Polycarbonate/Titania Composites Incorporating TiO2 with Different Nanoscale Morphologies for Enhanced Environmental Stress Cracking Resistance in Dioctyl Phthalate
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Melt Mixing and Injection Molding
2.3. Microstructure and Chemical Characterization
2.4. Environmental Stress Cracking Testing
3. Results and Discussion
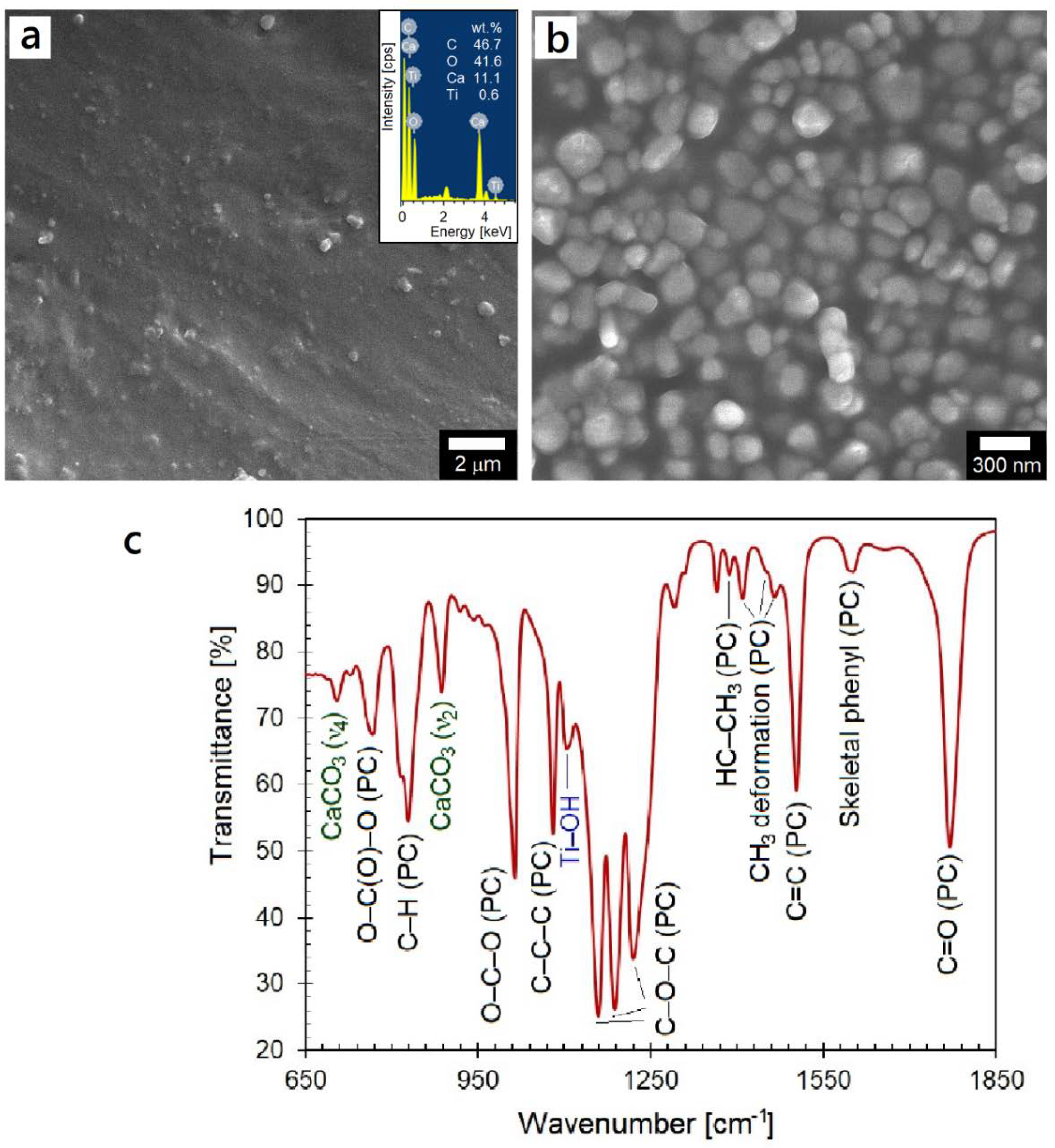
3.1. Characterization of Initial Materials
3.1.1. As-Received Polycarbonate Granules
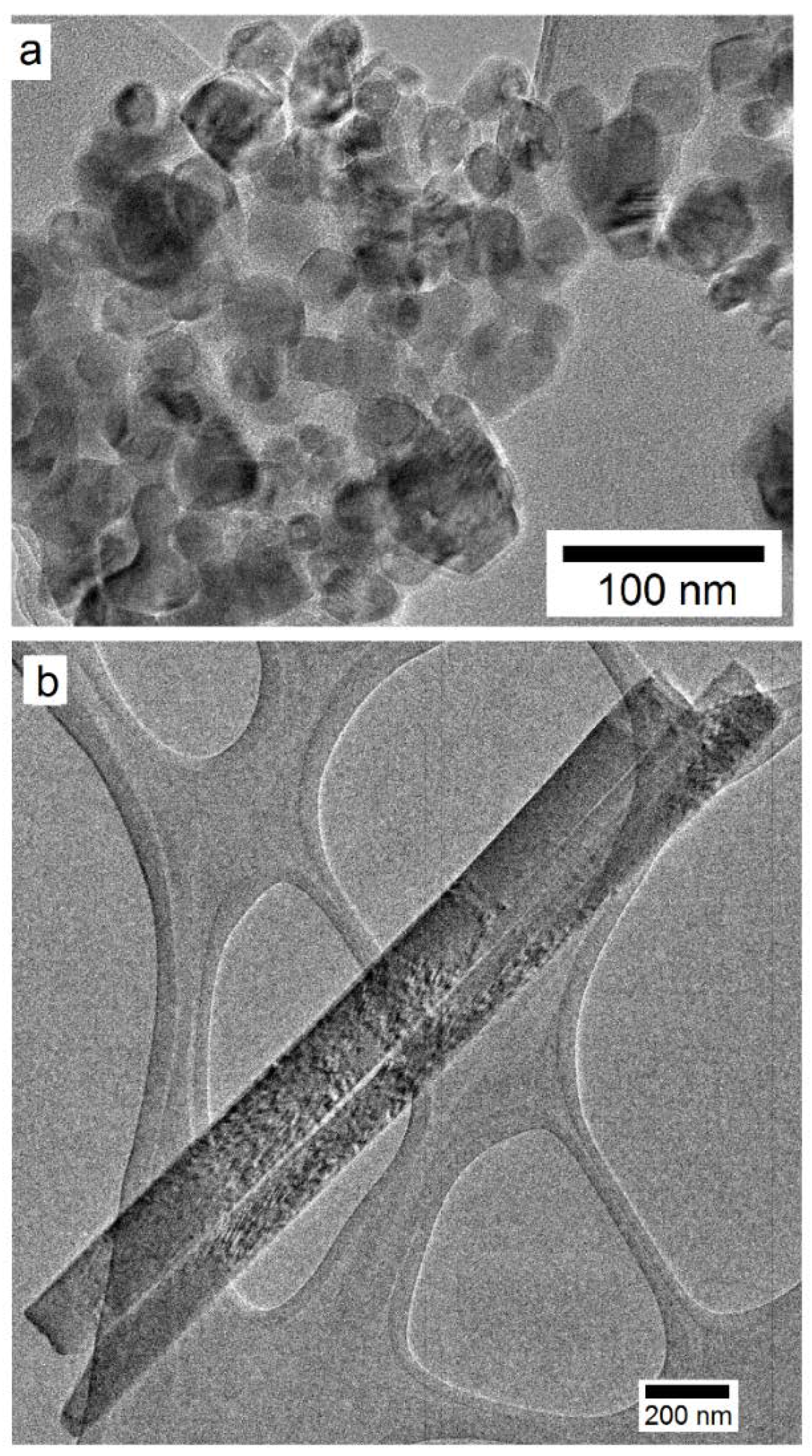
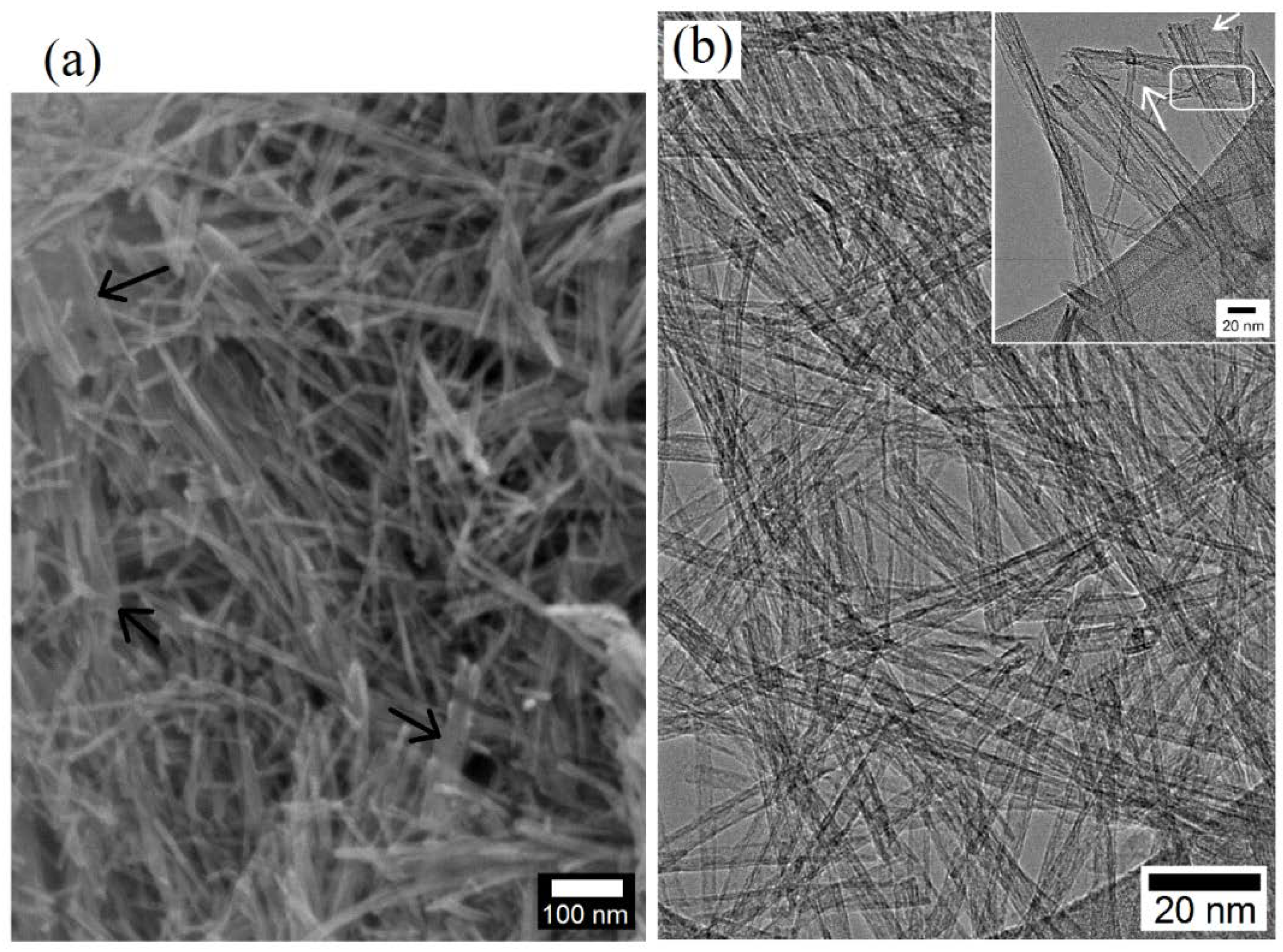
3.1.2. TiO2 Nanostructures
3.2. PC/TiO2 Nanocomposites
3.2.1. Chemical and Thermal Analyses
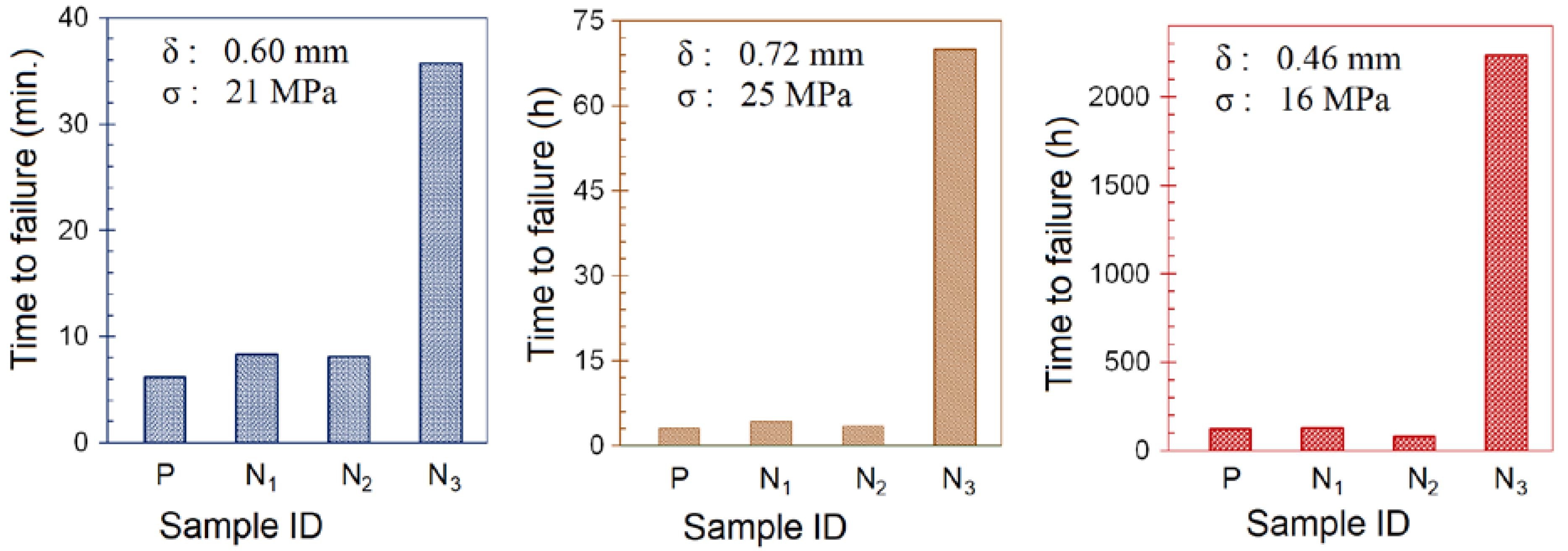
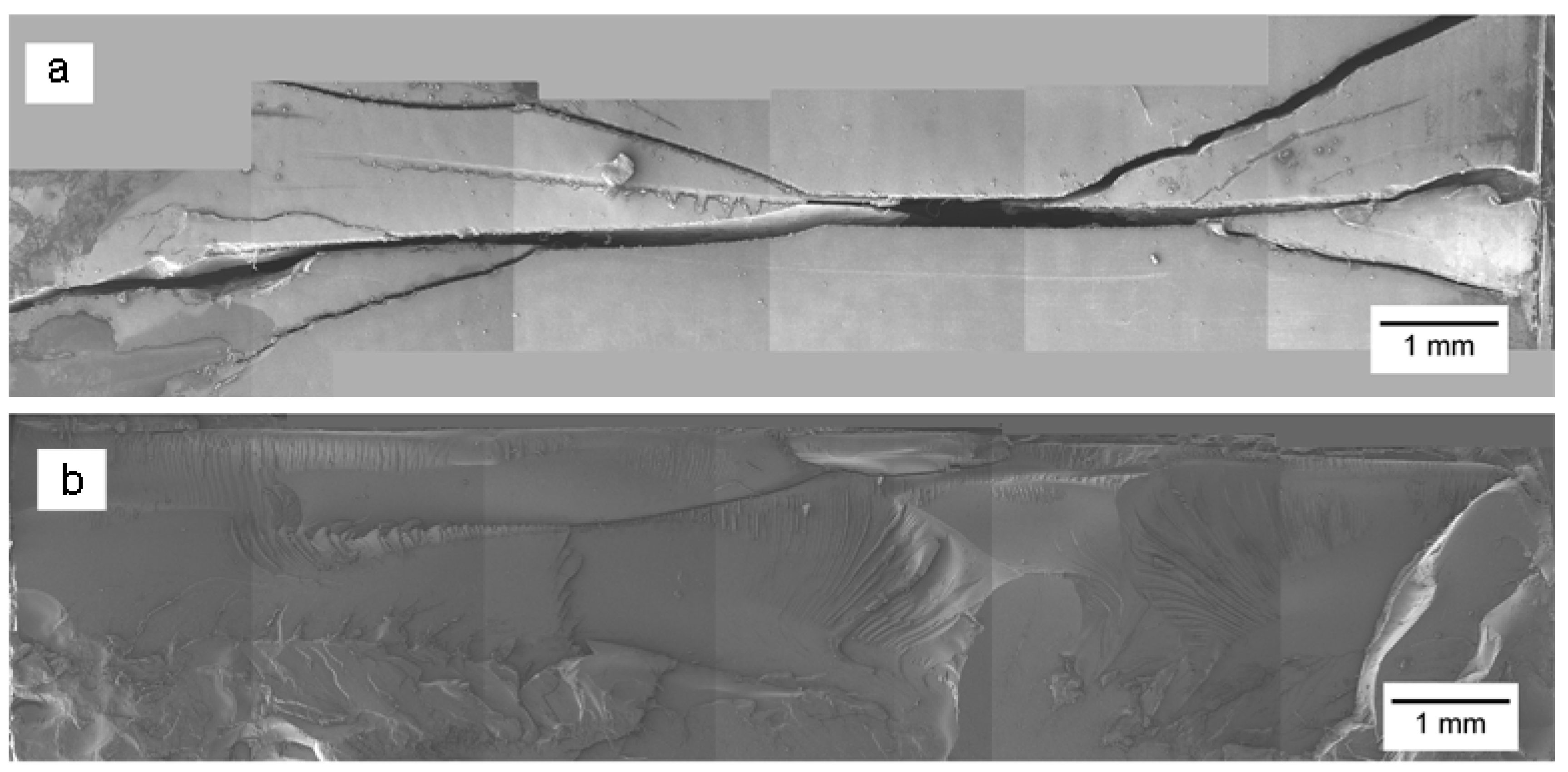
3.2.2. Environmental Stress Cracking (ESC) Tests
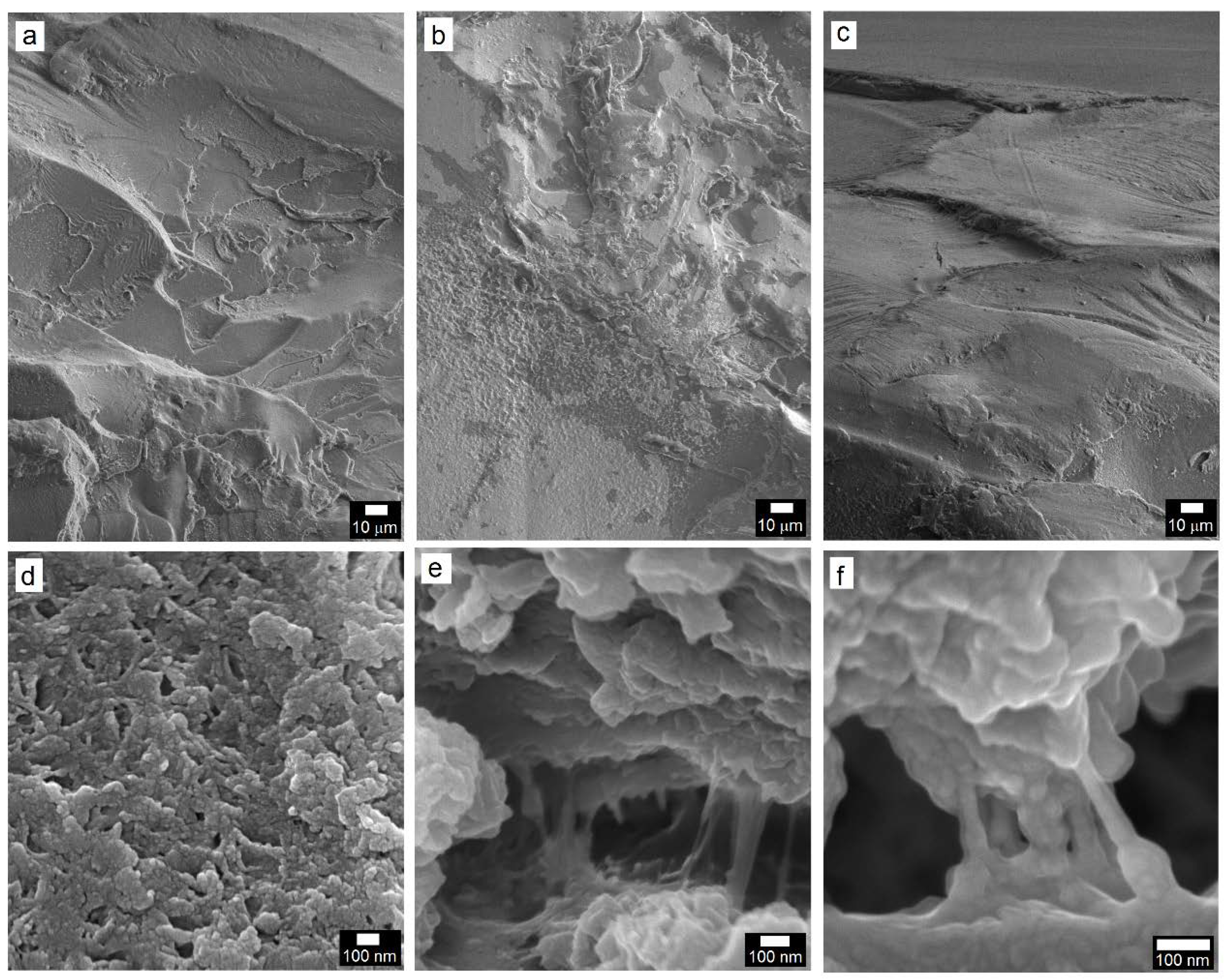
3.2.3. Microstructural Analysis of PC and PC/TiO2 Composites
3.3. Effect of TiO2 Hybrid Nanostructures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Polycarbonate Market Size Worth $35.4 Billion By 2030. In Polycarbonate Market Size & Share Report; Grand View Research: San Francisco, CA, USA, 2022; Available online: https://www.grandviewresearch.com/press-release/global-polycarbonate-market (accessed on 25 July 2022).
- Huang, M.Z.; Nomai, J.; Schlarb, A.K. The effect of different processing, injection molding (IM) and fused deposition modeling (FDM), on the environmental stress cracking (ESC) behavior of filled and unfilled polycarbonate (PC). Express Polym. Lett. 2021, 15, 194–202. [Google Scholar] [CrossRef]
- Silva, P.P.O.; Araujo, P.L.B.; Lima, T.B.S.; Araujo, E.S. The influence of environmental stress cracking (ESC) and gamma irradiation on the mechanical properties of polycarbonate: Study of synergistic effects. Mater. Res. 2022, 25, e20210342. [Google Scholar] [CrossRef]
- Xu, H.; Yang, J.; Zhang, X.; Li, T.; Huang, J.; Chen, M.; Dong, W. Polycarbonate blends with high environmental stress crack resistance, high strength and high toughness by introducing polyvinyl butyral at small fraction. Polymer 2022, 242, 124578. [Google Scholar] [CrossRef]
- Hopmann, C.; Borchmann, N.; Koch, S.; Alperstein, D. Influencing the environmental stress cracking resistance of amorphous thermoplastic parts by the examples of polycarbonate and water. Polym. Eng. Sci. 2019, 59, E361–E366. [Google Scholar] [CrossRef]
- Robeson, L.M. Environmental stress cracking: A review. Polym. Eng. Sci. 2013, 53, 453–467. [Google Scholar] [CrossRef]
- Saharudin, M.S.; Atif, R.; Shyha, I.; Inam, F. The degradation of mechanical properties in polymer nano-composites exposed to liquid media—A review. RSC Adv. 2016, 6, 1076–1089. [Google Scholar]
- Fang, M.; Zhang, N.; Huang, M.; Lu, B.; Lamnawar, K.; Liu, C.; Shen, C. Effects of hydrothermal aging of carbon fiber reinforced polycarbonate composites on mechanical performance and sand erosion test. Polymers 2020, 12, 2453. [Google Scholar] [CrossRef]
- Gohil, M.; Joshi, G. Perspective of polycarbonate composites and blends properties, applications and future development: A review. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Altalhi, T., Inamuddin, Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–424. [Google Scholar]
- Yan, C.; Zhang, J.; Han, J.; Wang, X.; Guan, Z.; Zhang, L.; Liu, C.; Shen, C. Improvement of environmental stress cracking resistance of polycarbonate by silicone coating. Polym. Test. 2017, 60, 6–11. [Google Scholar] [CrossRef]
- Nomai, J.; Schlarb, A.K. Environmental stress cracking (ESC) resistance of polycarbonate/SiO2 nanocomposites in different media. J. Appl. Polym. Sci. 2017, 134, 45451. [Google Scholar] [CrossRef]
- Meng, L.; Duwal, S.; Lane, J.M.D.; Ao, T.; Stoltzfus, B.; Knudson, M.; Park, C.; Chow, P.; Xiao, Y.; Fan, H.; et al. High pressure induced atomic and mesoscale phase behaviors of one-dimensional TiO2 anatase nanocrystals. MRS Bull. 2022, 47, 455. [Google Scholar] [CrossRef]
- Tominaka, S.; Tsujimoto, Y.; Matsushita, Y.; Yamaura, K. Synthesis of nanostructured reduced titanium oxide: Crystal structure transformation maintinaing nanomorphology. Angew. Chem. 2011, 50, 7418. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Chen, Y.; Lin, C. Corrosion protection of 316 L stainless steel by a TiO2 nanoparticle coating prepared by sol–gel method. Thin Solid Film. 2005, 489, 130–136. [Google Scholar] [CrossRef]
- Khanna, S.; Marathey, P.; Paneliya, S.; Vinchhi, P.; Chaudhari, R.; Vora, J. Fabrication of graphene/titania nanograss composite on shape memory alloy as photoanodes for photoelectrochemical studies: Role of the graphene. Int. J. Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Khanna, S.; Paneliya, S.; Marathey, P.; Shah, K.; Prajapati, P.; Chaudhari, R.; Vora, J. Investigation of thermophysical properties of synthesized N-Hexacosane-encapsulated titania phange change material for enhanced thermal storage application. In Recent Advances in Mechanical Infrastructures. Lecture Notes in Intelligent Transportation and Infrastructure; Parwani, A.K., Abhishek, P., Yadav, K., Eds.; Springer: Singapore, 2022; p. 107. [Google Scholar]
- Motaung, T.E.; Luyt, A.S.; Saladino, M.L.; Caponetti, E. Study of morphology, mechanical properties, and thermal degradation of polycarbonate-titania nanocomposites as function of titania crystalline phase and content. Polym. Compos. 2013, 34, 164–172. [Google Scholar] [CrossRef]
- Stuart, B.H. Temperature studies of polycarbonate using Fourier transform Raman spectroscopy. Polym. Bull. 1996, 36, 341–346. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. A TGA/FTIR and mass spectral study on the thermal degradation of bisphenol A polycarbonate. Polym. Degrad. Stab. 2004, 86, 419–430. [Google Scholar] [CrossRef]
- Yadav, R.; Naebe, M.; Wang, X.; Kandasubramanian, B. Structural and Thermal Stability of Polycarbonate Decorated Fumed Silica Nanocomposite via Thermomechanical Analysis and In-situ Temperature Assisted SAXS. Sci. Rep. 2017, 7, 7706. [Google Scholar] [CrossRef]
- Achour, A.; Arman, A.; Islam, M.; Zavarian, A.A.; Al-Zubaidi, A.B.; Szade, J. Synthesis and characterization of porous CaCO3 micro/nano-particles. Eur. Phys. J. Plus 2017, 132, 267. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Hu, G.Q.; Duan, X.F.; Sun, H.L.; Xue, Q.K. Microstructure and formation mechanism of titanium dioxide nanotubes. Chem. Phys. Lett. 2002, 365, 427–431. [Google Scholar] [CrossRef]
- Yao, B.D.; Chan, Y.F.; Zhang, X.Y.; Zhang, W.F.; Yang, Z.Y.; Wang, N. Formation mechanism of TiO2 nanotubes. Appl. Phys. Lett. 2003, 82, 281–283. [Google Scholar] [CrossRef]
- Nakahira, A.; Kubo, T.; Numako, C. Formation Mechanism of TiO2-Derived Titanate Nanotubes Prepared by the Hydrothermal Process. Inorg. Chem. 2010, 49, 5845–5852. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-J.; Chi, Z.-Y.; Wu, Y.-C. Morphology, Chemical Composition and Phase Transformation of Hydrothermal Derived Sodium Titanate. J. Am. Ceram. Soc. 2012, 95, 3297–3304. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Ishihara, H.; Sharma, R.; Biris, A.S. Photoelectroactivity and Raman spectroscopy of anodized titania (TiO2) photoactive water-splitting catalysts as a function of oxygen-annealing temperature. J. Mater. Chem. 2011, 21, 6337–6345. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Hinder, S.; Pillai, S.C. Improved high temperature stability of anatase TiO2 photocatalysts by N, F, P co-doping. Mater. Des. 2016, 96, 44–53. [Google Scholar] [CrossRef]
- Araújo, E.S.; Libardi, J.; Faia, P.M.; de Oliveira, H.P. Hybrid ZnO/TiO2 Loaded in Electrospun Polymeric Fibers as Photocatalyst. J. Chem. 2015, 2015, 476472. [Google Scholar] [CrossRef]
- Resta, V.; Quarta, G.; Lomascolo, M.; Maruccio, L.; Calcagnile, L. Raman and Photoluminescence spectroscopy of polycarbonate matrices irradiated with different energy 28Si+ ions. Vacuum 2015, 116, 82–89. [Google Scholar] [CrossRef]
- Lee, S.-N.; Stolarski, V.; Letton, A.; Laane, J. Studies of bisphenol-A–polycarbonate aging by Raman difference spectroscopy. J. Mol. Struct. 2000, 521, 19–23. [Google Scholar] [CrossRef]
- Javed, F.; Javed, S.; Akram, M.A.; Mujahid, M.; Islam, M.; Bhatti, A.S. Surface plasmon mediated optical properties of ZnO/Zu/TiO2 nanoheterostructure rod arranys. Mater. Sci. Eng. B 2018, 231, 32–39. [Google Scholar] [CrossRef]
- Mandair, G.S.; Morris, M.D. Contributions of Raman spectroscopy to the understanding of bone strength. BoneKEy Rep. 2015, 4, 620. [Google Scholar] [CrossRef]
- Harris, J.; Mey, I.; Hajir, M.; Mondeshki, M.; Wolf, S.E. Pseudomorphic transformation of amorphous calcium carbonate films follows spherulitic growth mechanisms and can give rise to crystal lattice tilting. CrystEngComm 2015, 17, 6831–6837. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Yao, L.; Zhai, J.; Shen, B.; Wang, H. Significantly improved dielectric properties and energy density of polymer nanocomposites via small loaded of BaTiO3 nanotubes. Compos. Sci. Technol. 2017, 147, 30–38. [Google Scholar] [CrossRef]
- Mahmood, N.; Islam, M.; Hameed, A.; Saeed, S. Polyamide 6/Multiwalled Carbon Nanotubes Nanocomposites with Modified Morphology and Thermal Properties. Polymers 2013, 5, 1380–1391. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Q.; Yang, H.; Xia, Y.; Jiang, Y. In-situ preparation of hierarchical flower-like TiO2/carbon nanostructures as fillers for polymer composites with enhanced dielectric properties. Sci. Rep. 2017, 7, 43970. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Islam, M.; Hameed, A.; Saeed, S.; Khan, A.N. Polyamide 6 based Composites Reinforced with Pristine or Functionalized Multi-Walled Carbon Nanotubes Produced using Melt Extrusion Technique. J. Compos. Mater. 2014, 48, 1197–1207. [Google Scholar] [CrossRef]
- Apaydin-Varol, E.; Polat, S.; Putun, A. Pyrolysis kinetics and thermal decomposition behavior of polycarbonate—A TGA-FTIR study. Therm. Sci. 2014, 18, 833–842. [Google Scholar] [CrossRef]
- Jing, H.; Higaki, Y.; Ma, W.; Xi, J.; Jinnai, H.; Otsuka, H.; Takahara, A. Preparation and characterization of polycarbonate nanocomposites based on surface-modified halloysite nanotubes. Polym. J. 2014, 46, 307–312. [Google Scholar] [CrossRef]
- Jiang, W.; Tjong, S. Thermal stability of polycarbonate composites reinforced with potassium titanate whiskers: Effect of coupling agent addition. Polym. Degrad. Stab. 1999, 66, 241–246. [Google Scholar] [CrossRef]
- Arnold, J.C. Environmental Effects on Crack Growth in Composites; Elsevier: Amsterdam, The Netherlands, 2007; Volume 6, pp. 428–470. [Google Scholar]
- Satapathy, B.K.; Weidisch, R.; Potschke, P.; Janke, A. Tough-to-brittle transition in multiwalled carbon nanotube (MWNT)/polycarbonate nanocomposites. Compos. Sci. Technol. 2007, 67, 867–879. [Google Scholar] [CrossRef]
- Tsuda, T.; Ogasawara, T.; Deng, F.; Takeda, N. Direct measurements of interfacial shear strength of multi-walled carbon nanotube/PEEK composite using a nano-pullout method. Compos. Sci. Technol. 2011, 71, 1295–1300. [Google Scholar] [CrossRef]
- Al-Lafi, W.; Jin, J.; Song, M. Mechanical response of polycarbonate nanocomposites to high velocity impact. Eur. Polym. J. 2016, 85, 354–362. [Google Scholar] [CrossRef] [Green Version]







| Intensity | Frequency (cm−1) | Phase | Vibration Mode |
|---|---|---|---|
| vs | 145 | Anatase TiO2 | Eg mode |
| sh | 197 | Anatase TiO2 | B1g mode |
| m | 399 | Anatase TiO2 | A1g mode |
| m | 514 | Anatase TiO2 | B1g mode |
| m | 639 | Anatase TiO2 | Eg mode |
| w | 400 | Anatase TiO2/PC | A1g mode; O–C–O bend |
| w | 453 | Rutile TiO2 | Eg mode |
| w | 581 | Polycarbonate | Phenyl ring vibration |
| vw | 614 | Rutile TiO2 | |
| s | 638 | Anatase TiO2/PC | Eg mode; Phenyl ring def. (i.p.) |
| s | 706 | Polycarbonate | Phenyl ring def. (o.p.) |
| m | 735 | Polycarbonate | C–H bend (o.p.) |
| sh | 817 | Polycarbonate | CH wag (o.p.) |
| w | 830 | Rutile TiO2 | B2g mode |
| sh | 837 | Polycarbonate | Phenyl ring vibration |
| vs | 890 | Polycarbonate | C–CH3 stretch |
| w | 897 | Polycarbonate | O–C(O)–O stretch |
| w | 940 | Polycarbonate | CH wag (o.p.) |
| w | 1008 | Polycarbonate | Ring stretch |
| sh | 1025 | Polycarbonate | C–C stretching |
| w, sh | 1084 | Calcite (CaCO3) | υ1 Symmetric stretch (CO3)2– |
| s | 1112 | Polycarbonate | CH wag (i.p.)/C–O–C stretch |
| s | 1180 | Polycarbonate | CH wag |
| s | 1238 | Polycarbonate | C–O stretch |
| s, sh | 1245 | Polycarbonate | C–O–C stretch |
| w | 1297 | Polycarbonate | C–O–C stretch |
| m | 1368 | Polycarbonate | CH3 bend |
| w | 1446 | Polycarbonate | CH3 symmetric bend |
| w | 1468 | Polycarbonate | CH3 asymmetric bend |
| s | 1605 | Polycarbonate | Phenyl ring stretch |
| w | 1780 | Polycarbonate | C=O stretch |
| ID | Temp. for Different Weight Loss (°C) | Melting Peak (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T−5 | T−10 | T−20 | T−30 | T−50 | T−70 | Onset | Peak | End | Width | |
| PC | 482.1 | 497.0 | 509.2 | 515.8 | 525.5 | 555.2 | 456.1 | 500.6 | 532.0 | 75.9 |
| PC/TiO2-0.5NP | 479.9 | 494.8 | 506.9 | 513.1 | 522.5 | 551.3 | 459.9 | 501.1 | 505.7 | 45.8 |
| PC/TiO2-0.1NW | 462.0 | 472.5 | 486.2 | 495.9 | 510.4 | 531.1 | 450.3 | 477.3 | 498.8 | 48.5 |
| PC/TiO2-0.05HN | 489.7 | 501.4 | 511.8 | 517.7 | 526.5 | 555.8 | 477.0 | 497.8 | 508.1 | 31.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, Y.; Achour, A.; Akram, M.A.; Islam, M. Polycarbonate/Titania Composites Incorporating TiO2 with Different Nanoscale Morphologies for Enhanced Environmental Stress Cracking Resistance in Dioctyl Phthalate. Polymers 2022, 14, 3693. https://doi.org/10.3390/polym14173693
Khalid Y, Achour A, Akram MA, Islam M. Polycarbonate/Titania Composites Incorporating TiO2 with Different Nanoscale Morphologies for Enhanced Environmental Stress Cracking Resistance in Dioctyl Phthalate. Polymers. 2022; 14(17):3693. https://doi.org/10.3390/polym14173693
Chicago/Turabian StyleKhalid, Yasir, Amine Achour, Muhammad Aftab Akram, and Mohammad Islam. 2022. "Polycarbonate/Titania Composites Incorporating TiO2 with Different Nanoscale Morphologies for Enhanced Environmental Stress Cracking Resistance in Dioctyl Phthalate" Polymers 14, no. 17: 3693. https://doi.org/10.3390/polym14173693
APA StyleKhalid, Y., Achour, A., Akram, M. A., & Islam, M. (2022). Polycarbonate/Titania Composites Incorporating TiO2 with Different Nanoscale Morphologies for Enhanced Environmental Stress Cracking Resistance in Dioctyl Phthalate. Polymers, 14(17), 3693. https://doi.org/10.3390/polym14173693







