Study of the Potential Accumulation of the Pesticide Alpha-Endosulfan by Microplastics in Water Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Samples
2.3. Procedure
2.3.1. Optimization of the Liquid-Liquid Extraction of α-Endosulfan from Aqueous Solution
2.3.2. GC Analysis
2.3.3. Stability and Homogenization Tests of α-Endosulfan Aqueous Solutions
2.3.4. Preliminary Evaluation of Adsorption Affinity of Six Systems Microplastics/α-Endosulfan
2.3.5. Batch Adsorption Experiments
3. Results and Discussion
3.1. Liquid-Liquid Extraction of α-Endosulfan with Different Solvents
3.2. Study of Stability of and Homogenization of α-Endosulfan Aqueous Solutions
3.3. Preliminary Studies of Adsorption Affinity of Six Microplastics/α-Endosulfan Systems
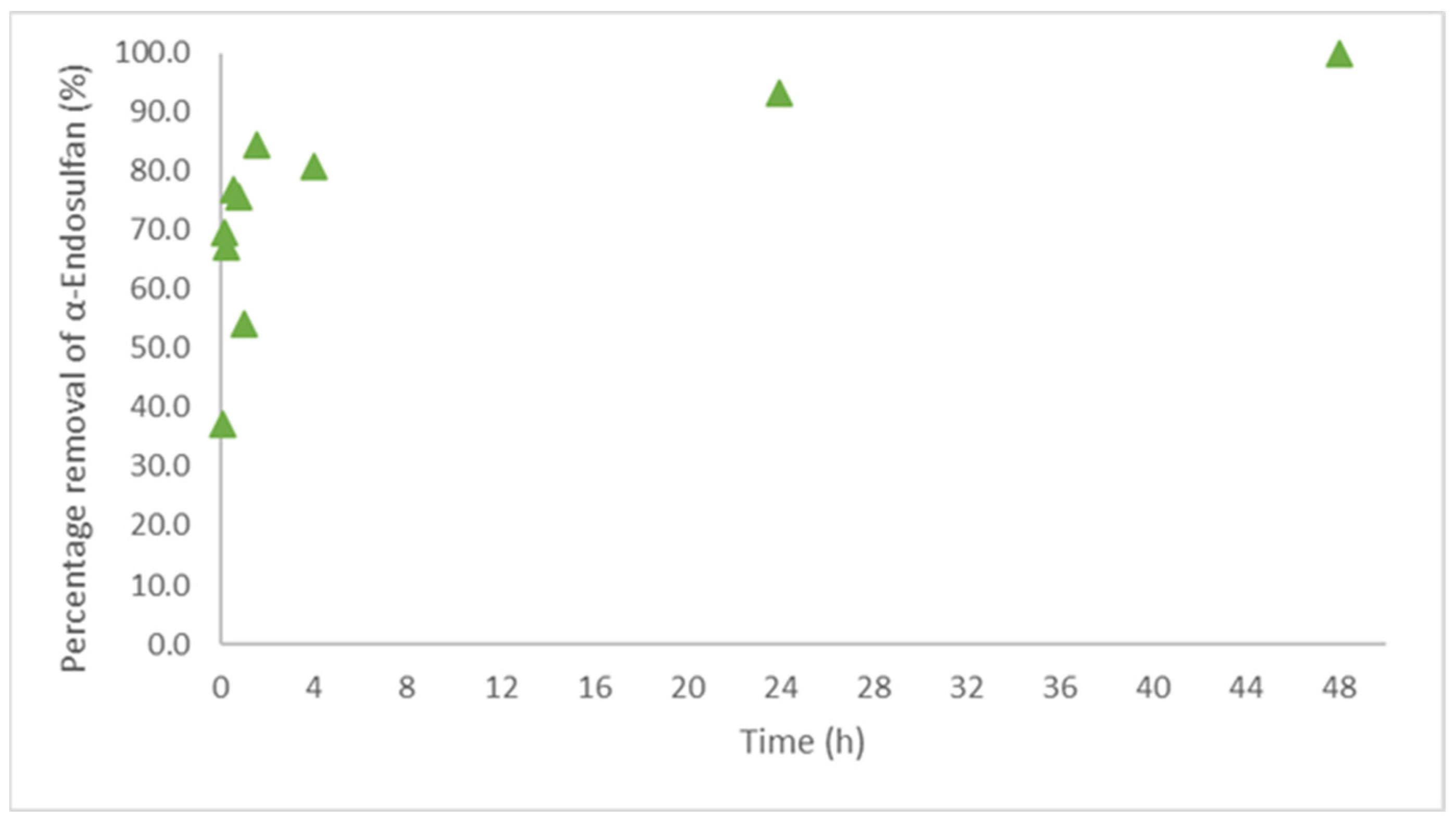
3.4. Sorption Kinetics
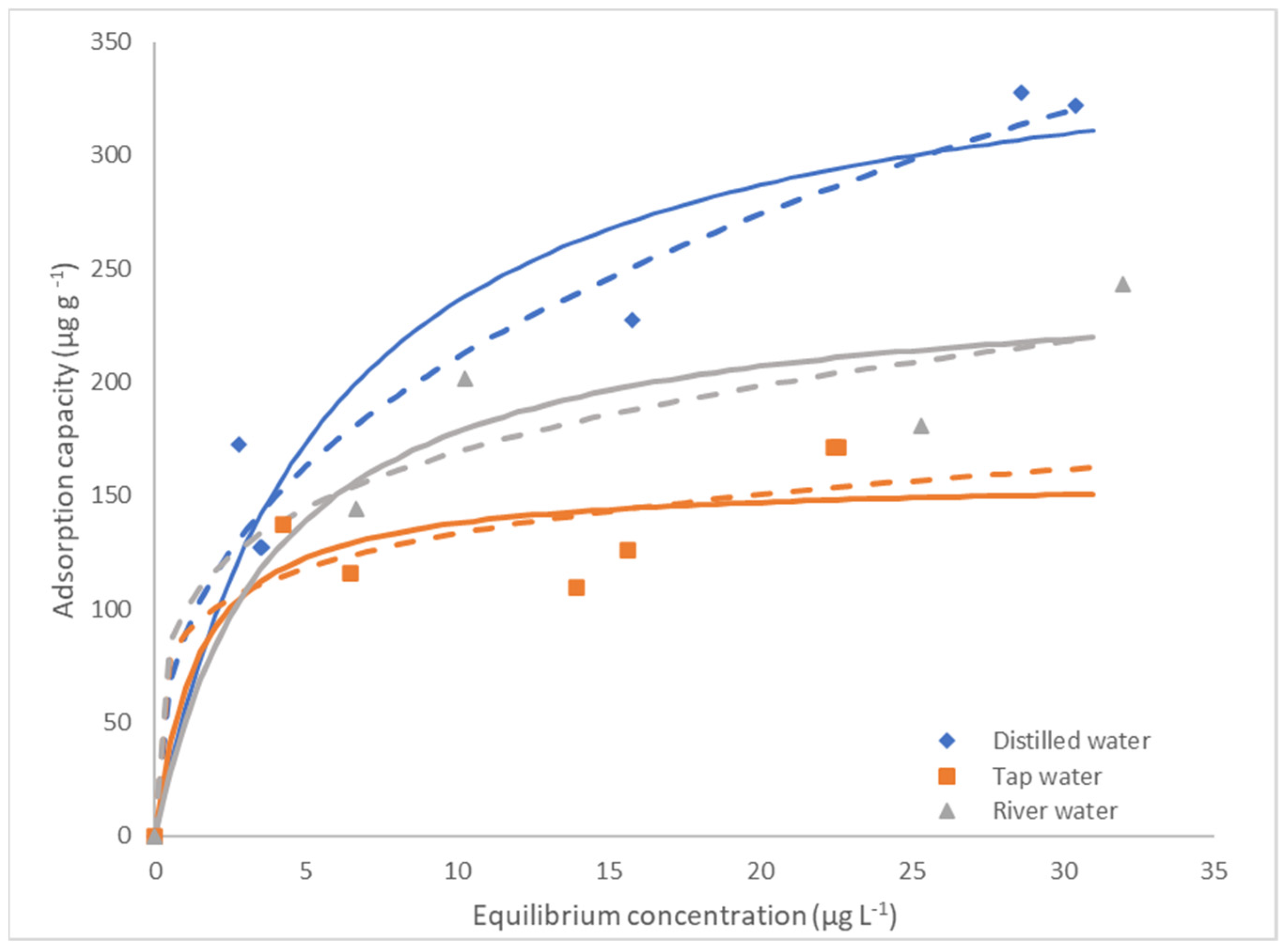
3.5. Equilibrium Studies
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Pinto da Costa, J.; Reis, V.; Paço, A.; Costa, M.; Duarte, A.C.; Rocha-Santos, T. Micro(nano)plastics—Analytical challenges towards risk evaluation. TRAC—Trend Anal. Chem. 2019, 111, 173–184. [Google Scholar] [CrossRef]
- Wang, T.; Yu, C.; Chu, Q.; Wang, F.; Lan, T.; Wang, J. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere 2020, 244, 125491. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef] [PubMed]
- Estahbanati, S.; Fahrenfeld, N.L. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere 2016, 162, 277–284. [Google Scholar] [CrossRef]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Wu, M.; Yang, C.; Du, C.; Liu, H. Microplastics in waters and soils: Occurrence, analytical methods and ecotoxicological effects. Ecotoxicol. Environ. Saf. 2020, 202, 110910. [Google Scholar] [CrossRef]
- Besseling, E.; Quik, J.T.K.; Sun, M.; Koelmans, A.A. Fate of nano- and microplastic in freshwater systems: A modeling study. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; Lopez-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Behbehani, M. An assessment of microplastic inputs into the aquatic environment from wastewater streams. Mar. Pollut. Bull. 2020, 160, 111538. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Azadkhah, S.; Farahani, H.; Uddin, S.; Khan, F.R. Microplastics in wastewater outlets of Bandar Abbas city (Iran): A potential point source of microplastics into the Persian Gulf. Chemosphere 2021, 262, 128039. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Clark, M.; Thompson, R.; Hughes, K.A. Microplastics in marine sediments near Rothera Research Station, Antarctica. Mar. Pollut. Bull. 2018, 133, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Lots, F.A.E.; Behrens, P.; Vijver, M.G.; Horton, A.A.; Bosker, T. A large-scale investigation of microplastic contamination: Abundance and characteristics of microplastics in European beach sediment. Mar. Pollut. Bull. 2017, 123, 219–226. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Leng, Y.; Wang, J. Defense responses in earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics in soils. Ecotoxicol. Environ. Saf. 2020, 187, 109788. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Du, J.; Xu, S.; Zhou, Q.; Li, H.; Fu, L.; Tang, J.; Wang, Y.; Peng, X.; Xu, Y.; Du, X. A review of microplastics in the aquatic environmental: Distribution, transport, ecotoxicology, and toxicological mechanisms. Environ. Sci. Pollut. Res. Int. 2020, 27, 11494–11505. [Google Scholar] [CrossRef]
- Chen, Q.; Allgeier, A.; Yin, D.; Hollert, H. Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions. Environ. Int. 2019, 130, 104938. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, J.W.; Pham, T.D.; Tarafdar, A.; Hong, S.; Chun, S.H.; Lee, S.H.; Kang, D.Y.; Kim, J.Y.; Kim, S.B.; et al. Microplastics in Food: A Review on Analytical Methods and Challenges. Int. J. Environ. Res. Public Health 2020, 17, 6710. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Habibi, N.; Sajid, S.; Dupont, S.; Behbehani, M. A Preliminary Assessment of Size-Fractionated Microplastics in Indoor Aerosol-Kuwait’s Baseline. Toxics 2022, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Uddin, S.; Fowler, S.W.; Behbehani, M. Microplastics in the atmosphere: A review. JEEA 2022, 1, 6. [Google Scholar] [CrossRef]
- Selonen, S.; Dolar, A.; Jemec Kokalj, A.; Sackey, L.N.A.; Skalar, T.; Cruz Fernandes, V.; Rede, D.; Delerue-Matos, C.; Hurley, R.; Nizzetto, L.; et al. Exploring the impacts of microplastics and associated chemicals in the terrestrial environment—Exposure of soil invertebrates to tire particles. Environ. Res. 2021, 201, 111495. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Mohsen, M.; Wang, Q.; Zhang, L.; Sun, L.; Lin, C.; Yang, H. Microplastic ingestion by the farmed sea cucumber Apostichopus japonicus in China. Environ. Pollut. 2019, 245, 1071–1078. [Google Scholar] [CrossRef]
- Vroom, R.J.E.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Liu, X.; Qu, F.; Wang, X.; Wang, X.; Li, Y.; Sun, Y. Microplastics in the environment: A review of analytical methods, distribution, and biological effects. TRAC—Trend Anal. Chem. 2019, 111, 62–72. [Google Scholar] [CrossRef]
- Zakeri, M.; Naji, A.; Akbarzadeh, A.; Uddin, S. Microplastic ingestion in important commercial fish in the southern Caspian Sea. Mar. Pollut. Bull. 2020, 160, 111598. [Google Scholar] [CrossRef]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frere, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Brate, I.L.N.; Sun, C.; Shahadat Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 2017, 9, 1346–1360. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.; Le Goic, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental status of (micro)plastics contamination in Portugal. Ecotoxicol. Environ. Saf. 2020, 200, 110753. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barcelo, D. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: Multiple sources other than plastic mulching film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef]
- Li, W.; Wufuer, R.; Duo, J.; Wang, S.; Luo, Y.; Zhang, D.; Pan, X. Microplastics in agricultural soils: Extraction and characterization after different periods of polythene film mulching in an arid region. Sci. Total Environ. 2020, 749, 141420. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Bussian, B.M.; Pandelova, M.; Lehnik-Habrink, P.; Aichner, B.; Henkelmann, B.; Schramm, K.W. Persistent endosulfan sulfate is found with highest abundance among endosulfan I, II, and sulfate in German forest soils. Environ. Pollut. 2015, 206, 661–666. [Google Scholar] [CrossRef]
- Allinson, G.; Allinson, M.; Bui, A.; Zhang, P.; Croatto, G.; Wightwick, A.; Rose, G.; Walters, R. Pesticide and trace metals in surface waters and sediments of rivers entering the Corner Inlet Marine National Park, Victoria, Australia. Environ. Sci. Pollut. Res. Int. 2016, 23, 5881–5891. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Karunarathna, A.; Buckley, N.A.; Manuweera, G.; Sheriff, M.H.; Eddleston, M. Influence of pesticide regulation on acute poisoning deaths in Sri Lanka. Bull. World Health Organ. 2003, 81, 789–798. [Google Scholar] [PubMed]
- Mathanakeerthi, S.; Sadheesh, S.; Nandha kumar, M.; Gowtham, S.; Manoj Kumar, V. Adsorption of endosulfan from aqueous solution using graphene clay matrix (GCM). Mater. Today Proc. 2021, 45, 5665–5671. [Google Scholar] [CrossRef]
- Weber, J.; Halsall, C.J.; Muir, D.; Teixeira, C.; Small, J.; Solomon, K.; Hermanson, M.; Hung, H.; Bidleman, T. Endosulfan, a global pesticide: A review of its fate in the environment and occurrence in the Arctic. Sci. Total Environ. 2010, 408, 2966–2984. [Google Scholar]
- Mudhoo, A.; Bhatnagar, A.; Rantalankila, M.; Srivastava, V.; Sillanpää, M. Endosulfan removal through bioremediation, photocatalytic degradation, adsorption and membrane separation processes: A review. Chem. Eng. Jorn. 2019, 360, 912–928. [Google Scholar] [CrossRef]
- Vera, J.; Fernandes, V.C.; Correia-Sá, L.; Mansilha, C.; Delerue-Matos, C.; Domingues, V.F. Occurrence of Selected Known or Suspected Endocrine-Disrupting Pesticides in Portuguese Surface Waters Using SPME-GC-IT/MS. Separations 2021, 8, 81. [Google Scholar] [CrossRef]
- Paiga, P.; Sousa, S.; Vera, J.; Bitencourt, L.; Vieira, J.; Jorge, S.; Silva, J.G.; Correia, M.; Domingues, V.F.; Delerue-Matos, C. Multi-residue analysis of fifty pesticides in river waters and in wastewaters. Environ. Sci. Pollut. Res. Int. 2021, 28, 66787–66803. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TRAC—Trend Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Lee, H.; Byun, D.-E.; Kim, J.M.; Kwon, J.-H. Desorption of Hydrophobic Organic Chemicals from Fragment-Type Microplastics. Ocean Sci. 2018, 53, 631–639. [Google Scholar] [CrossRef]
- Chen, S.; Tan, Z.; Qi, Y.; Ouyang, C. Sorption of tri-n-butyl phosphate and tris(2-chloroethyl) phosphate on polyethylene and polyvinyl chloride microplastics in seawater. Mar. Pollut. Bull. 2019, 149, 110490. [Google Scholar] [CrossRef]
- Xu, P.; Ge, W.; Chai, C.; Zhang, Y.; Jiang, T.; Xia, B. Sorption of polybrominated diphenyl ethers by microplastics. Mar. Pollut. Bull. 2019, 145, 260–269. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Zhou, X.; Kong, X.; Tao, S.; Xing, B. Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition. Environ. Sci. Technol. 2012, 46, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, J.; Tan, Z.; Gao, Y.; Zhan, Z.; Chen, Q.; Cai, L. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Weber, J. Physicochemical Process for Water Quality Control; J. W. Sons: Hoboken, NJ, USA, 1972; pp. 199–255. [Google Scholar]
- Tang, S.; Lin, L.; Wang, X.; Sun, X.; Yu, A. Adsorption of fulvic acid onto polyamide 6 microplastics: Influencing factors, kinetics modeling, site energy distribution and interaction mechanisms. Chemosphere 2021, 272, 129638. [Google Scholar] [CrossRef] [PubMed]
- Endosulfan. Monograph Annex B 2-: Physical and Chemical Properties Monograph July 1999; III Chapter 2. Available online: http://chm.pops.int/portals/0/docs/from_old_website/documents/meetings/poprc/submissions/Endosulfan_2008/Endosulfan_EU_ADDENDUM_B2_%20July2001-san.pdf (accessed on 5 July 2022).
- Fries, E.; Zarfl, C. Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE). Environ. Sci. Pollut. Res. Int. 2012, 19, 1296–1304. [Google Scholar] [CrossRef]
- Allen, T.; Farley, S.; Draper, J.; Clement, C.; Polidoro, B. Variations in Sorption of Organochlorine Pesticides and PCBs across Six Different Plastic Polymers. J. Environ. Toxicol. Stud. 2018, 2, 1. [Google Scholar]
- Yurtsever, M.; Oz, N.; Aksu, A.; Balkis, N.; Altug, G.; Taskin, O.S. Hydrophobic Pesticide Endosulfan (α + β) and Endrin Sorption on Different Types of Microplastics. J. Chem. Soc. Pak. 2020, 42, 789–797. [Google Scholar]
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption Behavior and Mechanisms of Organic Contaminants to Nano and Microplastics. Molecules 2020, 25, 1827. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Bjorn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Wu, B.; Taylor, C.M.; Knappe, D.R.; Nanny, M.A.; Barlaz, M.A. Factors controlling alkylbenzene sorption to municipal solid waste. Environ. Sci. Technol. 2001, 35, 4569–4576. [Google Scholar] [CrossRef]
- Pascall, M.A.; Zabik, M.E.; Zabik, M.J.; Hernandez, R.J. Uptake of polychlorinated biphenyls (PCBs) from an aqueous medium by polyethylene, polyvinyl chloride, and polystyrene.e films. J. Agric. Food Chem. 2005, 53, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, J.; Zhai, W.; Liu, D.; Zhou, Z.; Wang, P. The influence of polyethylene microplastics on pesticide residue and degradation in the aquatic environment. J. Hazard. Mater. 2020, 394, 122517. [Google Scholar] [CrossRef] [PubMed]
- Puckowski, A.; Cwiek, W.; Mioduszewska, K.; Stepnowski, P.; Bialk-Bielinska, A. Sorption of pharmaceuticals on the surface of microplastics. Chemosphere. 2021, 263, 127976. [Google Scholar] [CrossRef] [PubMed]
- Karapanagioti, H.K.; Klontza, I. Testing phenanthrene distribution properties of virgin plastic pellets and plastic eroded pellets found on Lesvos island beaches (Greece). Mar. Environ. Res. 2008, 65, 283–290. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic Resin Pellets as a transport Medium for Toxic Chemicals in the Marine Environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Edeline, F. L’épuration Physico-Chimique des Eaux: Théorie et Technologie; Cebedoc: Vienne, France, 1998; pp. 251–271. [Google Scholar]
- Gao, X.; Hassan, I.; Peng, Y.; Huo, S.; Ling, L. Behaviors and influencing factors of the heavy metals adsorption onto microplastics: A review. J. Clean. Prod. 2021, 319, 128777. [Google Scholar] [CrossRef]
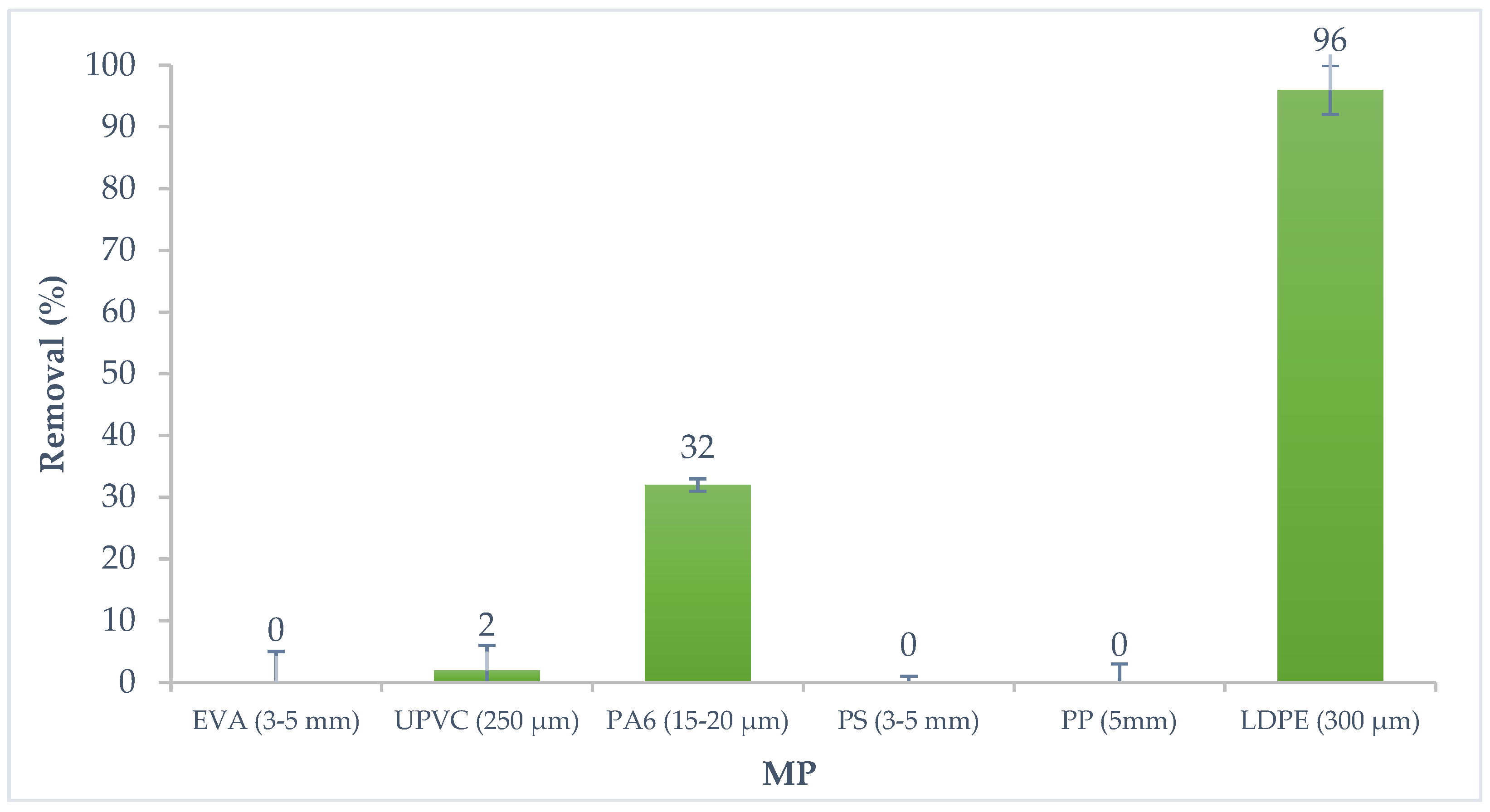


| MP | Particle Size | Particle Shape | Color | MP Experimental Concentration (g·L−1) |
|---|---|---|---|---|
| low-density polyethylene (LDPE) | 300 μm | powder | colorless | 1.00 |
| polyethylene-co-vinyl acetate (EVA) | 3–5 mm | granule | yellow | 1.13 |
| unplasticized polyvinylchloride (UPVC) | 250 μm | powder | colorless | 1.03 |
| Polyamide 6 (PA6) | 15–20 μm | spheroidal | colorless | 1.10 |
| polystyrene (PS) | 3–5 mm | granule | colorless | 1.15 |
| polypropylene (PP) | 5 mm | granule | colorless | 1.01 |
| Organic Solvent | C0 (µg·L−1) | Recovery Ratio (%) | RSD (%) |
|---|---|---|---|
| n-hexane | 150 | 95 | 1 |
| Ethyl acetate | 86 | 1 | |
| Dichloromethane | 68 | 3 |
| Water | Freundlich | Langmuir | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (Dimensionless) | kF (µg·g−1 (L· μg−1)1/n) | χ2Red | SSE | R2adj | qm (µg·g−1) | kL (L· μg−1) | χ2Red | SSE | R2adj | |
| Distilled | 2.67 ± 0.38 | 89.0 ± 14.0 | 609.2 | 3046.2 | 0.955 | 366.4 ± 39.4 | 0.18 ± 0.06 | 1109.9 | 5549.7 | 0.919 |
| Tap | 5.72 ± 3.74 | 89.1 ± 27.1 | 498.1 | 2490.7 | 0.851 | 157.4 ± 22.3 | 0.71 ± 0.79 | 607.4 | 3037.1 | 0.818 |
| Douro river | 4.38 ± 2.35 | 100.0 ± 36.3 | 801.2 | 2403.6 | 0.908 | 246.8 ± 38.4 | 0.26 ± 0.19 | 762.1 | 2286.2 | 0.912 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinho, S.D.; Fernandes, V.C.; Figueiredo, S.A.; Delerue-Matos, C. Study of the Potential Accumulation of the Pesticide Alpha-Endosulfan by Microplastics in Water Systems. Polymers 2022, 14, 3645. https://doi.org/10.3390/polym14173645
Martinho SD, Fernandes VC, Figueiredo SA, Delerue-Matos C. Study of the Potential Accumulation of the Pesticide Alpha-Endosulfan by Microplastics in Water Systems. Polymers. 2022; 14(17):3645. https://doi.org/10.3390/polym14173645
Chicago/Turabian StyleMartinho, Sílvia D., Virgínia Cruz Fernandes, Sónia A. Figueiredo, and Cristina Delerue-Matos. 2022. "Study of the Potential Accumulation of the Pesticide Alpha-Endosulfan by Microplastics in Water Systems" Polymers 14, no. 17: 3645. https://doi.org/10.3390/polym14173645
APA StyleMartinho, S. D., Fernandes, V. C., Figueiredo, S. A., & Delerue-Matos, C. (2022). Study of the Potential Accumulation of the Pesticide Alpha-Endosulfan by Microplastics in Water Systems. Polymers, 14(17), 3645. https://doi.org/10.3390/polym14173645










