Effect of Diatomite on the Thermal Degradation Behavior of Polypropylene and Formation of Graphene Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Diatomite/Polypropylene Blends
2.2.2. Graphene Synthesis by One–Pot Pyrolysis
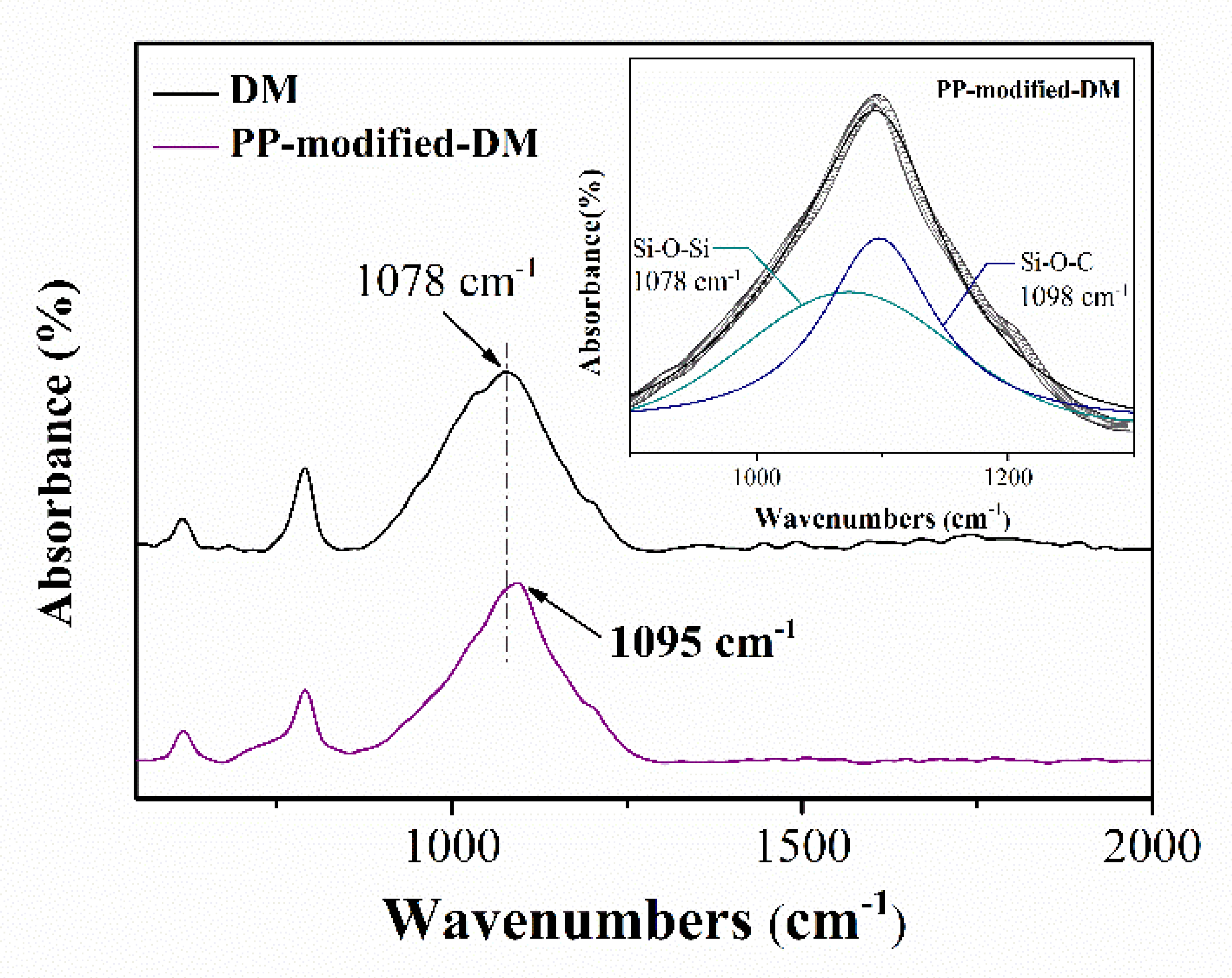
2.2.3. Characterization
Morphology and Microstructure of Graphene
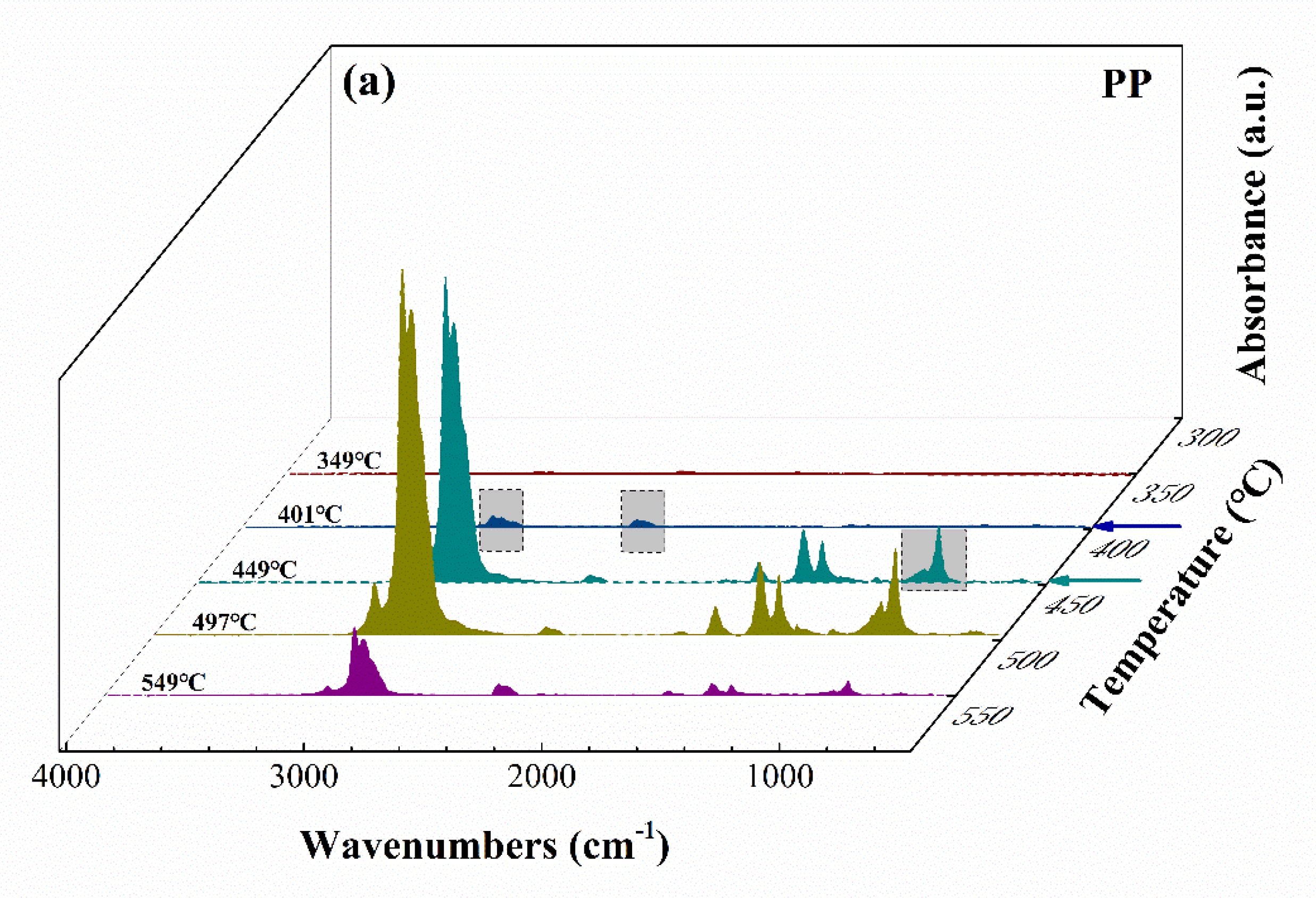
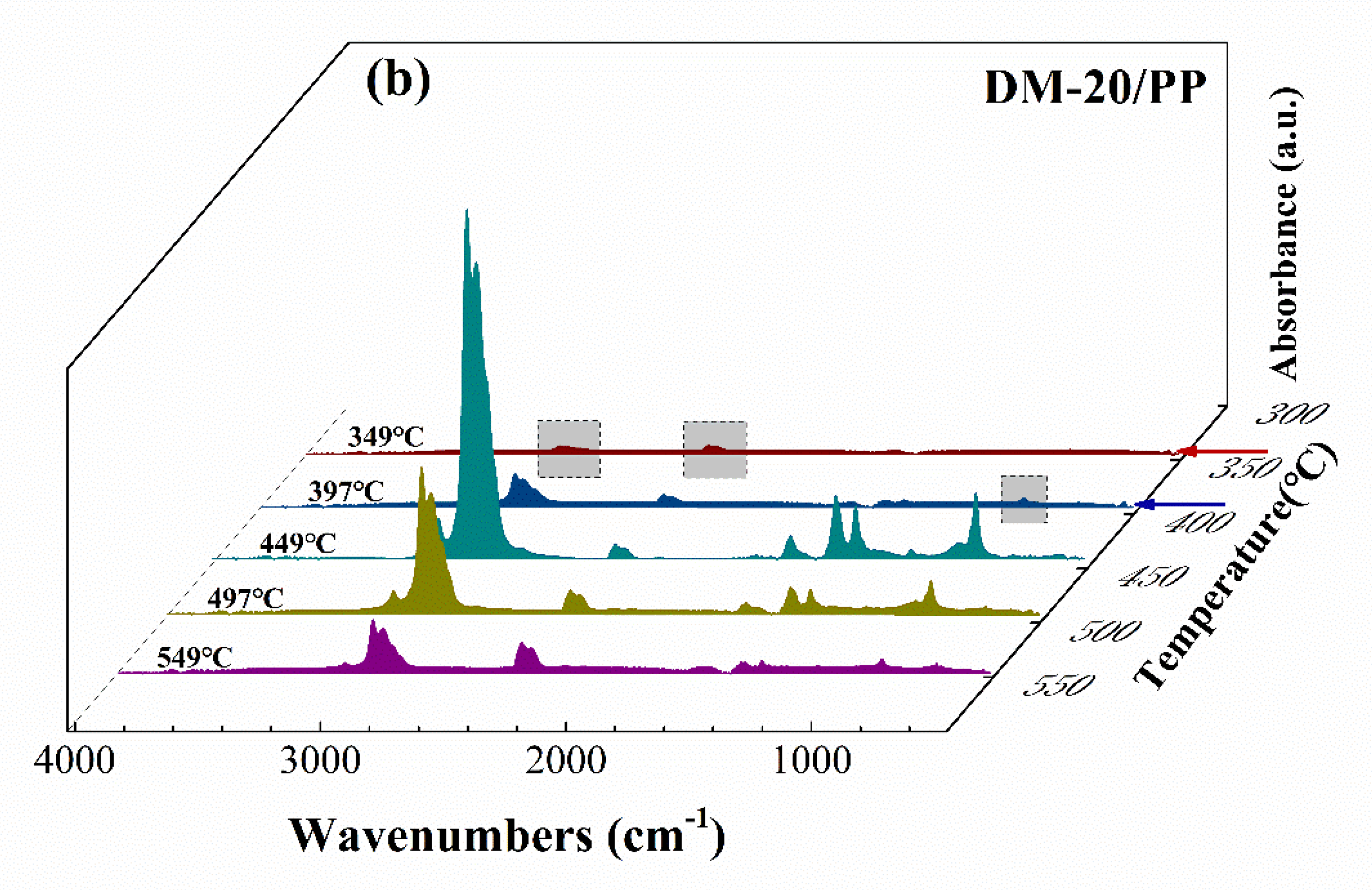
TG–FTIR Analysis
GC–MS Analysis
3. Results and Discussion
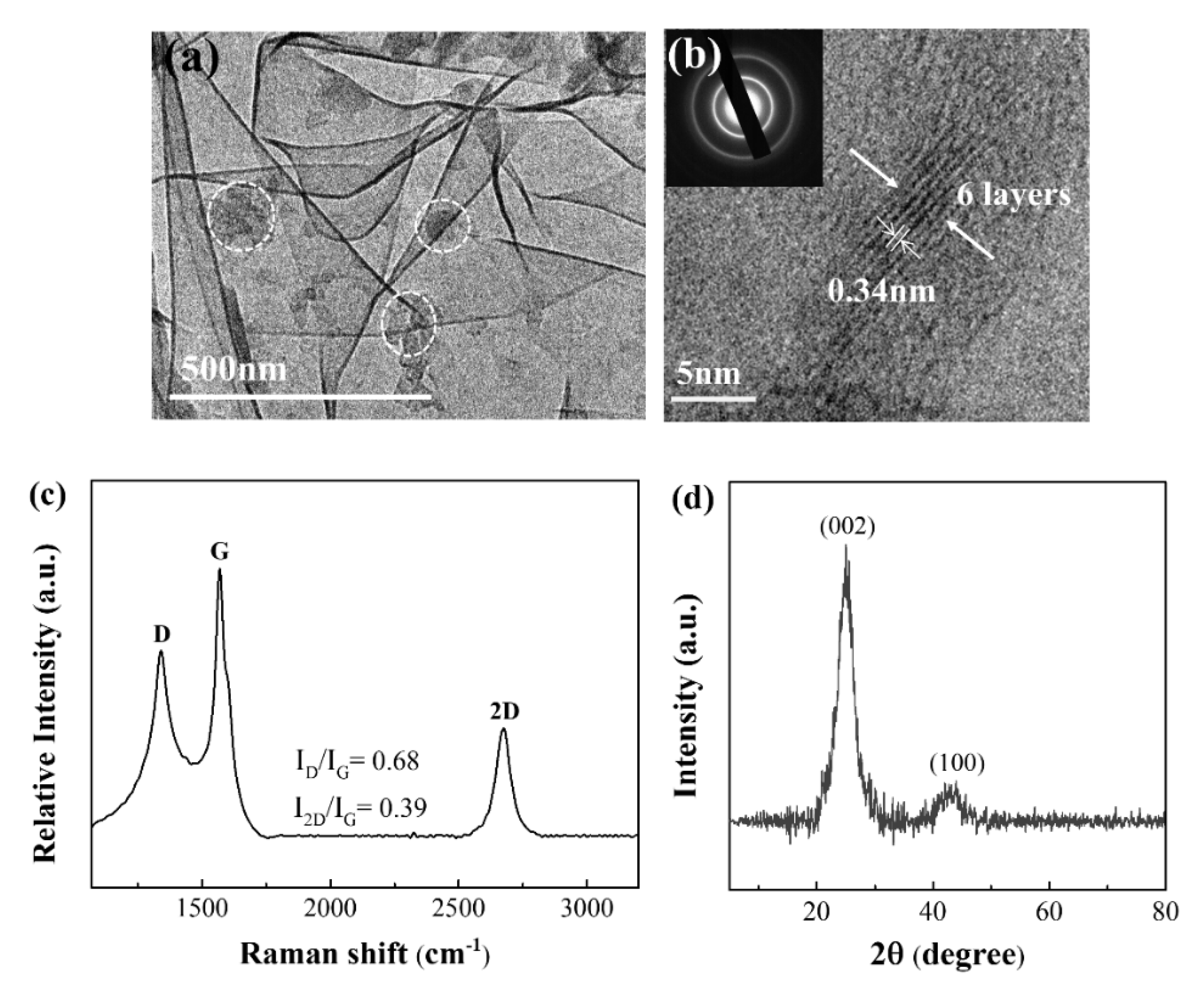
3.1. Characterizations of Morphology and Structure of Graphene
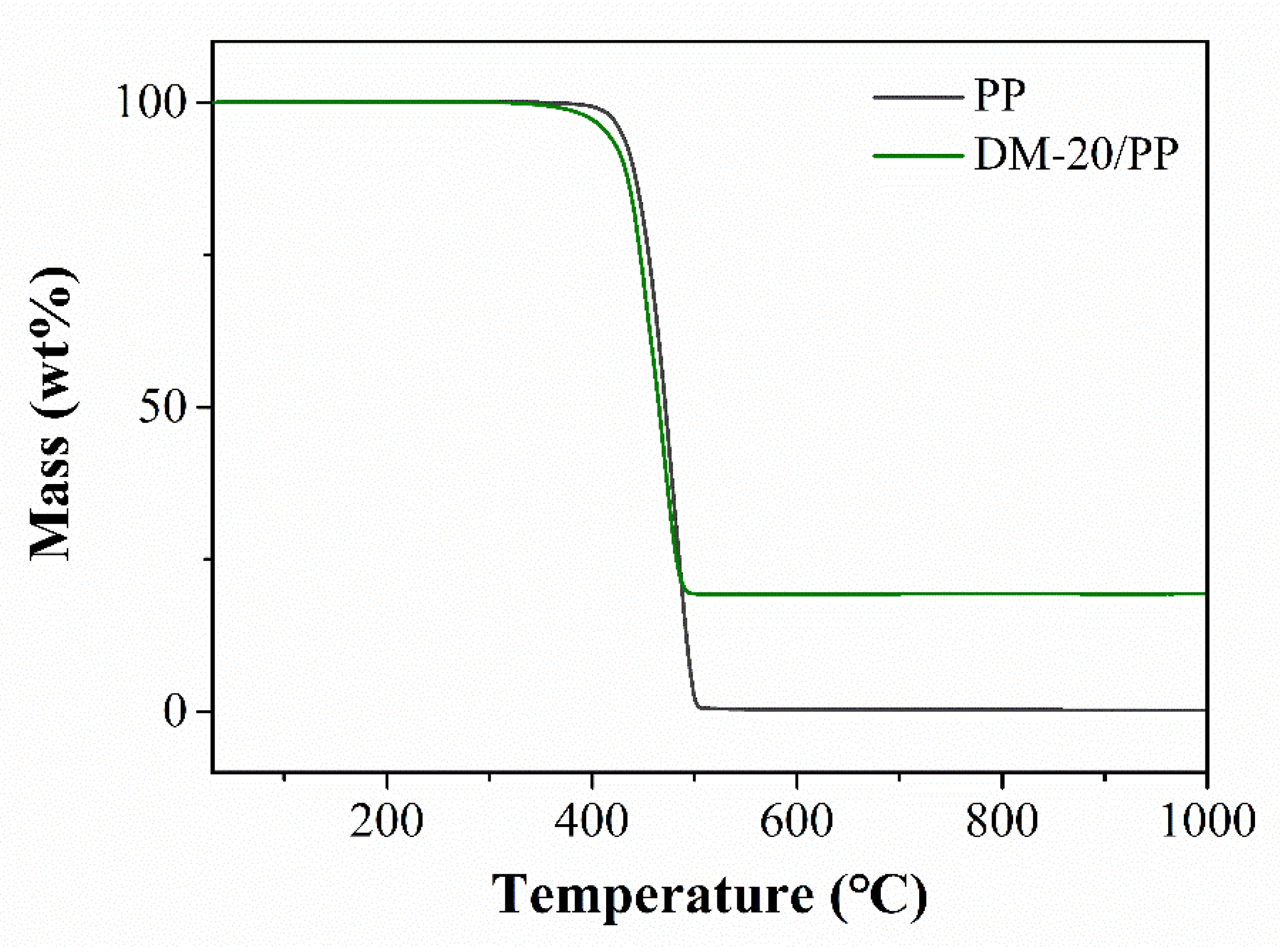
3.2. Effect of DM on the Thermal Degradation Behavior of PP
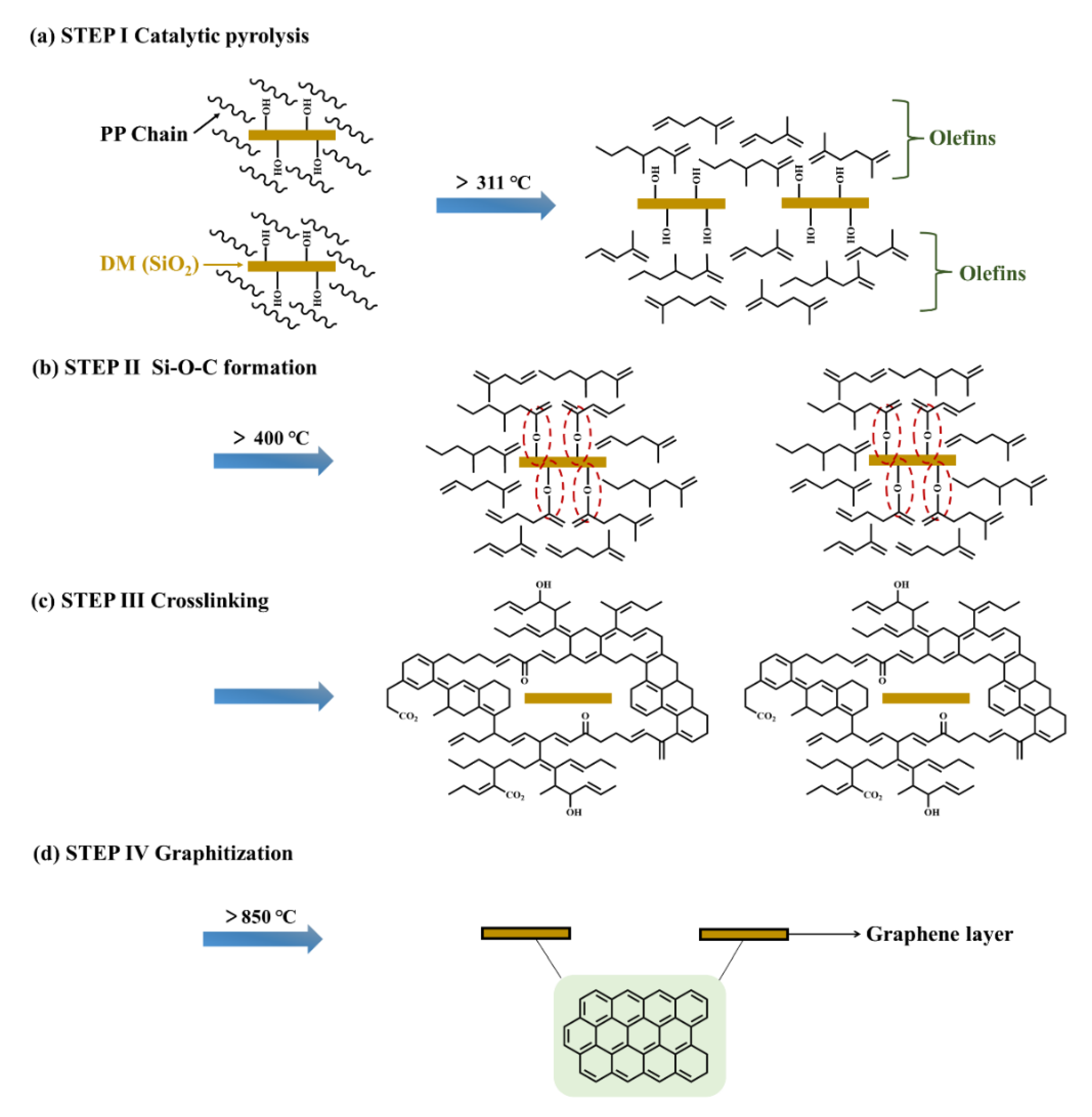
3.3. Discussion about the Possible Mechanism of Graphene Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Ruan, T.T.; Chen, Y.; Jin, F.; Peng, L.; Zhou, Y.; Wang, D.L.; Dou, S.X. Graphene–based composites for electrochemical energy storage. Energy Storage Mater. 2020, 24, 22–51. [Google Scholar] [CrossRef]
- Wang, J.; Song, F.; Ding, Y.; Shao, M. The incorporation of graphene to enhance mechanical properties of polypropylene self–reinforced polymer composites. Mater. Des. 2020, 195, 109073. [Google Scholar] [CrossRef]
- Song, N.; Cao, D.; Luo, X.; Wang, Q.; Ding, P.; Shi, L. Highly thermally conductive polypropylene/graphene composites for thermal management. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105912. [Google Scholar] [CrossRef]
- Islam, A.; Mukherjee, B.; Pandey, K.K.; Keshri, A.K. Ultra–Fast, Chemical–Free, Mass Production of High Quality Exfoliated Graphene. ACS Nano 2021, 15, 1775–1784. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large–Area Synthesis of High–Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Kordatos, A.; Kelaidis, N.; Giamini, S.A.; Marquez–Velasco, J.; Xenogiannopoulou, E.; Tsipas, P.; Kordas, G.; Dimoulas, A. AB stacked few layer graphene growth by chemical vapor deposition on single crystal Rh(111) and electronic structure characterization. Appl. Surf. Sci. 2016, 369, 251–256. [Google Scholar] [CrossRef]
- Sagar, R.R.; Zhang, X.; Xiong, C. Growth of graphene on copper and nickel foils via chemical vapour deposition using ethylene. Mater. Res. Innov. 2014, 18, 706–710. [Google Scholar] [CrossRef]
- Addou, R.; Dahal, A.; Sutter, P.; Batzill, M. Monolayer graphene growth on Ni(111) by low temperature chemical vapor deposition. Appl. Phys. Lett. 2012, 100, 21601. [Google Scholar] [CrossRef]
- Nandamuri, G.; Roumimov, S.; Solanki, R. Chemical vapor deposition of graphene films. Nanotechnology 2010, 21, 145604. [Google Scholar] [CrossRef]
- Chen, C.-S.; Hsieh, C.-K. Effects of acetylene flow rate and processing temperature on graphene films grown by thermal chemical vapor deposition. Thin Solid Film. 2015, 584, 265–269. [Google Scholar] [CrossRef]
- Lahiri, J.; S Miller, T.; J Ross, A.; Adamska, L.; Oleynik, I.I.; Batzill, M. Graphene growth and stability at nickel surfaces. New J. Phys. 2011, 13, 25001. [Google Scholar] [CrossRef]
- Akhtar, F.; Dabrowski, J.; Lisker, M.; Zaumseil, P.; Schulze, S.; Jouvray, A.; Caban, P.; Mai, A.; Wenger, C.; Lukosius, M. Large-scale chemical vapor deposition of graphene on polycrystalline nickel films: Effect of annealing conditions. Thin Solid Film. 2019, 690, 137565. [Google Scholar] [CrossRef]
- McCarty, K.F.; Feibelman, P.J.; Loginova, E.; Bartelt, N.C. Kinetics and thermodynamics of carbon segregation and graphene growth on Ru(0001). Carbon 2009, 47, 1806–1813. [Google Scholar] [CrossRef]
- Zhang, Y.; Gomez, L.; Ishikawa, F.N.; Madaria, A.; Ryu, K.; Wang, C.A.; Badmaev, A.; Zhou, C.W. Comparison of Graphene Growth on Single-Crystalline and Polycrystalline Ni by Chemical Vapor Deposition. J. Phys. Chem. Lett. 2010, 1, 3101–3107. [Google Scholar] [CrossRef]
- Chen, Z.L.; Qi, Y.; Chen, X.D.; Zhang, Y.F.; Liu, Z.F. Direct CVD Growth of Graphene on Traditional Glass: Methods and Mechanisms. Adv. Mater. 2019, 31, 18. [Google Scholar] [CrossRef]
- Saito, K.; Ogino, T. Direct Growth of Graphene Films on Sapphire (0001) and (11(2)over–bar0) Surfaces by Self–Catalytic Chemical Vapor Deposition. J. Phys. Chem. C 2014, 118, 5523–5529. [Google Scholar] [CrossRef]
- Chen, K.; Li, C.; Shi, L.; Gao, T.; Song, X.; Bachmatiuk, A.; Zou, Z.; Deng, B.; Ji, Q.; Ma, D.; et al. Growing three–dimensional biomorphic graphene powders using naturally abundant diatomite templates towards high solution processability. Nat. Commun. 2016, 7, 13440. [Google Scholar] [CrossRef]
- Kim, H.K.; Mattevi, C.; Calvo, M.R.; Oberg, J.C.; Artiglia, L.; Agnoli, S.; Hirjibehedin, C.F.; Chhowalla, M.; Saiz, E. Activation energy paths for graphene nucleation and growth on Cu. ACS Nano 2012, 6, 3614. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Weinberg, G.; Zhang, Q.; Lunkenbein, T.; Klein-Hoffmann, A.; Kurnatowska, M.; Plodinec, M.; Li, Q.; Chi, L.; Schloegl, R.; et al. Direct Observation of Graphene Growth and Associated Copper Substrate Dynamics by in Situ Scanning Electron Microscopy. ACS Nano 2015, 9, 1506–1519. [Google Scholar] [CrossRef]
- Bachmatiuk, A.; Borrnert, F.; Grobosch, M.; Schaffel, F.; Wolff, U.; Scott, A.; Zaka, M.; Warner, J.H.; Klingeler, R.; Knupfer, M.; et al. Investigating the Graphitization Mechanism of SiO2 Nanoparticles in Chemical Vapor Deposition. ACS Nano 2009, 3, 4098–4104. [Google Scholar] [CrossRef]
- Hong, G.; Wu, Q.H.; Ren, J.G.; Lee, S.T. Mechanism of non–metal catalytic growth of graphene on silicon. Appl. Phys. Lett. 2012, 100, 5. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Wen, X.; Jiang, Z.; Chen, X.; Mijowska, E.; Tang, T. Upcycling Waste Polypropylene into Graphene Flakes on Organically Modified Montmorillonite. Ind. Eng. Chem. Res. 2014, 53, 4173–4181. [Google Scholar] [CrossRef]
- Gurung, R.; Patil, B.; Sharon, M.; Sharon, M.; Mewada, A.; Mishra, N. Conversion of polypropylene to two–dimensional graphene, one–dimensional carbon nano tubes and zero–dimensional C–dots, all exhibiting typical sp2–hexagonal carbon rings. Iet Circuits Devices Syst. 2018, 9, 59–66. [Google Scholar]
- Sharma, S.; Kalita, G.; Hirano, R.; Shinde, S.M.; Papon, R.; Ohtani, H.; Tanemura, M. Synthesis of graphene crystals from solid waste plastic by chemical vapor deposition. Carbon 2014, 72, 66–73. [Google Scholar] [CrossRef]
- Byun, S.-J.; Lim, H.; Shin, G.-Y.; Han, T.-H.; Oh, S.H.; Ahn, J.-H.; Choi, H.C.; Lee, T.-W. Graphenes Converted from Polymers. J. Phys. Chem. Lett. 2011, 2, 493–497. [Google Scholar] [CrossRef]
- Bockhorn, H.; Hornung, A.; Hornung, U.; Schwaller, D. Kinetic study on the thermal degradation of polypropylene and polyethylene. J. Anal. Appl. Pyrolysis 1999, 48, 93–109. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Shu, H.; Tao, X.M.; Ding, F. What are the active carbon species during graphene chemical vapor deposition growth? Nanoscale 2015, 7, 1627–1634. [Google Scholar] [CrossRef]
- Bortoluzzi, J.H.; Cristiano, R.; Gallardo, H.A.; Carasek, E.; Soldi, V. Use of the SPME–GC–MS technique to study the thermal degradation of isotactic polypropylene Effects of temperature and reaction time and analysis of the reaction mechanism. e–Polymers 2008, 8, 193–202. [Google Scholar] [CrossRef]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2021, 803, 149911. [Google Scholar] [CrossRef]
- Stanic, S.; Koch, T.; Schmid, K.; Knaus, S.; Archodoulaki, V.M. Upcycling of polypropylene with various concentrations of peroxydicarbonate and dilauroyl peroxide and two processing steps. J. Appl. Polym. Sci. 2021, 138, 50659. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, G.; Zhou, Y.; Su, Y.; Dong, X.; Wang, D. Research Advances of Multiscale Structure Mediation of Polypropylene: Nucleation Effect and Blending. Polym. Bull. 2021, 6, 35–47. [Google Scholar]
- Song, R.; Jiang, Z.; Bi, W.; Cheng, W.; Lu, J.; Huang, B.; Tang, T. The combined catalytic action of solid acids with nickel for the transformation of polypropylene into carbon nanotubes by pyrolysis. Chem. A Eur. J. 2007, 13, 3234–3240. [Google Scholar] [CrossRef]
- Leon, C.; O’Brien, R.A.; McHugh, J.J.; Dasarathy, H.; Schimpf, W.C. Polyethylene and polypropylene as low cost carbon fiber (LCCF) precursors. In Advancing Affordable Materials Technology; Falcone, A., Nelson, K.M., Albers, R., Avery, W.B., Eds.; International Sampe Technical Conference Series: Boston, MA, USA, 2001; Volume 33, pp. 1289–1296. [Google Scholar]
- Jiang, H.; Liu, W.; Zhang, X.; Qiao, J. Chemical Recycling of Plastics by Microwave–Assisted High–Temperature Pyrolysis. Glob. Chall 2020, 4, 1900074. [Google Scholar] [CrossRef]
- Chan, J.H.; Balke, S.T. The thermal degradation kinetics of polypropylene.3. Thermogravimetric analyses. Polym. Degrad. Stab. 1997, 57, 135–149. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetics of the thermal and thermo–oxidative degradation of polystyrene, polyethylene and poly(propylene). Macromol. Chem. Phys. 2001, 202, 775–784. [Google Scholar] [CrossRef]
- Aboulkas, A.; El harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ohkawa, T.; Suzuki, M.; Tsuchiya, T.; Takeda, K. Semiquantitative analysis of the thermal degradation of polypropylene. J. Appl. Polym. Sci. 2010, 88, 1465–1472. [Google Scholar] [CrossRef]
- Achyut, K.P.; Singh, R.K. Catalytic performances of kaoline and silica alumina in the thermal degradation of polypropylene. J. Fuel Chem. Technol. 2011, 39, 198–202. [Google Scholar]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel–like fuels. J. Anal. Appl. Pyrolysis 2017, 127, 31–37. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, Y.; Huang, J.; Fei, Z.; Liu, Q.; Chen, X.; Cui, M.; Qiao, X. Study on the Mechanism and Kinetics of Waste Polypropylene Cracking Oxidation over the Mn2O3/HY Catalyst by TG–MS and In Situ FTIR. Ind. Eng. Chem. Res. 2020, 59, 16569–16578. [Google Scholar] [CrossRef]
- Aisien, E.T.; Otuya, I.C.; Aisien, F.A. Thermal and catalytic pyrolysis of waste polypropylene plastic using spent FCC catalyst. Environ. Technol. Innov. 2021, 22, 12. [Google Scholar] [CrossRef]
- Dogu, O.; Pelucchi, M.; Van de Vijver, R.; Van Steenberge, P.H.M.; D’Hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future directions. Prog. Energy Combust. Sci. 2021, 84, 69. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B. Multigraphene Prepared by One–Pot Pyrolysis of Diatomite/Polypropylene Composites. Appl. Sci. 2022, 12, 2687. [Google Scholar] [CrossRef]
- Tang, H.; Ma, J.K.; Chen, L.; Jiang, L.W.; Xie, J.; Li, P.; He, J. GC–MS Characterization of Volatile Flavor Compounds in Stinky Tofu Brine by Optimization of Headspace Solid–Phase Microextraction Conditions. Molecules 2018, 23, 3155. [Google Scholar] [CrossRef]
- Zhao, W.; Xia, B.; Lin, L.; Xiao, X.; Liu, P.; Lin, X.; Jiang, K. Low–energy transmission electron diffraction and imaging of large–area graphene. Sci. Adv. 2017, 3, e1603231. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Sharma, A.; Kyotani, T.; Tomita, A. Comparison of structural parameters of PF carbon from XRD and HRTEM techniques. Carbon 2000, 38, 1977–1984. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Sun, X.; Zhang, H.; Wang, C.; Ma, Y. High performance supercapacitor electrodes based on deoxygenated graphite oxide by ball milling. Electrochim. Acta 2013, 109, 874–880. [Google Scholar] [CrossRef]
- Kong, S.; Seo, H.; Shin, H.; Baik, J.-H.; Oh, J.; Kim, Y.-O.; Lee, J.-C. Improvement in mechanical and thermal properties of polypropylene nanocomposites using an extremely small amount of alkyl chain–grafted hexagonal boron nitride nanosheets. Polymer 2019, 180, 121714. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, A. Polypropylene clay nanocomposites. Rev. Chem. Eng. 2013, 29, 439–448. [Google Scholar] [CrossRef]
- Lichtenhan, J.D.; Pielichowski, K.; Blanco, I. POSS–Based Polymers. Polymers 2019, 11, 1727. [Google Scholar] [CrossRef] [Green Version]
- Merijs Meri, R.; Zicans, J.; Ivanova, T.; Berzina, R.; Japins, G.; Locs, J. Structure and Properties of Recycled Aromatic Thermoplastic Polyester Nanocomposites. Key Eng. Mater. 2012, 527, 44–49. [Google Scholar] [CrossRef]
- Ogunniran, E.S.; Sadiku, R.; Sinha Ray, S.; Luruli, N. Morphology and Thermal Properties of Compatibilized PA12/PP Blends with Boehmite Alumina Nanofiller Inclusions. Macromol. Mater. Eng. 2012, 297, 627–638. [Google Scholar] [CrossRef]
- Ajorloo, M.; Fasihi, M.; Ohshima, M.; Taki, K. How are the thermal properties of polypropylene/graphene nanoplatelet composites affected by polymer chain configuration and size of nanofiller? Mater. Des. 2019, 181, 108068. [Google Scholar] [CrossRef]
- Nisar, J.; Khan, M.A.; Ali, G.; Iqbal, M.; Shah, A.; Shah, M.R.; Sirajuddin; Sherazi, S.T.H.; Shah, L.A.; Rehman, N.U. Pyrolysis of polypropylene over zeolite mordenite ammonium: Kinetics and products distribution. J. Polym. Eng. 2019, 39, 785–793. [Google Scholar] [CrossRef]
- Choi, D.; Kil, H.S.; Lee, S. Fabrication of low–cost carbon fibers using economical precursors and advanced processing technologies. Carbon 2019, 142, 610–649. [Google Scholar] [CrossRef]
- Barton, B.E.; Patton, J.; Hukkanen, E.; Behr, M.; Lin, J.C.; Beyer, S.; Zhang, Y.Q.; Brehm, L.; Haskins, B.; Bell, B.; et al. The chemical transformation of hydrocarbons to carbon using SO3 sources. Carbon 2015, 94, 465–471. [Google Scholar] [CrossRef]
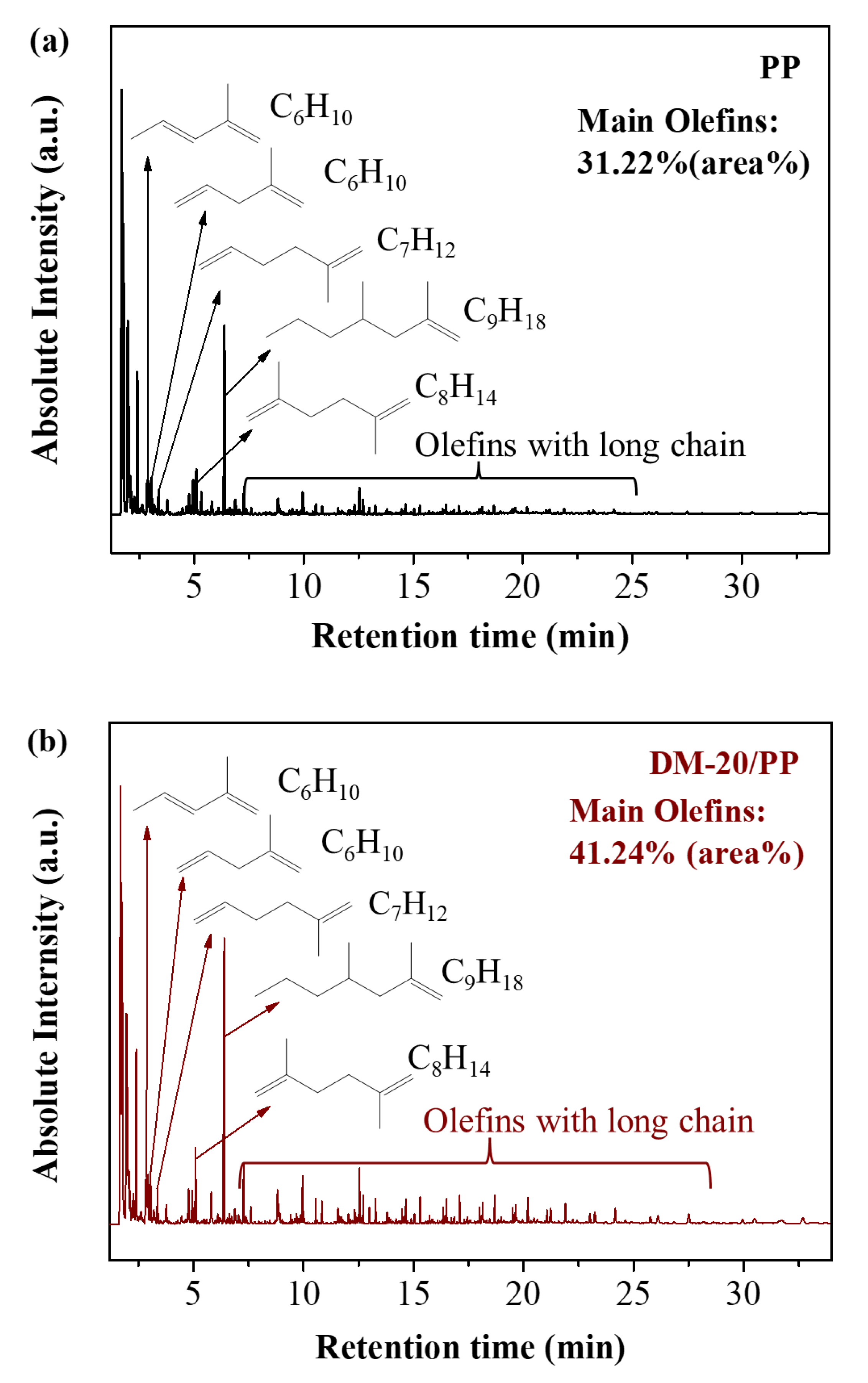
| Main Degradation Products | PP Sample (Area%) 1 | DM–20/PP Sample (Area%) 1 |
|---|---|---|
| Propylene | 28.72 | 24.56 |
| 2–Methyl–propene | 14.86 | 13.19 |
| 1–Pentene | 9.31 | 11.35 |
| 2–Methyl–1,4–pentadiene | 2.00 | 2.06 |
| trans–2–Methyl–1,3–pentadiene | 1.40 | 1.59 |
| 2–Methyl–1,5–hexadiene | 1.08 | 1.26 |
| 2,5–Dimethyl–1,5–hexadiene | 2.16 | 2.58 |
| 2,4–Dimethyl–1–heptene | 8.70 | 11.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, B. Effect of Diatomite on the Thermal Degradation Behavior of Polypropylene and Formation of Graphene Products. Polymers 2022, 14, 3764. https://doi.org/10.3390/polym14183764
Chen Y, Wang B. Effect of Diatomite on the Thermal Degradation Behavior of Polypropylene and Formation of Graphene Products. Polymers. 2022; 14(18):3764. https://doi.org/10.3390/polym14183764
Chicago/Turabian StyleChen, Yankun, and Biao Wang. 2022. "Effect of Diatomite on the Thermal Degradation Behavior of Polypropylene and Formation of Graphene Products" Polymers 14, no. 18: 3764. https://doi.org/10.3390/polym14183764
APA StyleChen, Y., & Wang, B. (2022). Effect of Diatomite on the Thermal Degradation Behavior of Polypropylene and Formation of Graphene Products. Polymers, 14(18), 3764. https://doi.org/10.3390/polym14183764






