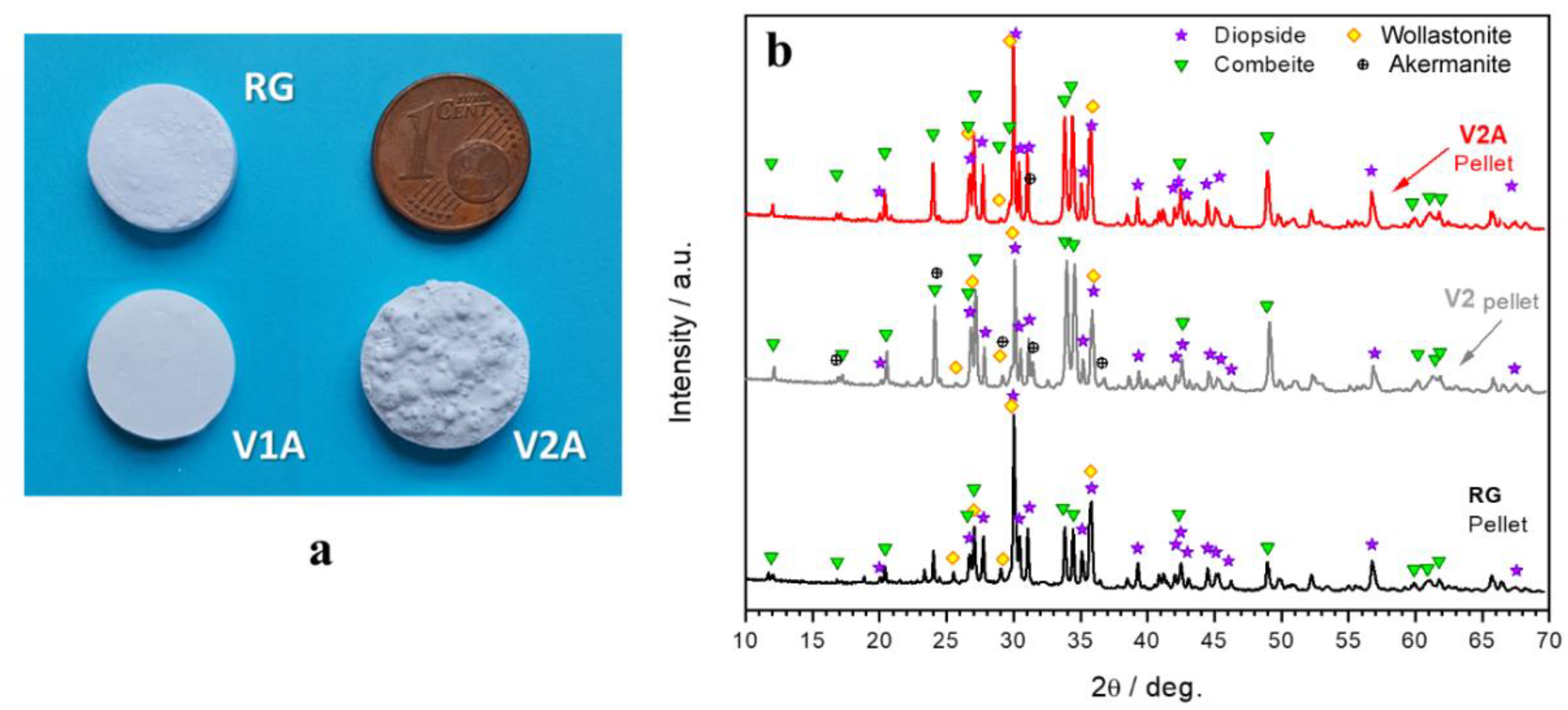
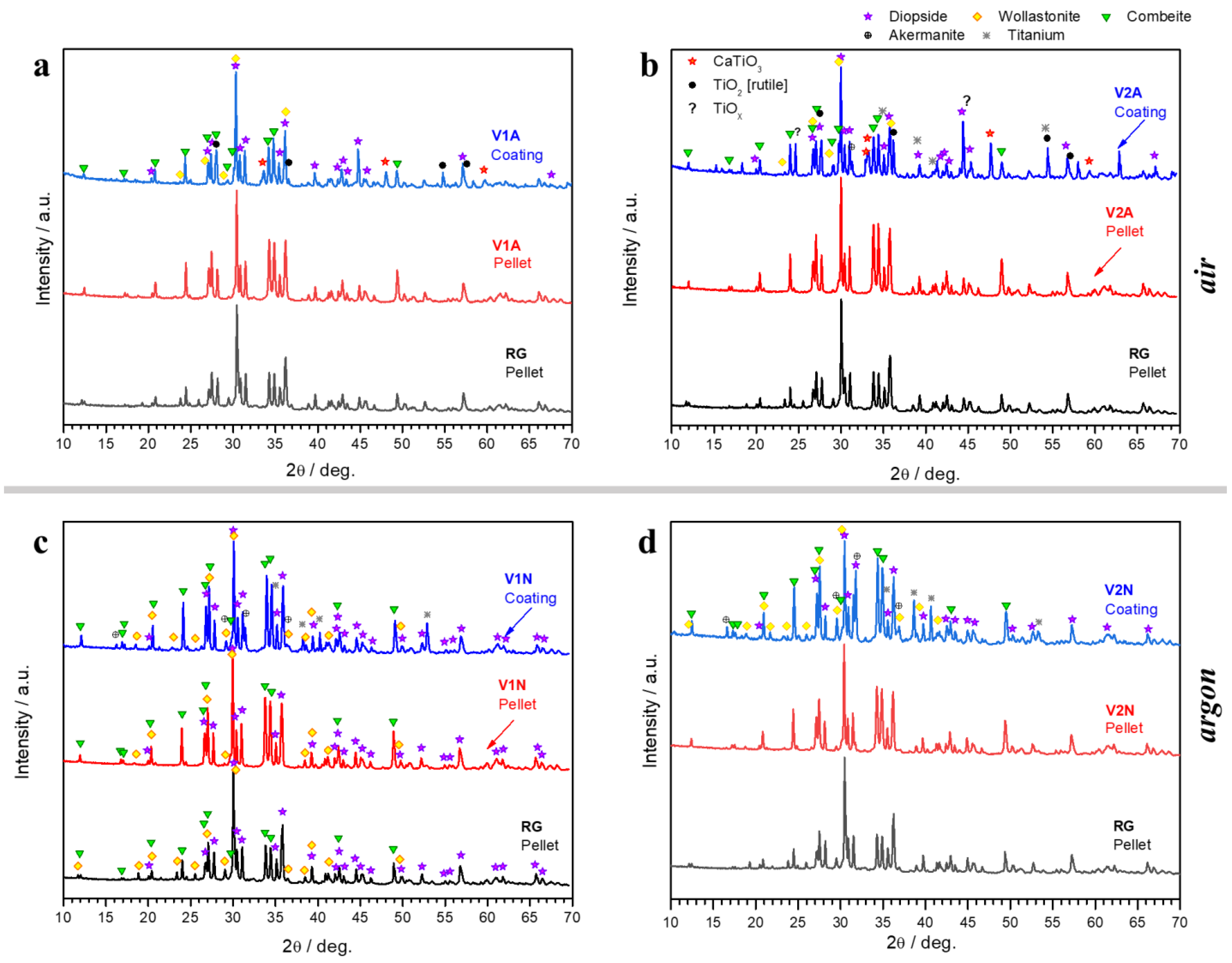
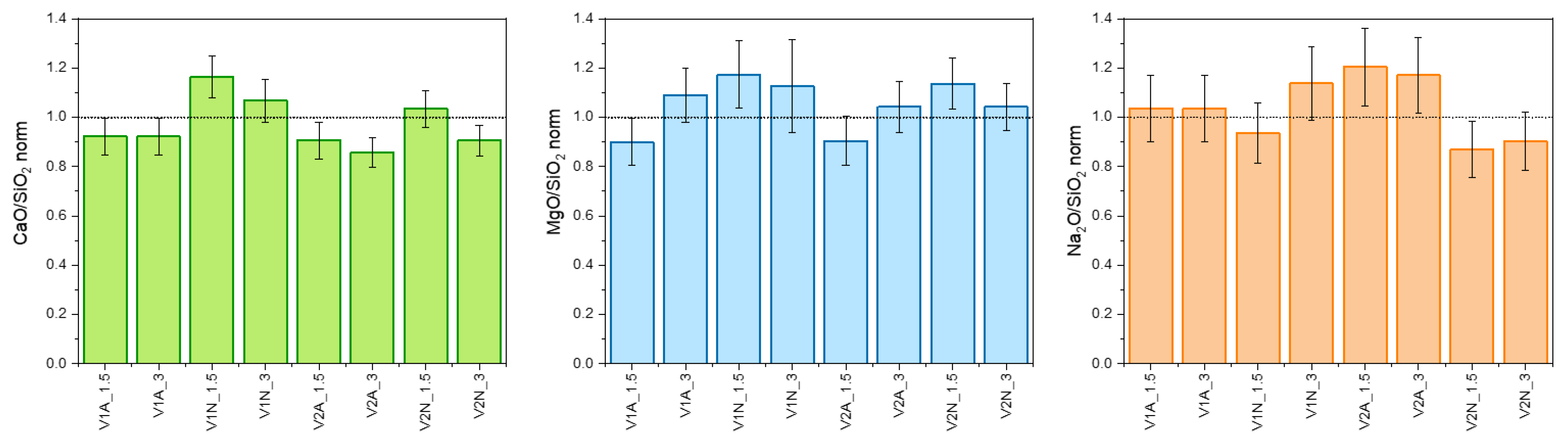
3.1. Phase Development in Glass–Ceramic Pellets and Coatings
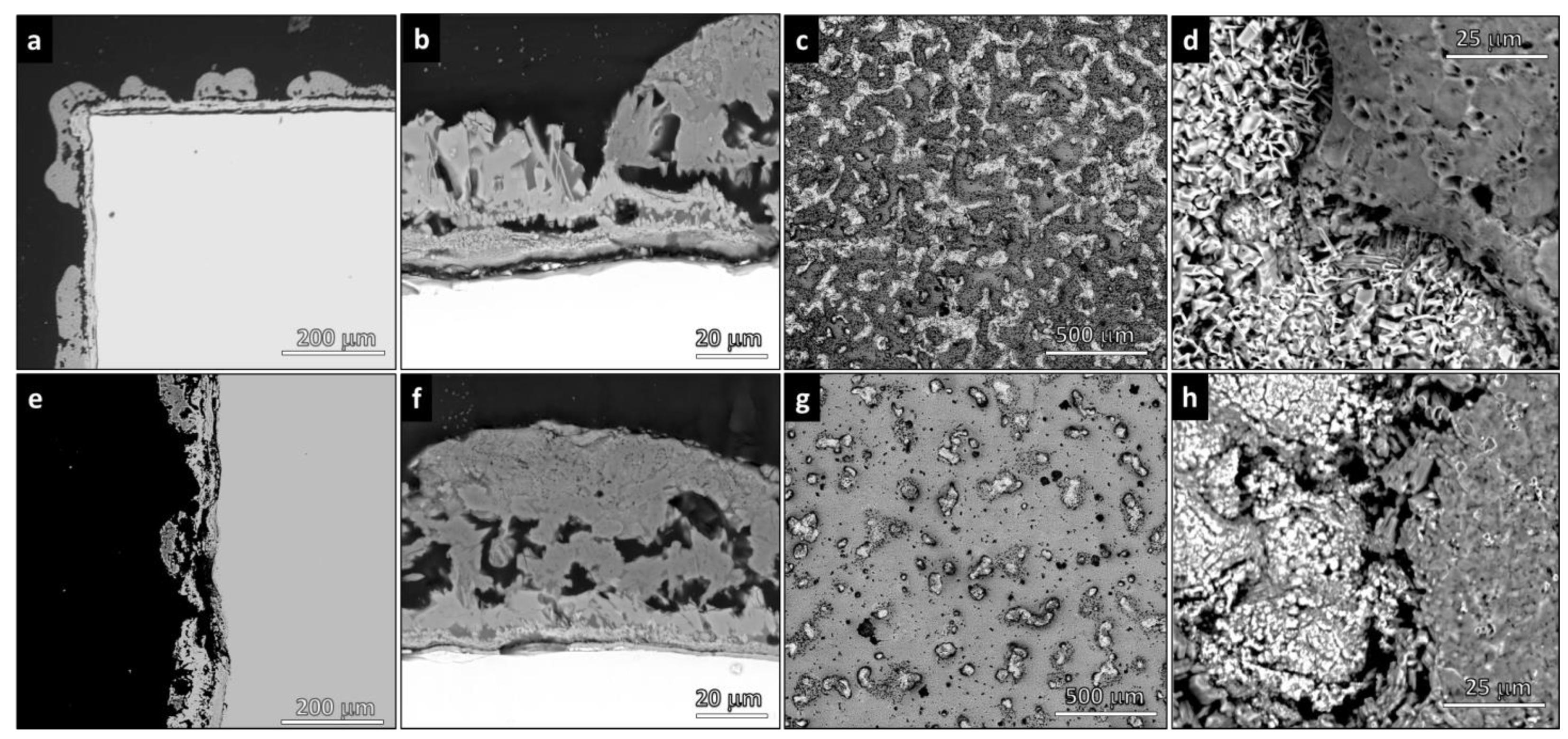
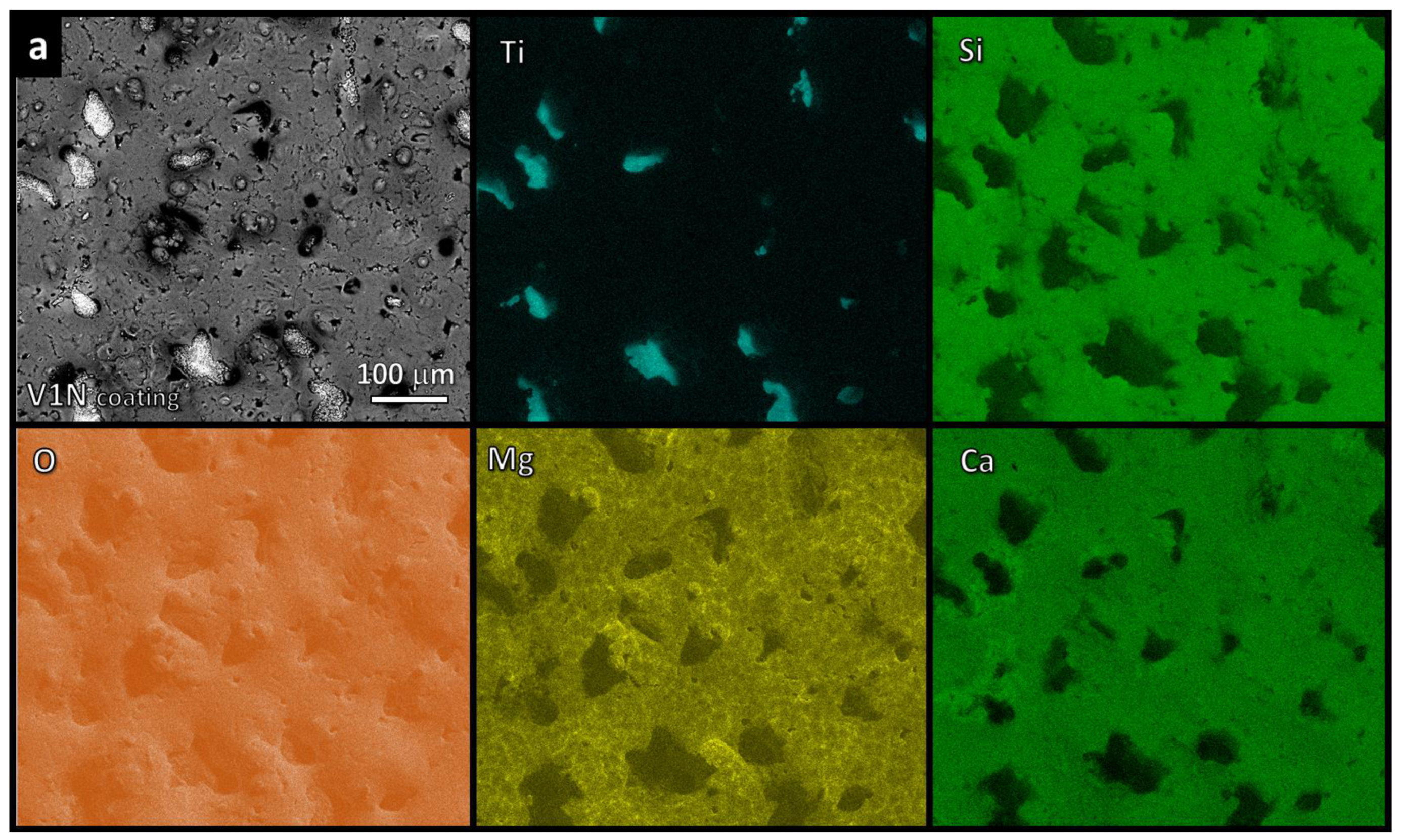
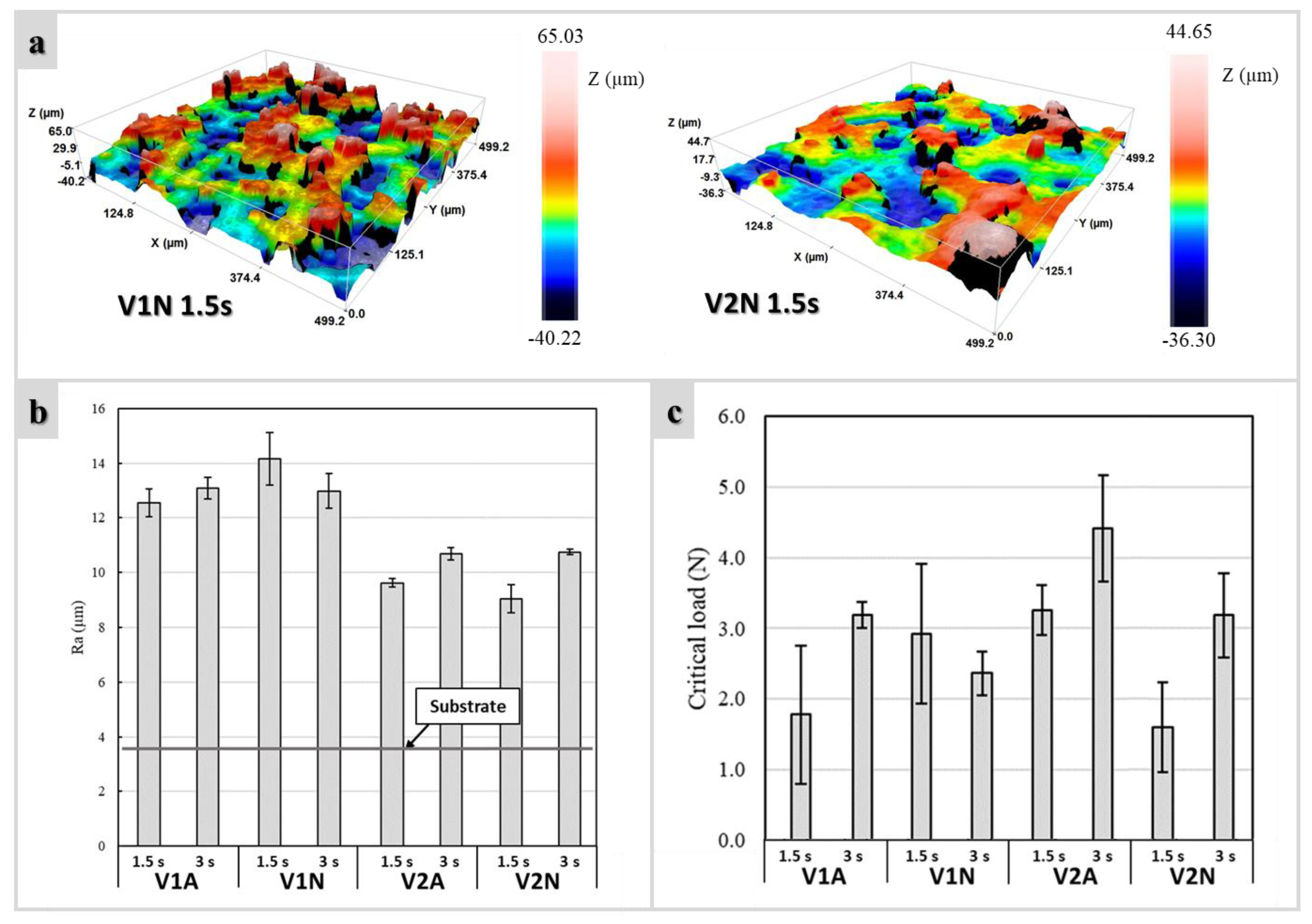
3.2. Morphology of Coatings
3.3. Scratch Tests
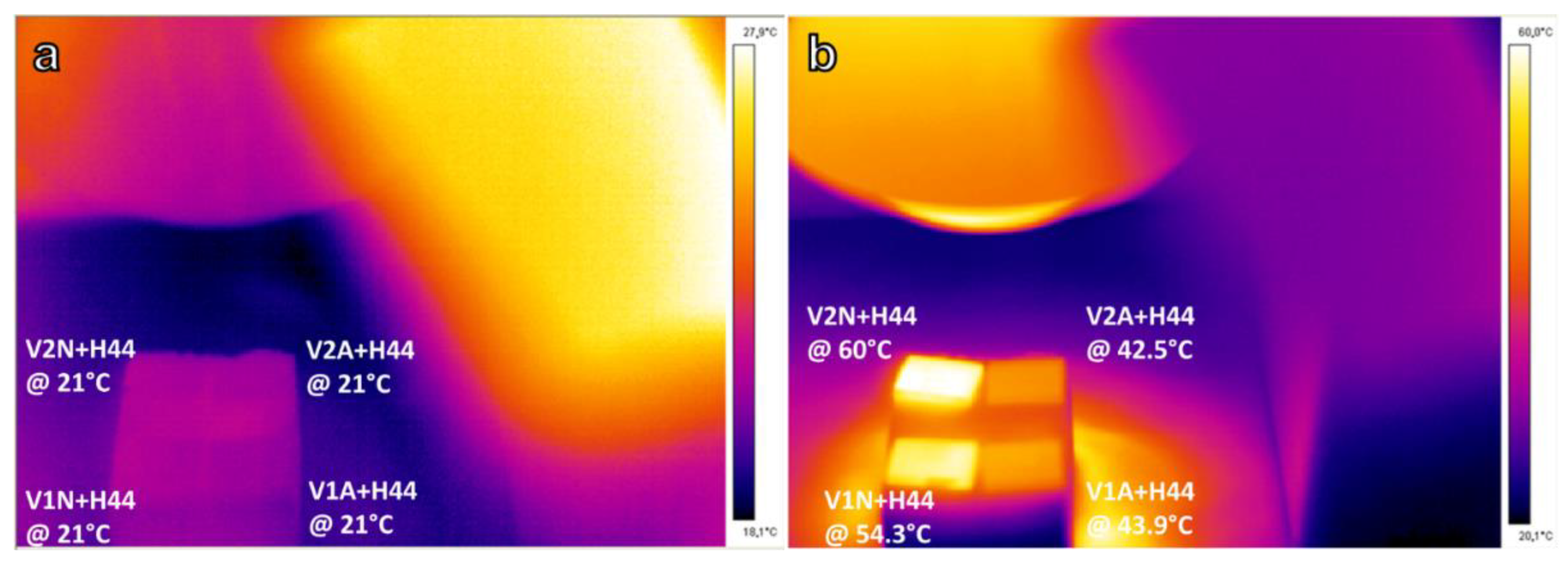
3.4. Infrared Heating
4. Discussion
4.1. Phase Evolution
4.2. Roughness, Scratch Resistance, and Functionalization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived-ceramics: 40 years of re-search and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 805–837. [Google Scholar]
- Bernardo, E.; Fiocco, L.; Parcianello, G.; Storti, E.; Colombo, P. Advanced ceramics from preceramic polymers modified at the nano-scale: A review. Materials 2014, 7, 1927–1956. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, E.; Carlotti, J.-F.; Dias, P.M.; Hess, U.; Rezwan, K. Novel akermanite-based bioceramics from preceramic polymers and oxide fillers. Ceram. Int. 2014, 40, 1029–1035. [Google Scholar] [CrossRef]
- Fiocco, L.; Elsayed, H.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Bioactive wollastonite-diopside foams from preceramic polymers and reactive oxide fillers. Materials 2015, 8, 2480–2494. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Hu, F.; Yang, J.; Cheng, L.; Wang, Y. Polymer-derived yttrium silicate coatings on 2D C/SiC composites. J. Eur. Ceram. Soc. 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Biasetto, L.; Elsayed, H.; Bonollo, F.; Colombo, P. Polymer-derived sphene biocoating on cp-Ti substrates for orthopedic and dental implants. Surf. Coat. Technol. 2016, 301, 140–147. [Google Scholar] [CrossRef]
- Greil, P. Near net shape manufacturing of polymer derived ceramics. J. Eur. Ceram. Soc. 1998, 18, 1905–1914. [Google Scholar] [CrossRef]
- Petríková, I.; Parchovianský, M.; Švančárek, P.; Lenz, L.M.; Motz, G.; Galusek, D. Passive filler loaded polysilazane-derived glass/ceramic coating system applied to AISI 441 stainless steel, part 1: Processing and characterization. Int. J. Appl. Ceram. Technol. 2020, 17, 998–1009. [Google Scholar] [CrossRef]
- Parchovianský, M.; Petríková, I.; Švančárek, P.; Lenz, L.M.; Motz, G.; Galusek, D. Passive filler loaded polysilazane-derived glass/ceramic coating system applied to AISI 441 stainless steel, part 2: Oxidation behavior in synthetic air. Int. J. Appl. Ceram. Technol. 2020, 17, 1675–1687. [Google Scholar] [CrossRef]
- Ohl, C.; Kappa, M.; Wilker, V.; Scheffler, F.; Scheffler, M. Novel Open-Cellular Glass Foams for Optical Applications. J. Am. Ceram. Soc. 2011, 94, 436–441. [Google Scholar] [CrossRef]
- Elsayed, H.; Javed, H.; Sabato, A.G.; Smeacetto, F.; Bernardo, E. Novel glass-ceramic SOFC sealants from glass powders and a reactive silicone binder. J. Eur. Ceram. Soc. 2018, 38, 4245–4251. [Google Scholar] [CrossRef]
- Elsayed, H.; Picicco, M.; Dasan, A.; Kraxner, J.; Galusek, D.; Bernardo, E. Glass powders and reactive silicone binder: Interactions and application to additive manufacturing of bioactive glass-ceramic scaffolds. Ceram. Int. 2019, 45, 13740–13746. [Google Scholar] [CrossRef]
- Elsayed, H.; Picicco, M.; Dasan, A.; Kraxner, J.; Galusek, D.; Bernardo, E. Glass powders and reactive silicone binder: Application to digital light processing of bioactive glass-ceramic scaffolds. Ceram. Int. 2020, 46, 25299–25305. [Google Scholar] [CrossRef]
- Romero, A.R.; Elsayed, H.; Kraxner, J.; Bernardo, E. Glass Reactive Sintering. Encycl. Mater. Tech. Ceram. Glasses 2021, 2, 728–745. [Google Scholar]
- Biasetto, L.; Elsayed, H. Sphene silicate ceramic coatings on cpTi substrates: Process upgrade. J. Surf. Technol. 2017, 321, 416. [Google Scholar] [CrossRef]
- Maminskas, J.; Pilipavicius, J.; Staisiunas, E.; Baranovas, G.; Alksne, M.; Daugela, P.; Juodzbalys, G. Novel Yttria-Stabilized Zirconium Oxide and Lithium Disilicate Coatings on Titanium Alloy Substrate for Implant Abutments and Biomedical Application. Materials 2020, 13, 2070. [Google Scholar] [CrossRef]
- Emissivity Coefficients Common Products. Available online: https://www.engineeringtoolbox.com/emissivity-coefficients-d_447.html (accessed on 8 July 2022).
- Scheffler, M.; Takahashi, T.; Kaschta, J.; Muensted, H.; Buhler, P.; Greil, P. Pyrolytic decom-position of preceramic organo polysiloxanes. In Innovative Processing and Synthesis of Ceramics, Glasses, and Composites IV; Ceramic Transactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; Volume 115, pp. 239–250. [Google Scholar]
- Saha, A.; Raj, R.; Williamson, D.L. A Model for the Nanodomains in Polymer-Derived SiCO. J. Am. Ceram. Soc. 2006, 89, 2188–2195. [Google Scholar] [CrossRef]
- Dasan, A.; Elsayed, H.; Kraxner, J.; Galusek, D.; Colombo, P.; Bernardo, E. Engineering of silicone-based mixtures for the digital light processing of Åkermanite scaffolds. J. Eur. Ceram. Soc. 2020, 40, 2566–2572. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816. [Google Scholar] [CrossRef]
- Brunello, G.; Elsayed, H.; Biasetto, L. Bioactive glass and silicate-based ceramic coatings on metallic implants: Open challenge or outdated topic? Materials 2019, 12, 2929. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Soeparto, A.; Zreiqat, H. Incorporation of titanium into calcium silicate improved their chemical stability and biological properties. J. Biomed. Mater. Res. Part A. 2008, 86, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhu, S.; Guo, F.; Zhu, J.; Li, M.; Ma, J.; Zhu, Q. The effect of adhesive strength of hydroxyapatite coating on the stability of hydroxyapatite-coated prostheses in vivo at the early stage of implantation. Arch. Med. Sci. 2012, 98, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.C.; Yen, S.K. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mat. Sci. Eng. C 2002, 20, 153–160. [Google Scholar] [CrossRef]
- Li, H.; Jin, H.; Zhang, Q.; Yang, J.; Jin, Z. SiC/C machinable ceramics surface hardening by silicon infiltration. Scr. Mater. 2010, 63, 1177–1180. [Google Scholar] [CrossRef]
- Bellucci, D.; Bianchi, M.; Graziani, G.; Gambardella, A.; Berni, M.; Russo, A.; Cannillo, V. Pulsed Electron Deposition of nanostructured bioactive glass coatings for biomedical ap-plications. Ceram. Int. 2017, 43, 15862–15867. [Google Scholar] [CrossRef]
- Altomare, L.; Bellucci, D.; Bolelli, G.; Bonferroni, B.; Cannillo, V.; De Nardo, L.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Sola, A.; et al. Microstructure and in vitro behaviour of 45S5 bioglass coatings deposited by high velocity suspension flame spraying (HVSFS). J. Mater. Sci. Mater. Med. 2011, 22, 1303–1319. [Google Scholar] [CrossRef] [PubMed]
- Biasetto, L.; Bertolini, R.; Elsayed, H.; Ghiotti, A.; Bruschi, S. Use of cryogenic machining to improve the adhesion of sphene bioceramic coatings on titanium substrates for dental and orthopaedic applications. Ceram. Int. 2019, 45, 5941–5951. [Google Scholar] [CrossRef]
- Fu, S.; Hu, H.; Chen, J.; Zhu, Y.; Zhao, S. Silicone resin derived larnite/C scaffolds via 3D printing for potential tumour therapy and bone regeneration. Chem. Eng. J. 2020, 382, 122928. [Google Scholar] [CrossRef]
- Zhu, T.; Zhu, M.; Zhu, Y. Fabrication of forsterite scaffolds with photothermal induced an-tibacterial activity by 3D printing and polymer-derived ceramics strategy. Ceram. Int. 2020, 46, 13607–13614. [Google Scholar] [CrossRef]
- Dogrul, F.; Bortolin, S.; Del Col, D.; Dengo, N.; Pedron, D.; Michalek, M.; Elsayed, H.; Galusek, D.; Bernardo, E. Polymer-derived Biosilicate-C composite foams: Phase development and photothermal effect. J. Eur. Ceram. Soc. 2021, 41, 380–388. [Google Scholar] [CrossRef]
- Xu, J.; Yao, K.; Xu, Z. Nanomaterials with a photothermal effect for antibacterial activities: An overview. Nanoscale 2019, 11, 8680–8691. [Google Scholar] [CrossRef] [PubMed]





| RG | Silica-Defective Glasses | ||
|---|---|---|---|
| V1 (Low Silica Reduction) | V2 (High Silica Reduction) | ||
| Chemical composition (wt%) | |||
| SiO2 | 58.8 | 55.5 | 51.6 |
| MgO | 8.6 | 9.3 | 10.1 |
| CaO | 21.8 | 23.6 | 25.7 |
| Na2O | 9.9 | 10.6 | 11.5 |
| Li2O | 0.9 | 1.0 | 1.1 |
| Particle size distribution | |||
| d10 (µm) | 3.7 | 3.8 | 4.3 |
| d50 (µm) | 11.3 | 12.0 | 11.8 |
| d90 (µm) | 29.2 | 32.7 | 30.9 |
| Silicone–glass ratios (wt%) | |||
| Firing in air | 13.5–86.5 (V1A) | 25–75 (V2A) | |
| Firing in argon | 17.5–82.5 (V1N) | 31–69 (V2N) | |
| Sample | Lc [N] | Deposition Method | Reference |
|---|---|---|---|
| V1A-V2A | <5 | Airbrush | This work |
| V1N-V2N | <4.5 | Airbrush | This work |
| 45S5 and CaK | 0.8–4.5 | Pulsed electron deposition | [27] |
| 45S5 | 18–21 | High velocity suspension flame spraying | [28] |
| Sphene | <1 | Sol-gel | [23] |
| 5.6–8.6 | Airbrush | [15,29] | |
| HAp | 11 | Plasma spray | [24] |
| <2.5 | Electrochemical process | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, H.; Bertolini, R.; Biasetto, L.; Ożóg, P.; Kraxner, J.; Galusek, D.; Bernardo, E. Novel Functional Glass–Ceramic Coatings on Titanium Substrates from Glass Powders and Reactive Silicone Binders. Polymers 2022, 14, 4016. https://doi.org/10.3390/polym14194016
Elsayed H, Bertolini R, Biasetto L, Ożóg P, Kraxner J, Galusek D, Bernardo E. Novel Functional Glass–Ceramic Coatings on Titanium Substrates from Glass Powders and Reactive Silicone Binders. Polymers. 2022; 14(19):4016. https://doi.org/10.3390/polym14194016
Chicago/Turabian StyleElsayed, Hamada, Rachele Bertolini, Lisa Biasetto, Paulina Ożóg, Jozef Kraxner, Dušan Galusek, and Enrico Bernardo. 2022. "Novel Functional Glass–Ceramic Coatings on Titanium Substrates from Glass Powders and Reactive Silicone Binders" Polymers 14, no. 19: 4016. https://doi.org/10.3390/polym14194016
APA StyleElsayed, H., Bertolini, R., Biasetto, L., Ożóg, P., Kraxner, J., Galusek, D., & Bernardo, E. (2022). Novel Functional Glass–Ceramic Coatings on Titanium Substrates from Glass Powders and Reactive Silicone Binders. Polymers, 14(19), 4016. https://doi.org/10.3390/polym14194016











