Conductive Hydrogels Based on Industrial Lignin: Opportunities and Challenges
Abstract
:1. Introduction
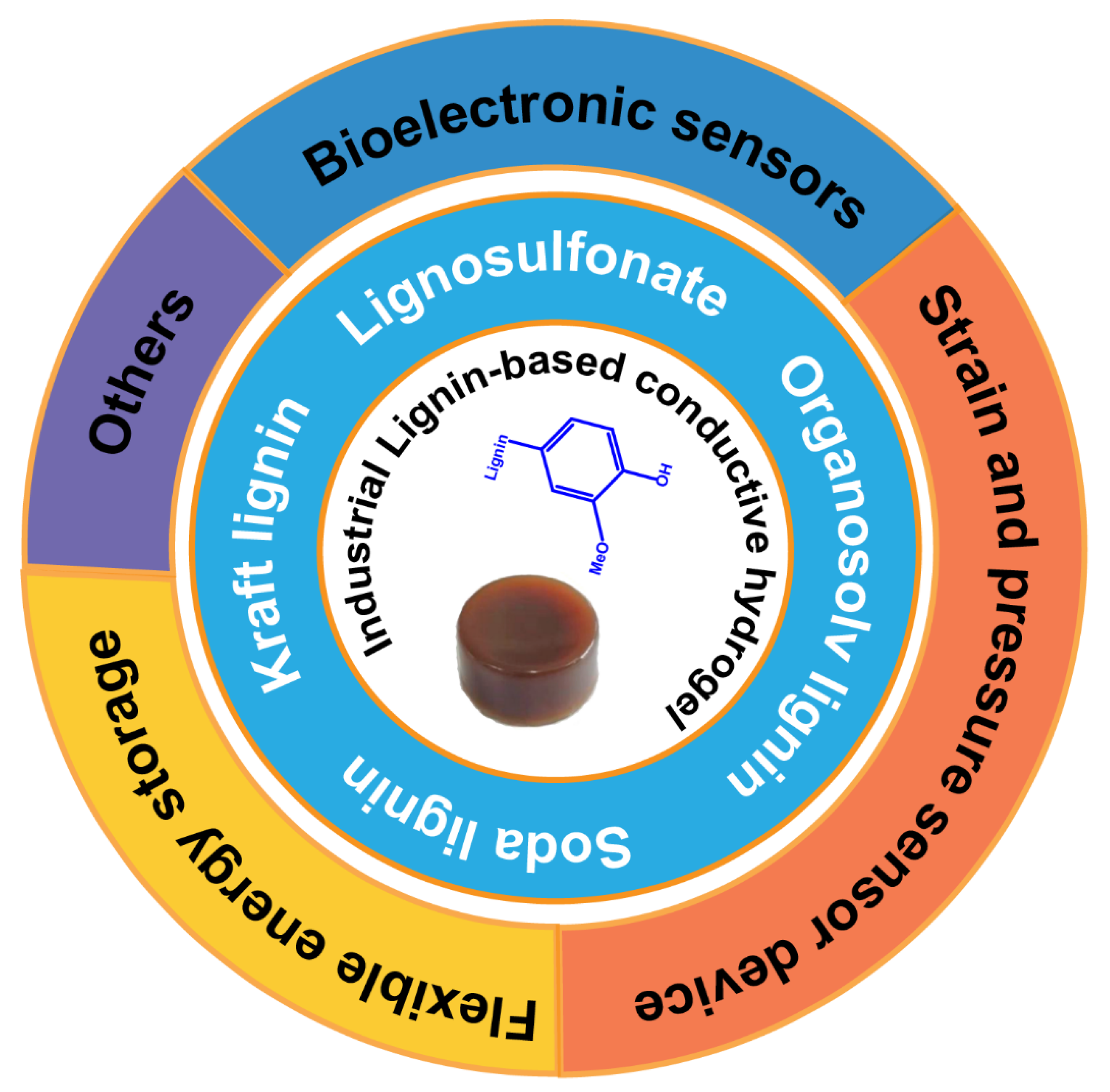
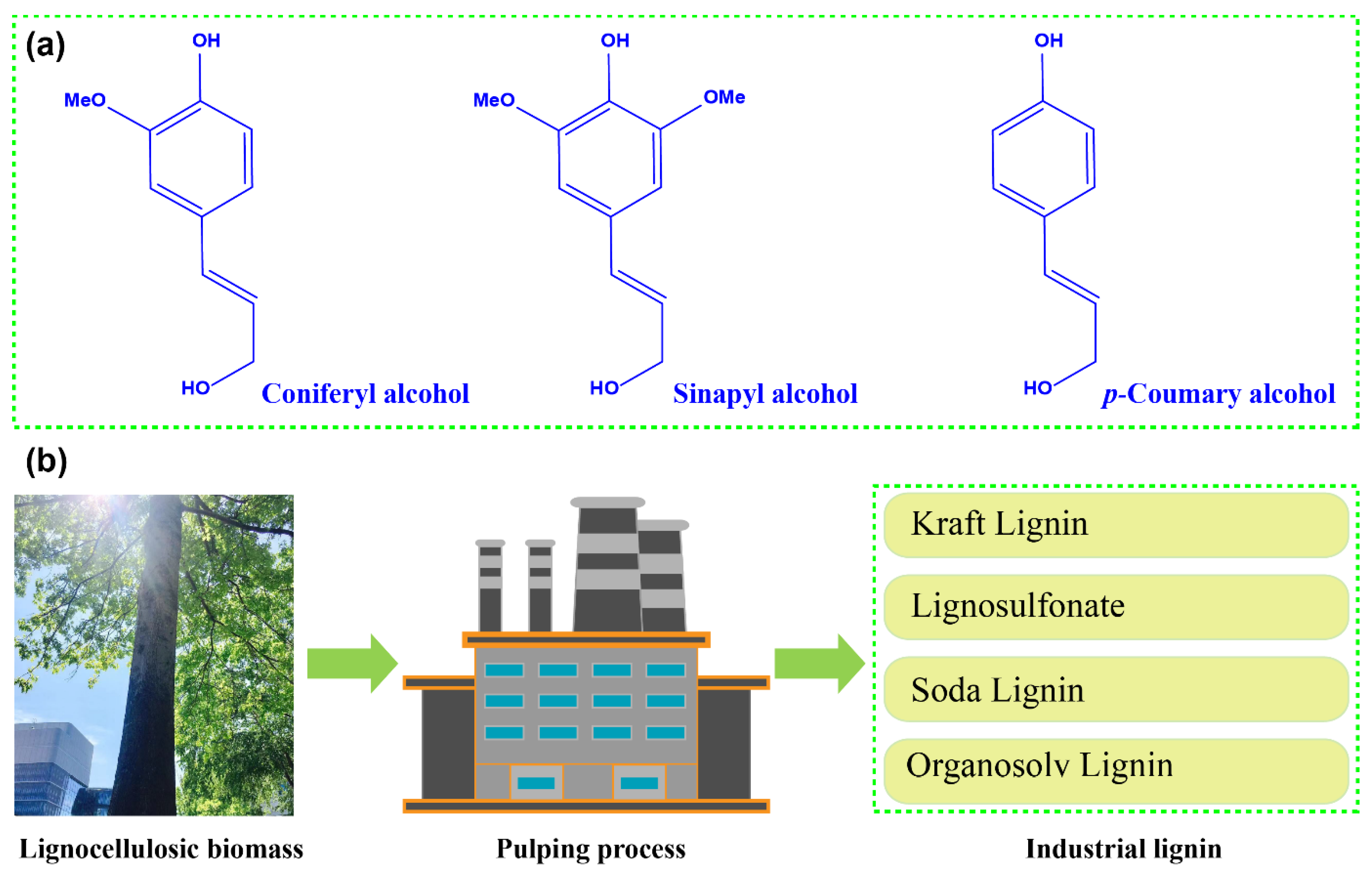
2. Lignin from the Pulping Process
2.1. Kraft Lignin
2.2. Lignosulfonate
2.3. Soda Lignin
2.4. Organosolv Lignin
3. Techniques for Preparation of Lignin-Based Hydrogel
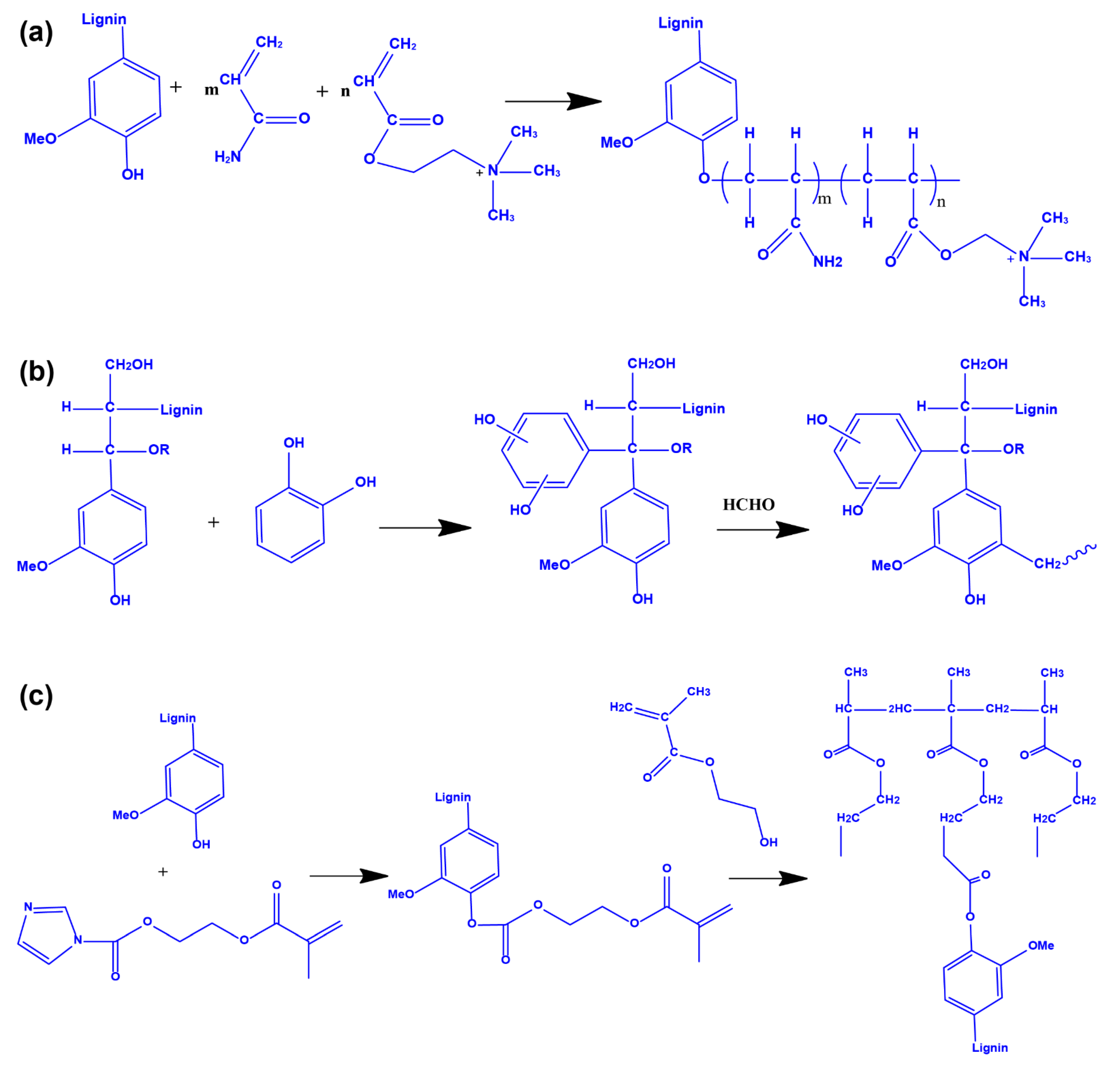
3.1. Preparation of Lignin Hydrogels by Physical Crosslinking
3.2. Interpenetrating Polymer Network and Polymerization Method
3.3. Crosslinking Copolymerization Method
3.4. Graft Crosslinking Polymerization Method
4. Properties of Lignin-Based Hydrogel
4.1. Biocompatibility and Biodegradability
4.2. Conductivity
4.3. Porous Structure
4.4. Mechanical Properties
4.5. Water Uptake and Retention
5. Application of Industrial Lignin-Based Conductive Hydrogels
5.1. Bioelectronic Sensors

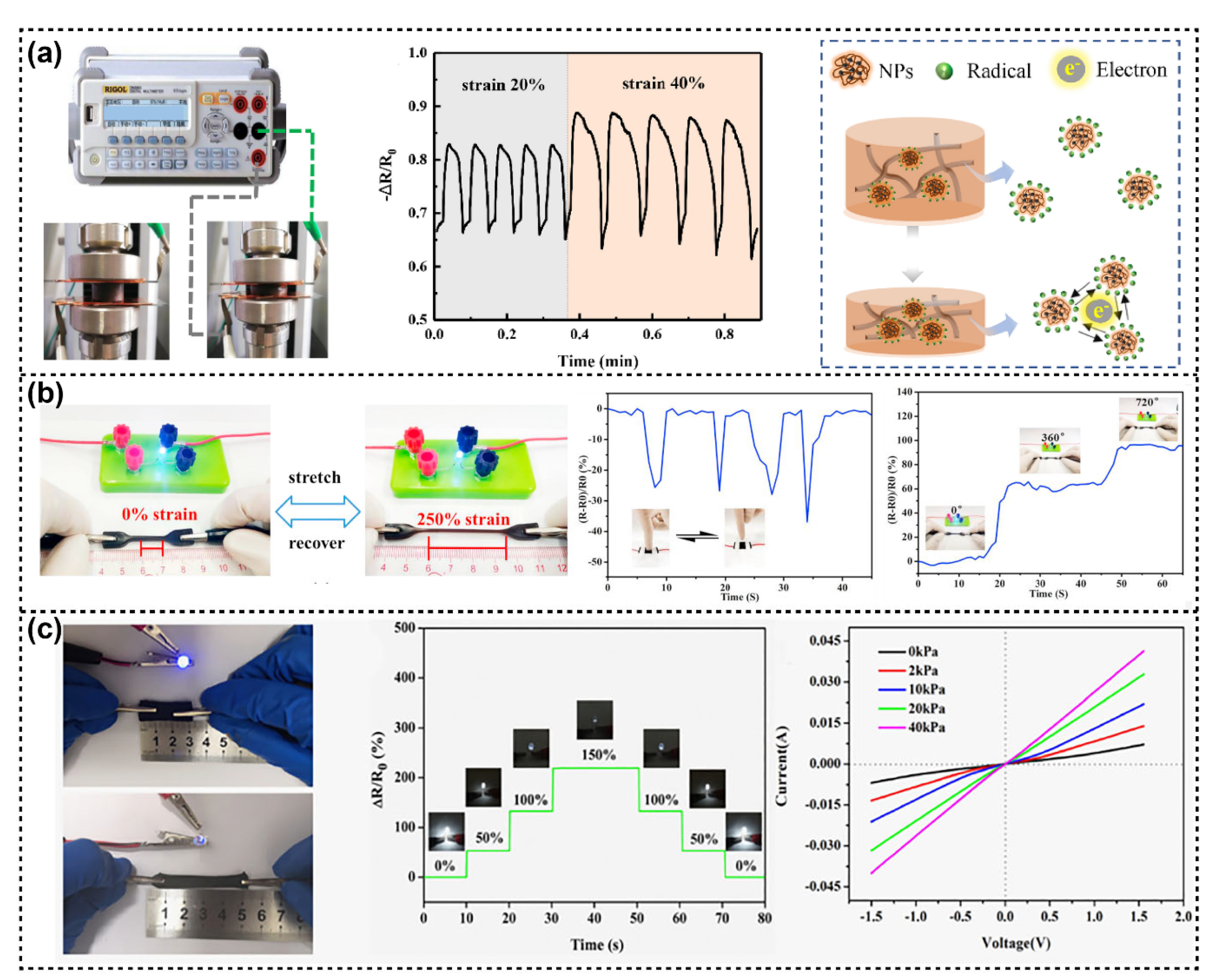
5.2. Strain and Pressure Sensor Devices
5.3. Flexible Energy Storage
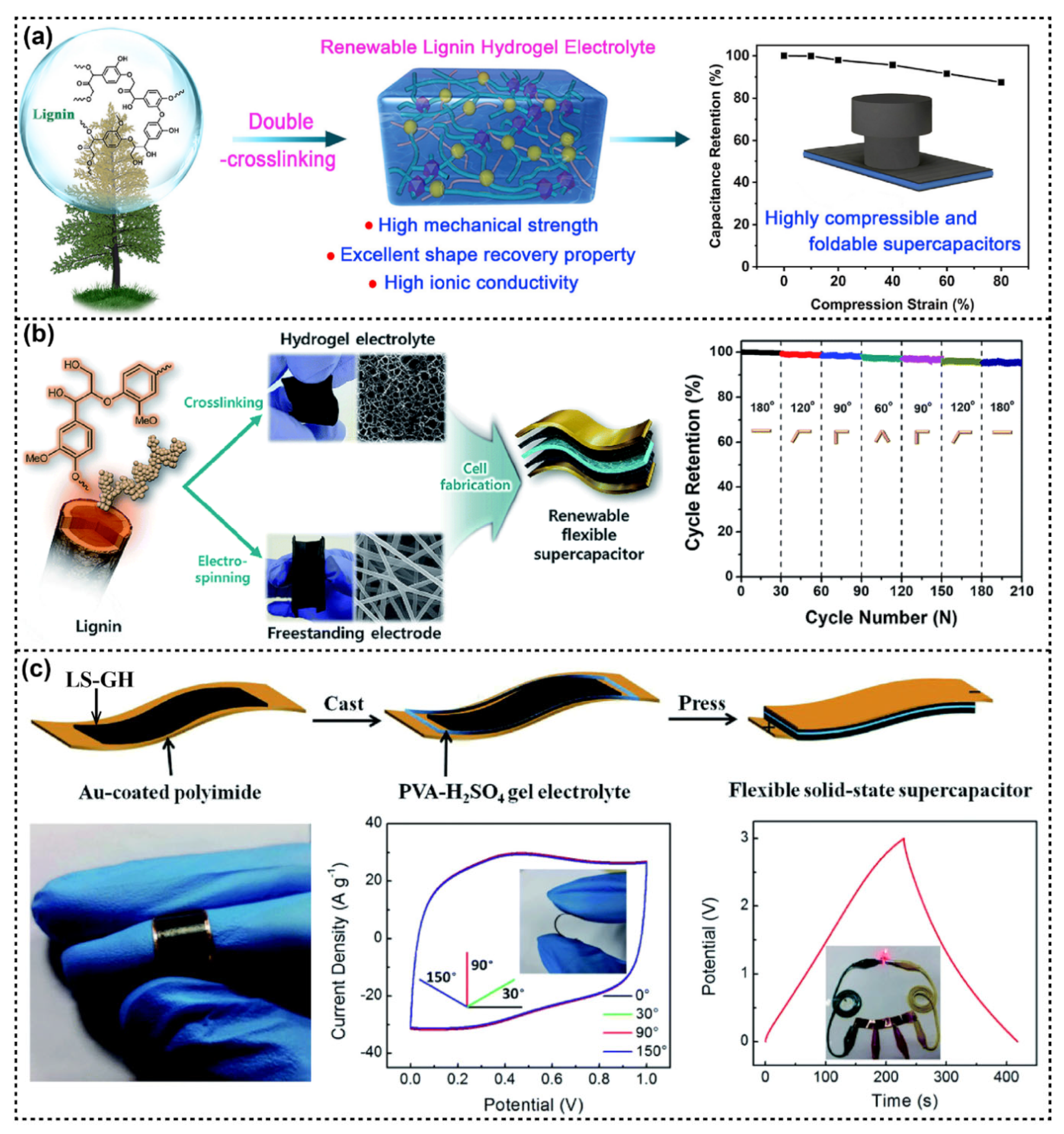
5.4. Other Applications
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, Q.; Chen, J.; Wang, T.; Peng, X.; Liu, J.; Wang, X.; Wang, J.; Zeng, H. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2020, 2, 843–865. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, A.; Ullah, K.; Rehman, N.U. Insight into hydrogels. Des. Monomers Polym. 2016, 19, 456–478. [Google Scholar] [CrossRef]
- Long, T.; Li, Y.; Fang, X.; Sun, J. Salt-Mediated Polyampholyte Hydrogels with High Mechanical Strength, Excellent Self-Healing Property, and Satisfactory Electrical Conductivity. Adv. Funct. Mater. 2018, 28, 1804416. [Google Scholar] [CrossRef]
- Ohm, Y.; Pan, C.; Ford, M.J.; Huang, X.; Majidi, C. An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics. Nat. Electron. 2021, 4, 185–192. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Dai, L.; Ma, M.; Xu, J.K.; Si, C.; Ni, Y. All-Lignin-Based Hydrogel with Fast pH-Stimuli Responsiveness for Mechanical Switching and Actuation. Chem. Mater. 2020, 32, 4324–4330. [Google Scholar] [CrossRef]
- Josef, E.; Zilberman, M.; Bianco-Peled, H. Composite alginate hydrogels: An innovative approach for the controlled release of hydrophobic drugs. Acta Biomater. 2010, 6, 4642–4649. [Google Scholar] [CrossRef]
- Lu, J.; Han, X.; Dai, L.; Li, C.; Si, C. Conductive cellulose nanofibrils-reinforced hydrogels with synergetic strength, toughness, self-adhesion, flexibility and adjustable strain responsiveness. Carbohydr. Polym. 2020, 250, 117010. [Google Scholar] [CrossRef]
- Santander, P.; Butter, B.; Oyarce, E.; Yáñez, M.; Xiao, L.P.; Sánchez, J. Lignin-based adsorbent materials for metal ion removal from wastewater: A review. Ind. Crops Prod. 2021, 167, 113510. [Google Scholar]
- Mondal, A.K.; Xu, D.; Wu, S.; Zou, Q.; Huang, F.; Ni, Y. Design of Fe3+-Rich, High-Conductivity Lignin Hydrogels for Supercapacitor and Sensor Applications. Biomacromolecules 2022, 23, 766–778. [Google Scholar] [CrossRef]
- Sun, X.; Yao, F.; Li, J. Nanocomposite hydrogel-based strain and pressure sensors: A review. J. Mater. Chem. A 2020, 8, 18605–18623. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; He, P.; Liu, K.; Xu, S. A Bionic Tactile Plastic Hydrogel-based Electronic Skin Constructed by a Nerve-like Nanonetwork Combining Stretchable, Compliant, and Self-healing Properties. Chem. Eng. J. 2020, 379, 122271. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, Y.; Wang, C.; Zhou, K.; Shen, C. Ultra-Stretchable, durable and conductive hydrogel with hybrid double network as high performance strain sensor and stretchable triboelectric nanogenerator. Nano Energy 2020, 76, 105035. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Shen, Y.; Tang, L.; Lu, B. Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. A 2019, 7, 26442–26455. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; Guo, R.; Ni, Y.; Liu, K.; Ouyang, X.; Chen, L.; Huang, L.; Cao, S.; Xie, M. An integrated transparent, UV-filtering organohydrogel sensor via molecular-level ion conductive channels Electronic supplementary information (ESI) available. J. Mater. Chem. A 2019, 7, 4525–4535. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Gregorich, N.; Ding, J.; Thies, M.C.; Davis, E.M. Novel composite hydrogels containing fractionated, purified lignins for aqueous-based separations. J. Mater. Chem. 2021, 9, 1025–1038. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.; Vilas-Vilela, J.L. Lignin-Based Hydrogels: Synthesis and Applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Leichang, C.; Yu, I.K.M.; Yaoyu, L.; Xiuxiu, R.; Tsang, D.C.W.; Hunt, A.J.; Yong, S.O.; Hocheol, S.; Shicheng, Z. Lignin valorization for the production of renewable chemicals: State-of-the-art review and future prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar]
- Grossman, A.; Vermerris, W. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2018, 56, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Gharehkhani, S.; Zhang, Y.; Fatehi, P. Lignin-derived platform molecules through TEMPO catalytic oxidation strategies. Prog. Energy Combust. Sci. 2019, 72, 59–89. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Olmedo-Martínez, J.L.; Vega-Rios, A.; Flores-Gallardo, S.; Zaragoza-Contreras, E.A. Lignin in storage and renewable energy applications: A review. J. Energy Chem. 2018, 27, 1422–1438. [Google Scholar] [CrossRef]
- Goring, D.A.I.; Vuong, R.; Gancet, C.; Chanzy, H. The flatness of lignosulfonate macromolecules as demonstrated by electron microscopy. J. Appl. Polym. Sci. 2010, 24, 931–936. [Google Scholar] [CrossRef]
- Norah, A.; Pedram, F. Synthetic and lignin-based surfactants: Challenges and opportunities. Carbon Resour. Convers. 2018, 1, 126–138. [Google Scholar]
- Galkin, M.V.; Samec, J.S. Lignin Valorization through Catalytic Lignocellulose Fractionation: A Fundamental Platform for the Future Biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef]
- Vishtal, A.G.; Kraslawski, A. Challenges in industrial applications of technical lignins. Bioresources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; Mcloughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- Bouxin, F.P.; Mcveigh, A.; Tran, F.; Westwood, N.J.; Jackson, S.D. Catalytic depolymerisation of isolated lignins to fine chemicals using a Pt/alumina catalyst: Part 1—Impact of the lignin structure. Green Chem. 2015, 17, 1235–1242. [Google Scholar] [CrossRef]
- Szalaty, T.J.; Klapiszewski, Ł.; Jesionowski, T. Recent developments in modification of lignin using ionic liquids for the fabrication of advanced materials—A review. J. Mol. Liq. 2020, 301, 112417. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Xu, C.; Wang, K.; Si, C. Lignin-based micro- and nanomaterials and composites in biomedical applications. ChemSusChem 2020, 13, 4266–4283. [Google Scholar] [CrossRef]
- Huang, S.; Su, S.; Gan, H.; Wu, L.; Lin, C.; Xu, D.; Zhou, H.; Lin, X.; Qin, Y. Facile fabrication and characterization of highly stretchable lignin-based hydroxyethyl cellulose self-healing hydrogel. Carbohydr. Polym. 2019, 223, 115080. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, K.; Venkatesan, M.; Desingh, R.P.; Mahalingam, A.; Sadhasivam, B.; Subramaniyam, R.; Dhamodharan, R. Biocompatible hydrogels of chitosan-alkali lignin for potential wound healing applications. Mater. Sci. Eng. C 2019, 102, 447–457. [Google Scholar] [CrossRef]
- Raschip, I.E.; Hitruc, E.G.; Vasile, C. Semi-interpenetrating polymer networks containing polysaccharides. II. Xanthan/lignin networks: A spectral and thermal characterization. High Perform. Polym. 2011, 23, 219–229. [Google Scholar] [CrossRef]
- Jiang, P.; Cheng, Y.; Yu, S.; Lu, J.; Wang, H. Study on the Effect of 1-Butanol Soluble Lignin on Temperature-Sensitive Gel. Polymers 2018, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Alyssa, Z.; Leila, P.; Pedram, F. Hardwood Kraft Lignin-Based Hydrogels: Production and Performance. ACS Omega 2018, 3, 8233–8242. [Google Scholar]
- Gao, G.; Xu, W.Z.; Kadla, J.F. Reversible pH-Responsive Hydrogels of Softwood Kraft Lignin and Poly[(2-dimethylamino)ethyl Methacrylate]-based Polymers. J. Wood Chem. Technol. 2014, 35, 73–90. [Google Scholar] [CrossRef]
- Xue, B.L.; Wen, J.L.; Sun, R.C. Ethanol organosolv lignin as a reactive filler for acrylamide-based hydrogels. J. Appl. Polym. Sci. 2015, 132, 42638. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Le, T.; Fletcher, D.F.; Dehghani, F. Tough and Processable Hydrogels Based on Lignin and Hydrophilic Polyurethane. ACS Appl. Bio Mater. 2018, 1, 2073–2081. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, Y.; Shao, S.; Ye, J.; Lin, L.; Iiyama, K. Lignin as a cross-linker of acrylic acid-grafted carboxymethyl lignocellulose. J. Wood Sci. 2010, 56, 470–476. [Google Scholar] [CrossRef]
- Passauer, L. Highly Swellable Lignin Hydrogels: Novel Materials with Interesting Properties. In Functional Materials from Renewable Sources; ACS Publications: Washington, DC, USA, 2012; pp. 211–228. [Google Scholar]
- Musilová, L.; Mráček, A.; Kovalcik, A.; Smolka, P.; Ponížil, P. Hyaluronan hydrogels modified by glycinated Kraft lignin: Morphology, swelling, viscoelastic properties and biocompatibility. Carbohydr. Polym. 2018, 181, 394. [Google Scholar] [CrossRef]
- Jiang, P.; Sheng, X.; Yu, S.; Li, H.; Lu, J.; Zhou, J.; Wang, H. Preparation and characterization of thermo-sensitive gel with phenolated alkali lignin. Sci. Rep. 2018, 8, 14450. [Google Scholar] [CrossRef] [PubMed]
- Eneko, L.; Mikel, I.; Toh, J.X.; Irwin, N.J.; Anastasia, R.; Anastasia, P.; Juan, D.R.; Alejandro, R.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar]
- Xin, W.; Shiyao, J.; Zhaosheng, H.; Yingying, L.; Xiumin, Q.; Yusheng, L.; Yebang, T. Permeable, robust and magnetic hydrogel beads: Water droplet templating synthesis and utilization for heavy metal ions removal. J. Mater. Sci. 2018, 53, 15009–15024. [Google Scholar]
- Liu, Z.; Wang, H.; Li, B.; Liu, C.; Jiang, Y.; Yu, G.; Mu, X. Biocompatible magnetic cellulose–chitosan hybrid gel microspheres reconstituted from ionic liquids for enzyme immobilization. J. Mater. Chem. 2012, 22, 15085–15091. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Guo, X.; Qiang, H.; Ge, C. Ultrasonic-assisted synthesis of sodium lignosulfonate-grafted poly(acrylic acid-co-poly(vinyl pyrrolidone)) hydrogel for drug delivery. RSC Adv. 2016, 6, 35550–35558. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Wang, J.; Zhang, X. Ultrasonic-assisted fabrication of montmorillonite-lignin hybrid hydrogel: Highly efficient swelling behaviors and super-sorbent for dye removal from wastewater. Colloids Surf. A 2017, 520, 903–913. [Google Scholar] [CrossRef]
- Ma, Y.; Lyu, L.; Guo, Y.; Fu, Y.; Shao, Q.; Wu, T.; Guo, S.; Sun, K.; Guo, X.; Wujcik, E.K. Porous lignin based poly (acrylic acid)/organo-montmorillonite nanocomposites: Swelling behaviors and rapid removal of Pb (II) ions. Polymer 2017, 128, 12–23. [Google Scholar] [CrossRef]
- Jin, C.; Song, W.; Liu, T.; Xin, J.; Hiscox, W.C.; Zhang, J.; Liu, G.; Kong, Z. Temperature and pH Responsive Hydrogels Using Methacrylated Lignosulfonate Cross-Linker: Synthesis, Characterization, and Properties. ACS Sustain. Chem. Eng. 2017, 6, 1763–1771. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, X.; Lin, C.; Lin, D.; Ni, Y.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Biocompatible, self-wrinkled, antifreezing and stretchable hydrogel-based wearable sensor with PEDOT: Sulfonated lignin as conductive materials. Chem. Eng. J. 2019, 370, 1039–1047. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Y.; Fu, Y.; Fang, G.; Yan, X.; Guo, Z. Swelling behaviors of porous lignin based poly (acrylic acid). Chemosphere Environ. Toxicol. Risk Assess. 2016, 163, 610–619. [Google Scholar] [CrossRef]
- Passauer, L.; Hallas, T.; Bäucker, E.; Ciesielski, G.; Lebioda, S.; Hamer, U. Biodegradation of Hydrogels from Oxyethylated Lignins in Model Soils. ACS Sustain. Chem. Eng. 2015, 3, 1955–1964. [Google Scholar] [CrossRef]
- Yamamoto, H.; Amaike, M.; Saitoh, H.; Sano, Y. Gel formation of lignin and biodegradation of the lignin gels by microorganisms. Mater. Sci. Eng. C 1999, 7, 143–147. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L.; Yu, G. Multifunctional Nanostructured Conductive Polymer Gels: Synthesis, Properties, and Applications. Acc. Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Lin, L.; Ji, M.; Zhang, S.; Yang, M.; Fu, Q. Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog. Polym. Sci. 2014, 39, 627–655. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Thomas, A. Carbon-Based Microbial-Fuel-Cell Electrodes: From Conductive Supports to Active Catalysts. Adv. Mater. 2017, 29, 1602547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, W.; Tao, Y.; Yang, Q.H. Towards superior volumetric performance: Design and preparation of novel carbon materials for energy storage. Energy Environ. Sci. 2015, 8, 1390–1403. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, H. Crystal phase-controlled synthesis, properties and applications of noble metal nanomaterials. Chem. Soc. Rev. 2016, 45, 63–82. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Cai, J.; Huang, J.; Qiu, X. Equip the hydrogel with armor: Strong and super tough biomass reinforced hydrogels with excellent conductivity and anti-bacterial performance. J. Mater. Chem. A 2019, 7, 26917–26926. [Google Scholar] [CrossRef]
- Keplinger, C.; Sun, J.Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M.; Suo, Z. Stretchable, Transparent, Ionic Conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef]
- Wang, M.X.; Chen, Y.M.; Gao, Y.; Hu, C.; Hu, J.; Tan, L.; Yang, Z. Rapid Self-Recoverable Hydrogels with High Toughness and Excellent Conductivity. ACS Appl. Mater. Interfaces 2018, 10, 26610–26617. [Google Scholar] [CrossRef]
- Yajie, S.; Yanli, M.; Guizhen, F.; Shixue, R.; Yujie, F. Controlled Pesticide Release from Porous Composite Hydrogels Based on Lignin and Polyacrylic Acid. Bioresources 2016, 11, 2361–2371. [Google Scholar]
- Mushi, N.E.; Kochumalayil, J.; Cervin, N.T.; Zhou, Q.; Berglund, L.A. Nanostructurally Controlled Hydrogel Based on Small-Diameter Native Chitin Nanofibers: Preparation, Structure, and Properties. ChemSusChem 2016, 9, 989–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinoski, R.M.; Shi, J. Hydrogels derived from lignocellulosic compounds: Evaluation of the compositional, structural, mechanical and antimicrobial properties. Ind. Crops Prod. 2019, 128, 323–330. [Google Scholar] [CrossRef]
- Li, Z.; Ge, Y. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 2021, 20, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, K.; Niu, L.; Zhang, Y.; Liu, Y.; Wang, C.; Chu, F. Highly mechanical properties nanocomposite hydrogels with biorenewable lignin nanoparticles—ScienceDirect. Int. J. Biol. Macromol. 2019, 128, 414–420. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, X.; Lin, C.; Ma, X.; Cao, S.; Ni, Y. Ultrafast gelling using sulfonated lignin-Fe3+ chelates to produce dynamic crosslinked hydrogel/coating with charming stretchable, conductive, self-healing, and ultraviolet-blocking properties. Chem. Eng. J. 2020, 396, 125341. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.; Jiang, W.; Zhang, S.; Tong, L.; Mao, J.; Wei, G.; Zuo, M.; Ni, Y. Lignin sulfonate induced ultrafast polymerization of double network hydrogels with anti-freezing, high strength and conductivity and their sensing applications at extremely cold conditions—ScienceDirect. Compos. Part B Eng. 2021, 217, 108879. [Google Scholar] [CrossRef]
- Wang, Q.; Lan, J.; Hua, Z.; Ma, X.; Chen, L.; Pan, X.; Li, Y.; Cao, S.; Ni, Y. An oriented Fe3+-regulated lignin-based hydrogel with desired softness, conductivity, stretchability, and asymmetric adhesiveness towards anti-interference pressure sensors. Int. J. Biol. Macromol. 2021, 184, 282–288. [Google Scholar] [CrossRef]
- Han, X.; Lv, Z.; Ran, F.; Dai, L.; Li, C.; Si, C. Green and stable piezoresistive pressure sensor based on lignin-silver hybrid nanoparticles/polyvinyl alcohol hydrogel. Int. J. Biol. Macromol. 2021, 176, 78–86. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.; Liu, W.; Huang, J.; Qiu, X. Synthesis of highly conductive hydrogel with high strength and super toughness. Polymer 2020, 202, 122643. [Google Scholar] [CrossRef]
- Mla, C.; Qta, B.; Xing, L.; Qza, B.; Hj, A.; Cca, B.; Swa, B.; Dma, B. Flexible conductive hydrogel fabricated with polyvinyl alcohol, carboxymethyl chitosan, cellulose nanofibrils, and lignin-based carbon applied as strain and pressure sensor—ScienceDirect. Int. J. Biol. Macromol. 2020, 166, 1526–1534. [Google Scholar]
- Schlee, P.; Hosseinaei, O.; Baker, D.; Landmer, A.; Tomani, P.; Mostazo-Lopez, M.J.; Cazorla-Amorós, D.; Herou, S.; Titirici, M.M. From waste to wealth: From Kraft lignin to free-standing supercapacitors. Carbon 2019, 145, 470–480. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Liu, T.; Ren, X.; Zhang, J.; Liu, J.; Ou, R.; Guo, C.; Yu, X.; Wang, Q.; Liu, Z. Highly compressible lignin hydrogel electrolytes via double-crosslinked strategy for superior foldable supercapacitors. J. Power Sources 2020, 449, 227532. [Google Scholar] [CrossRef]
- Park, J.H.; Rana, H.H.; Lee, J.Y.; Park, H.S. Renewable flexible supercapacitors based on all-lignin-based hydrogel electrolytes and nanofiber electrodes. J. Mater. Chem. A 2019, 7, 16962–16968. [Google Scholar] [CrossRef]
- Milczarek, G.; Inganas, O. Renewable Cathode Materials from Biopolymer/Conjugated Polymer Interpenetrating Networks. Science 2012, 335, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Sun, R. A metal-free and flexible supercapacitor based on redox-active lignosulfonate functionalized graphene hydrogels. J. Mater. Chem. A 2017, 5, 20643–20650. [Google Scholar] [CrossRef]
- Cui, L.; Li, Y.; Jia, M.; Cheng, C.; Jin, X. A Self-Assembled and Flexible Supercapacitor based on Redox-Active Lignin-Based Nitrogen-Doped Activated Carbon Functionalized Graphene Hydrogels. J. Electrochem. Soc. 2021, 168, 053504. [Google Scholar] [CrossRef]
- Gong, S.D.; Huang, Y.; Cao, H.J.; Lin, Y.H.; Li, Y.; Tang, S.H.; Wang, M.-S.; Li, X. A green and environment-friendly gel polymer electrolyte with higher performances based on the natural matrix of lignin. J. Power Sources 2016, 307, 624–633. [Google Scholar] [CrossRef]
- Hur, O.N.; Park, S.; Park, S.; Kang, B.H.; Lee, C.S.; Hong, J.Y.; Park, S.-H.; Bae, J. A study on fabrication of polypyrrole@lignin composite and electrical sensing and metal ion adsorption capabilities. Mater. Chem. Phys. 2022, 285, 126166. [Google Scholar] [CrossRef]
- Jędrzak, A.; Rębiś, T.; Klapiszewski, Ł.; Zdarta, J.; Milczarek, G.; Jesionowski, T. Carbon paste electrode based on functional GOx/silica-lignin system to prepare an amperometric glucose biosensor. Sens. Actuators B Chem. 2018, 256, 176–185. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Lv, X.; Wang, T.; Xue, B. Synthesis of novel lignosulfonate-modified graphene hydrogel for ultrahigh adsorption capacity of Cr (VI) from wastewater. J. Clean. Prod. 2021, 295, 126406. [Google Scholar] [CrossRef]
- Jiao, G.J.; Ma, J.; Li, Y.; Jin, D.; Zhou, J.; Sun, R. Removed heavy metal ions from wastewater reuse for chemiluminescence: Successive application of lignin-based composite hydrogels. J. Hazard. Mater. 2022, 421, 126722. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, Y.; Zhuang, J.; Xiang, Z.; Jiang, W.; He, S.; Xiao, H. Conductive Hydrogels Based on Industrial Lignin: Opportunities and Challenges. Polymers 2022, 14, 3739. https://doi.org/10.3390/polym14183739
Liu C, Li Y, Zhuang J, Xiang Z, Jiang W, He S, Xiao H. Conductive Hydrogels Based on Industrial Lignin: Opportunities and Challenges. Polymers. 2022; 14(18):3739. https://doi.org/10.3390/polym14183739
Chicago/Turabian StyleLiu, Chao, Yu Li, Jingshun Zhuang, Zhouyang Xiang, Weikun Jiang, Shuaiming He, and Huining Xiao. 2022. "Conductive Hydrogels Based on Industrial Lignin: Opportunities and Challenges" Polymers 14, no. 18: 3739. https://doi.org/10.3390/polym14183739
APA StyleLiu, C., Li, Y., Zhuang, J., Xiang, Z., Jiang, W., He, S., & Xiao, H. (2022). Conductive Hydrogels Based on Industrial Lignin: Opportunities and Challenges. Polymers, 14(18), 3739. https://doi.org/10.3390/polym14183739






