Optimization of the Physical Properties of HDPE/PU Blends through Improved Compatibility and Electron Beam Crosslinking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the HDPE/PU Blends
2.3. Characterization of the HDPE/PU Blends
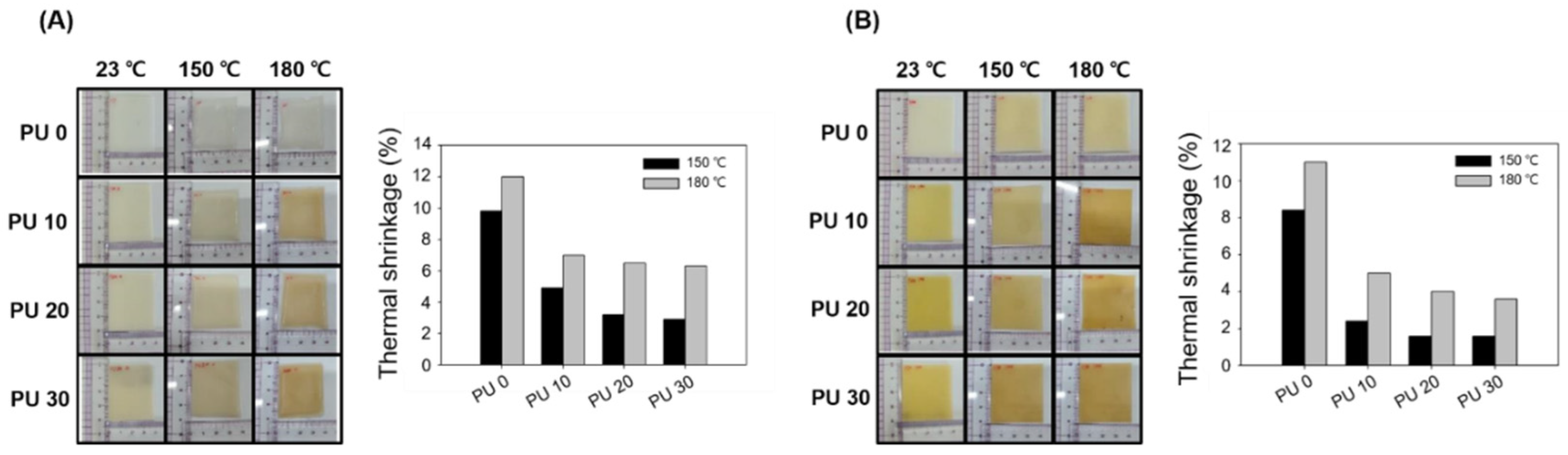
3. Results and Discussion
3.1. Preparation of the HDPE/PU Blends
3.2. Characterization of the HDPE/PU Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, J.-O.; Lim, Y.-M.; Park, J.-S. Improving Thermal Stability and Mechanical Performance of Polypropylene/Polyurethane Blend Prepared by Radiation-Based Techniques. Eur. Polym. J. 2017, 94, 366–375. [Google Scholar] [CrossRef]
- Abbassi, F.; Mbarek, M.; Almoneef, M.; Alimi, K. Photophysical Properties of the PVK-MEH-PPV/PCBM Composite for Organic Solar Cells Application: Synthesis, Characterization and Computational Study. Polymers 2021, 13, 2902. [Google Scholar] [CrossRef] [PubMed]
- Mohd, A.; Baba, N.B.; Umor, M.Z.; Mohamed, R.M. Composites of Polymer Blends and Their Applications Using Natural Fibres: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1068, 012006. [Google Scholar] [CrossRef]
- Barlow, J.W.; Paul, D.R. Polymer Blends and Alloys—A Review of Selected Considerations. Polym. Eng. Sci. 1981, 21, 985–996. [Google Scholar] [CrossRef]
- Paul, D.R.; Barlow, J.W. Polymer Blends. J. Macromol. Sci. Part C 1980, 18, 109–168. [Google Scholar] [CrossRef]
- Covas, J.A.; Pessan, L.A.; Machado, A.V.; Larocca, N.M. Polymer Blend Compatibilization by Copolymers and Functional Polymers. In Encyclopedia of Polymer Blends; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 315–356. ISBN 978-3-527-80524-2. [Google Scholar]
- Wright, D.B.; Patterson, J.P.; Pitto-Barry, A.; Lu, A.; Kirby, N.; Gianneschi, N.C.; Chassenieux, C.; Colombani, O.; O’Reilly, R.K. The Copolymer Blending Method: A New Approach for Targeted Assembly of Micellar Nanoparticles. Macromolecules 2015, 48, 6516–6522. [Google Scholar] [CrossRef]
- Paul, D.R.; Barlow, J.W. A Brief Review of Polymer Blend Technology. In Multiphase Polymers; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1979; Volume 176, pp. 315–335. ISBN 978-0-8412-0457-7. [Google Scholar]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Khanam, P.N.; AlMaadeed, M.A.A. Processing and Characterization of Polyethylene-Based Composites. Adv. Manuf. Polym. Compos. Sci. 2015, 1, 63–79. [Google Scholar] [CrossRef]
- Graziano, A.; Jaffer, S.; Sain, M. Review on Modification Strategies of Polyethylene/Polypropylene Immiscible Thermoplastic Polymer Blends for Enhancing Their Mechanical Behavior. J. Elastomers Plast. 2019, 51, 291–336. [Google Scholar] [CrossRef]
- Ltayef, M.; Almoneef, M.M.; Taouali, W.; Mbarek, M.; Alimi, K. Conception and Theoretical Study of a New Copolymer Basedon MEH-PPV and P3HT: Enhancement of the Optoelectronic Properties for Organic Photovoltaic Cells. Polymers 2022, 14, 513. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Park, J.-S.; Lim, Y.-M. Development of Styrene-Grafted Polyurethane by Radiation-Based Techniques. Materials 2016, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Ojha, N.; Pradhan, N.; Singh, S.; Barla, A.; Shrivastava, A.; Khatua, P.; Rai, V.; Bose, S. Evaluation of HDPE and LDPE Degradation by Fungus, Implemented by Statistical Optimization. Sci. Rep. 2017, 7, 39515. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.L.; Sweeting, O.J. Polyethylene: Preparation, Structure, And Properties. Chem. Rev. 1957, 57, 665–742. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Suzuki, K.-I.; Maeda, S. Enhanced Strain Hardening in Elongational Viscosity for HDPE/Crosslinked HDPE Blend. I. Characteristics of Crosslinked HDPE. J. Appl. Polym. Sci. 2002, 86, 73–78. [Google Scholar] [CrossRef]
- Benham, E.; McDaniel, M. Ethylene Polymers, HDPE. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; ISBN 978-0-471-44026-0. [Google Scholar]
- Khare, N.P.; Seavey, K.C.; Liu, Y.A.; Ramanathan, S.; Lingard, S.; Chen, C.-C. Steady-State and Dynamic Modeling of Commercial Slurry High-Density Polyethylene (HDPE) Processes. Ind. Eng. Chem. Res. 2002, 41, 5601–5618. [Google Scholar] [CrossRef]
- Ronca, S. Chapter 10—Polyethylene. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 247–278. ISBN 978-0-323-35824-8. [Google Scholar]
- Karagöz, İ.; Tuna, Ö. Effect of Melt Temperature on Product Properties of Injection-Molded High-Density Polyethylene. Polym. Bull. 2021, 78, 6073–6091. [Google Scholar] [CrossRef]
- Yilmaz, G.; Ellingham, T.; Turng, L.-S. Improved Processability and the Processing-Structure-Properties Relationship of Ultra-High Molecular Weight Polyethylene via Supercritical Nitrogen and Carbon Dioxide in Injection Molding. Polymers 2017, 10, 36. [Google Scholar] [CrossRef]
- Lee, J.-G.; Jeong, J.-O.; Jeong, S.-I.; Park, J.-S. Radiation-Based Crosslinking Technique for Enhanced Thermal and Mechanical Properties of HDPE/EVA/PU Blends. Polymers 2021, 13, 2832. [Google Scholar] [CrossRef]
- Bengtsson, M.; Gatenholm, P.; Oksman, K. The Effect of Crosslinking on the Properties of Polyethylene/Wood Flour Composites. Compos. Sci. Technol. 2005, 65, 1468–1479. [Google Scholar] [CrossRef]
- Kazemi-Najafi, S.; Englund, K.R. Effect of Highly Degraded High-Density Polyethylene (HDPE) on Processing and Mechanical Properties of Wood Flour-HDPE Composites. J. Appl. Polym. Sci. 2013, 129, 3404–3410. [Google Scholar] [CrossRef]
- Mbarek, M.; Abbassi, F.; Alimi, K. The effect of cross-linking yield of PVK on the vibrational and emissive properties of new copolymer based on vinylcarbazole and phenylene-vinylene units. J. Mol. Struct. 2016, 1120, 125–131. [Google Scholar] [CrossRef]
- Abbassi, F.; Mbarek, M.; Kreher, D.; Alimi, K. Cross linking-dependent properties of PVK-MEH-PPV bi-block copolymer: Vibrational, thermal and optical properties. J. Phys. Chem. Solids 2019, 126, 274–279. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, H.Y.; Lee, S.Y.; Cho, S.O. Effects of Electron Beam Irradiation on Mechanical and Thermal Shrinkage Properties of Boehmite/HDPE Nanocomposite Film. Nanomaterials 2021, 11, 777. [Google Scholar] [CrossRef]
- Gheysari, D.; Behjat, A. Radiation Crosslinking of LDPE and HDPE with 5 and 10 MeV Electron Beams. Eur. Polym. J. 2001, 37, 2011–2016. [Google Scholar] [CrossRef]
- Gheysari, D.; Behjat, A.; Haji-Saeid, M. The Effect of High-Energy Electron Beam on Mechanical and Thermal Properties of LDPE and HDPE. Eur. Polym. J. 2001, 37, 295–302. [Google Scholar] [CrossRef]
- Behnood, A.; Modiri Gharehveran, M. Morphology, Rheology, and Physical Properties of Polymer-Modified Asphalt Binders. Eur. Polym. J. 2019, 112, 766–791. [Google Scholar] [CrossRef]
- Abzan, N.; Kharaziha, M.; Labbaf, S.; Saeidi, N. Modulation of the Mechanical, Physical and Chemical Properties of Polyvinylidene Fluoride Scaffold via Non-Solvent Induced Phase Separation Process for Nerve Tissue Engineering Applications. Eur. Polym. J. 2018, 104, 115–127. [Google Scholar] [CrossRef]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of Polymers: A Review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef]
- Fortelný, I.; Jůza, J. The Effects of Copolymer Compatibilizers on the Phase Structure Evolution in Polymer Blends—A Review. Materials 2021, 14, 7786. [Google Scholar] [CrossRef]
- Resch, J.; Dreier, J.; Bonten, C.; Kreutzbruck, M. Miscibility and Phase Separation in PMMA/SAN Blends Investigated by Nanoscale AFM-IR. Polymers 2021, 13, 3809. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.-C.; Su, C.-C.; Yang, C.-F. Chemical Interaction-Induced Evolution of Phase Compatibilization in Blends of Poly(Hydroxy Ether of Bisphenol-A)/Poly(1,4-Butylene Terephthalate). Materials 2018, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Nara, S.; Oyama, H.T. Effects of Partial Miscibility on the Structure and Properties of Novel High Performance Blends Composed of Poly(p-Phenylene Sulfide) and Poly(Phenylsulfone). Polym. J. 2014, 46, 568–575. [Google Scholar] [CrossRef]
- Samper-Madrigal, M.D.; Fenollar, O.; Dominici, F.; Balart, R.; Kenny, J.M. The Effect of Sepiolite on the Compatibilization of Polyethylene–Thermoplastic Starch Blends for Environmentally Friendly Films. J. Mater. Sci. 2015, 50, 863–872. [Google Scholar] [CrossRef]
- Wu, X.; Wu, C.; Wang, G.; Jiang, P.; Zhang, J. A Crosslinking Method of UHMWPE Irradiated by Electron Beam Using TMPTMA as Radiosensitizer. J. Appl. Polym. Sci. 2013, 127, 111–119. [Google Scholar] [CrossRef]
- Sabet, M.; Savory, R.M.; Hassan, A.; Ratnam, C.T. The Effect of TMPTMA Addition on Electron-Beam Irradiated LDPE, EVA and Blend Properties. Int. Polym. Process. 2013, 28, 386–392. [Google Scholar] [CrossRef]
- Tan, H.; Moet, A.; Hiltner, A.; Baer, E. Thermoreversible Gelation of Atactic Polystyrene Solutions. Macromolecules 1983, 16, 28–34. [Google Scholar] [CrossRef]
- Somarathna, H.M.C.C.; Raman, S.N.; Mohotti, D.; Mutalib, A.A.; Badri, K.H. The Use of Polyurethane for Structural and Infrastructural Engineering Applications: A State-of-the-Art Review. Constr. Build. Mater. 2018, 190, 995–1014. [Google Scholar] [CrossRef]
- Cong, L.; Yang, F.; Guo, G.; Ren, M.; Shi, J.; Tan, L. The Use of Polyurethane for Asphalt Pavement Engineering Applications: A State-of-the-Art Review. Constr. Build. Mater. 2019, 225, 1012–1025. [Google Scholar] [CrossRef]
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer Composite Materials: A Comprehensive Review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Zhang, Y.; Rodrigue, D.; Ait-Kadi, A. High Density Polyethylene Foams. IV. Flexural and Tensile Moduli of Structural Foams. J. Appl. Polym. Sci. 2003, 90, 2139–2149. [Google Scholar] [CrossRef]

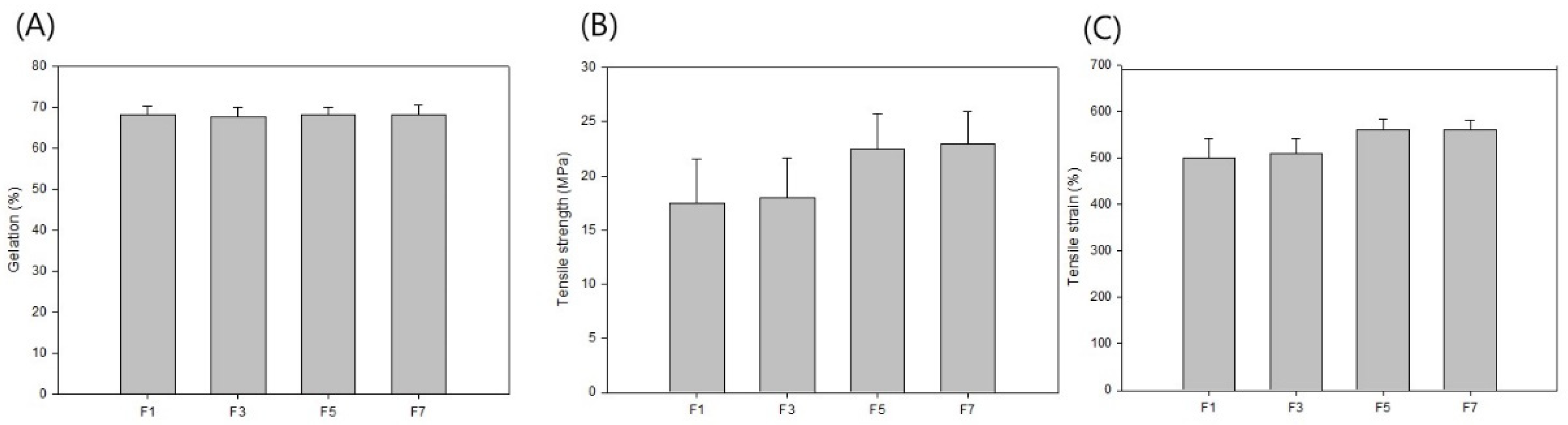
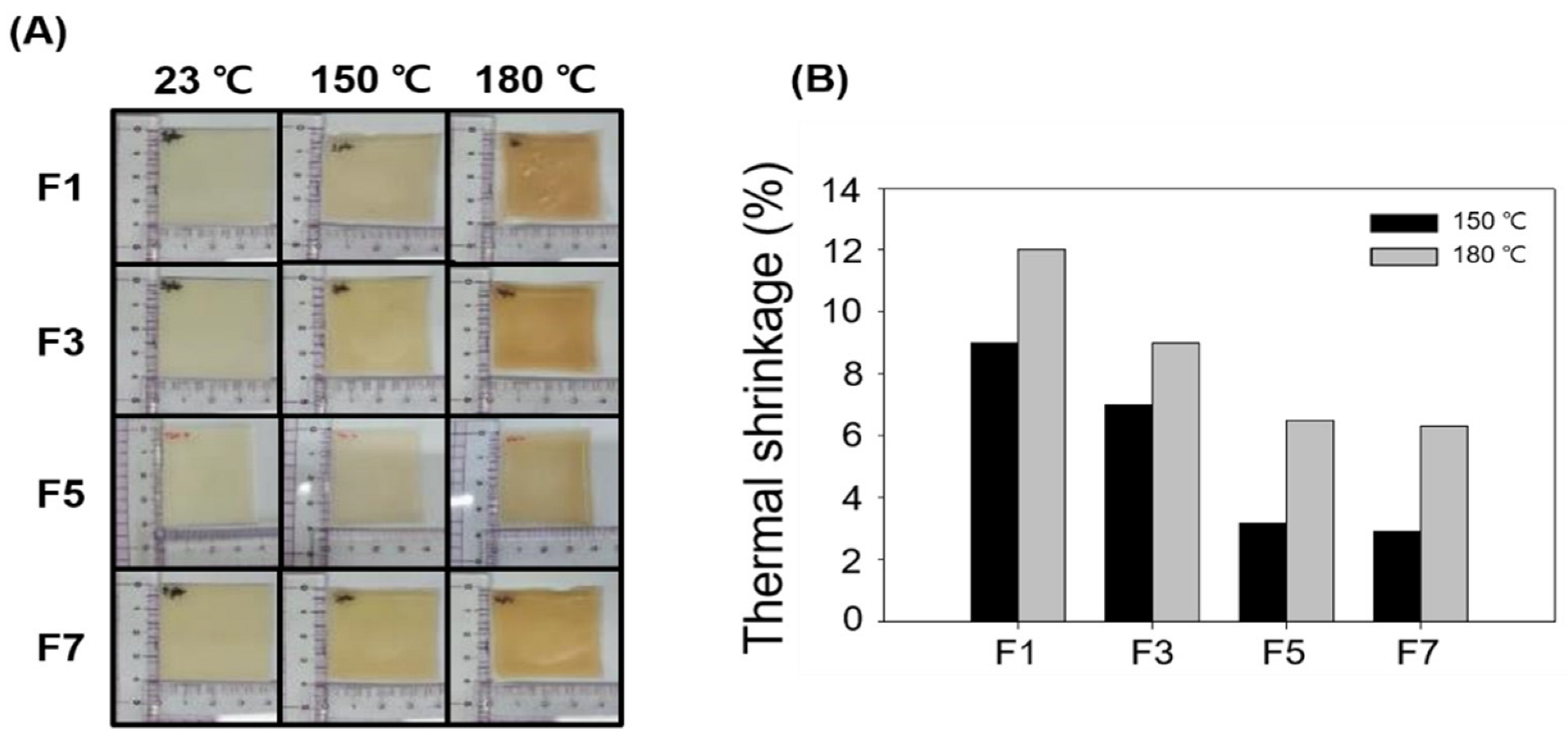

| F1 | F3 | F5 | F7 | |
|---|---|---|---|---|
| HDPE | 100 g | 100 g | 100 g | 100 g |
| PU | 20 phr | 20 phr | 20 phr | 20 phr |
| PE-g-MA | 1 phr | 3 phr | 5 phr | 7 phr |
| TMPTMA | 3 phr | 3 phr | 3 phr | 3 phr |
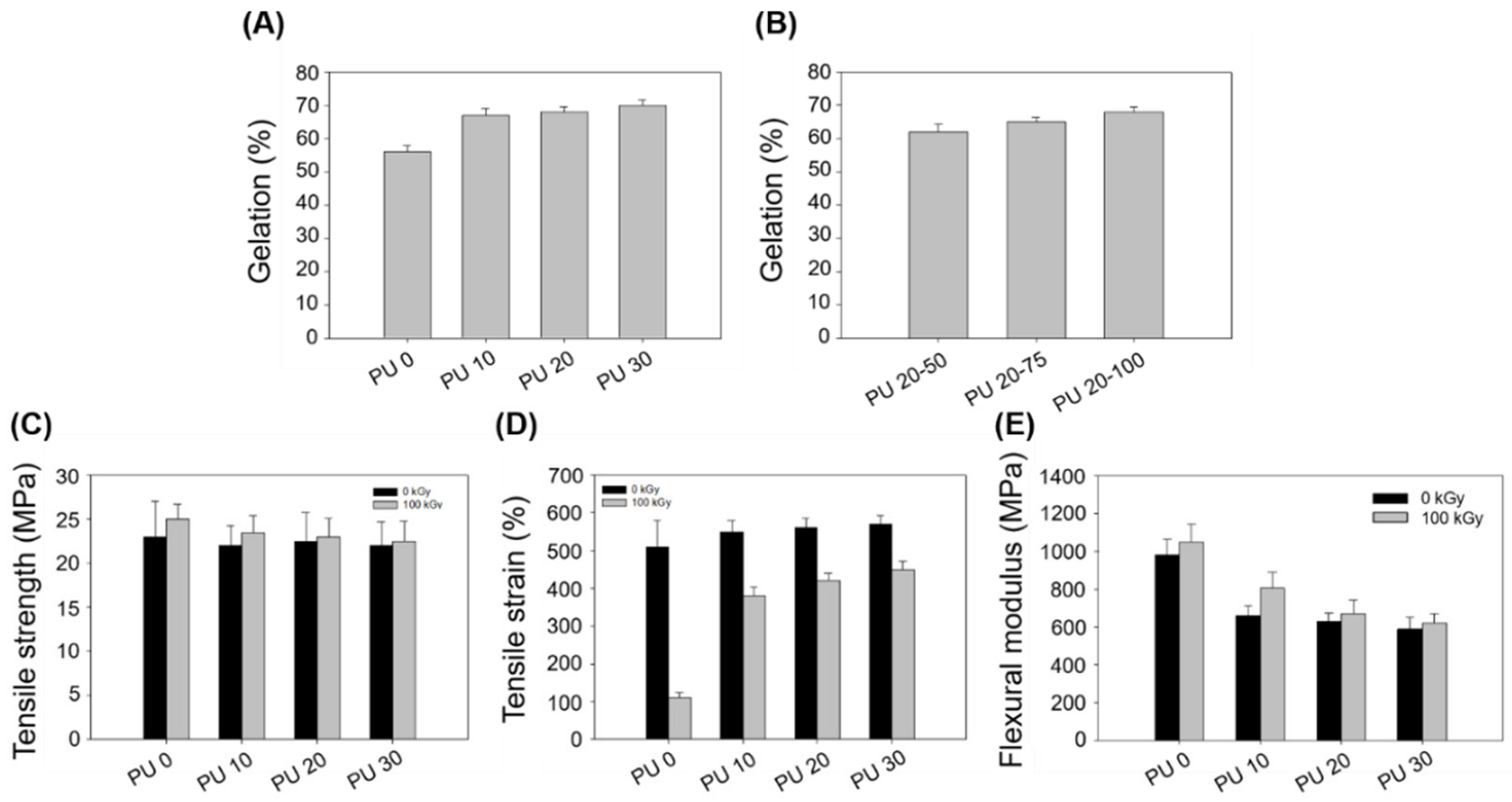
| PU 0 | PU 10 | PU 20 | PU 30 | |
|---|---|---|---|---|
| HDPE | 100 g | 100 g | 100 g | 100 g |
| PU | - | 10 phr | 20 phr | 30 phr |
| PE-g-MA | - | 5 phr | 5 phr | 5 phr |
| TMPTMA | - | 3 phr | 3 phr | 3 phr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-O.; Oh, Y.-H.; Jeong, S.-I.; Park, J.-S. Optimization of the Physical Properties of HDPE/PU Blends through Improved Compatibility and Electron Beam Crosslinking. Polymers 2022, 14, 3607. https://doi.org/10.3390/polym14173607
Jeong J-O, Oh Y-H, Jeong S-I, Park J-S. Optimization of the Physical Properties of HDPE/PU Blends through Improved Compatibility and Electron Beam Crosslinking. Polymers. 2022; 14(17):3607. https://doi.org/10.3390/polym14173607
Chicago/Turabian StyleJeong, Jin-Oh, Yong-Hyeon Oh, Sung-In Jeong, and Jong-Seok Park. 2022. "Optimization of the Physical Properties of HDPE/PU Blends through Improved Compatibility and Electron Beam Crosslinking" Polymers 14, no. 17: 3607. https://doi.org/10.3390/polym14173607
APA StyleJeong, J.-O., Oh, Y.-H., Jeong, S.-I., & Park, J.-S. (2022). Optimization of the Physical Properties of HDPE/PU Blends through Improved Compatibility and Electron Beam Crosslinking. Polymers, 14(17), 3607. https://doi.org/10.3390/polym14173607







