Heavy Metal Ions Trigger a Fluorescent Quenching in DNA–Organic Semiconductor Hybrid Assemblies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Pb2+: 5′-GGTTGGTGTGGTTGG-3′;
- Hg2+: 5′-TTCTTTCTTCCCCTTGTTTGTT-3′;
- Cd2+: 5′-GGGTTCACAGTCCGTT-3′;
- As3+: 5′-ATGCAAACCCTTAAGAAAGTGGTCGTCCAAAAAACCATTG-3′.
2.2. Preparation of Hybrid Assemblies
2.3. Reaction with Heavy Metal Ions
2.4. Exploration of the Conditions for the Detection of Heavy Metal Ions
2.5. Reaction with HNO3
2.6. Detection of Heavy Metal Ions in Actual Samples
2.7. Characterization
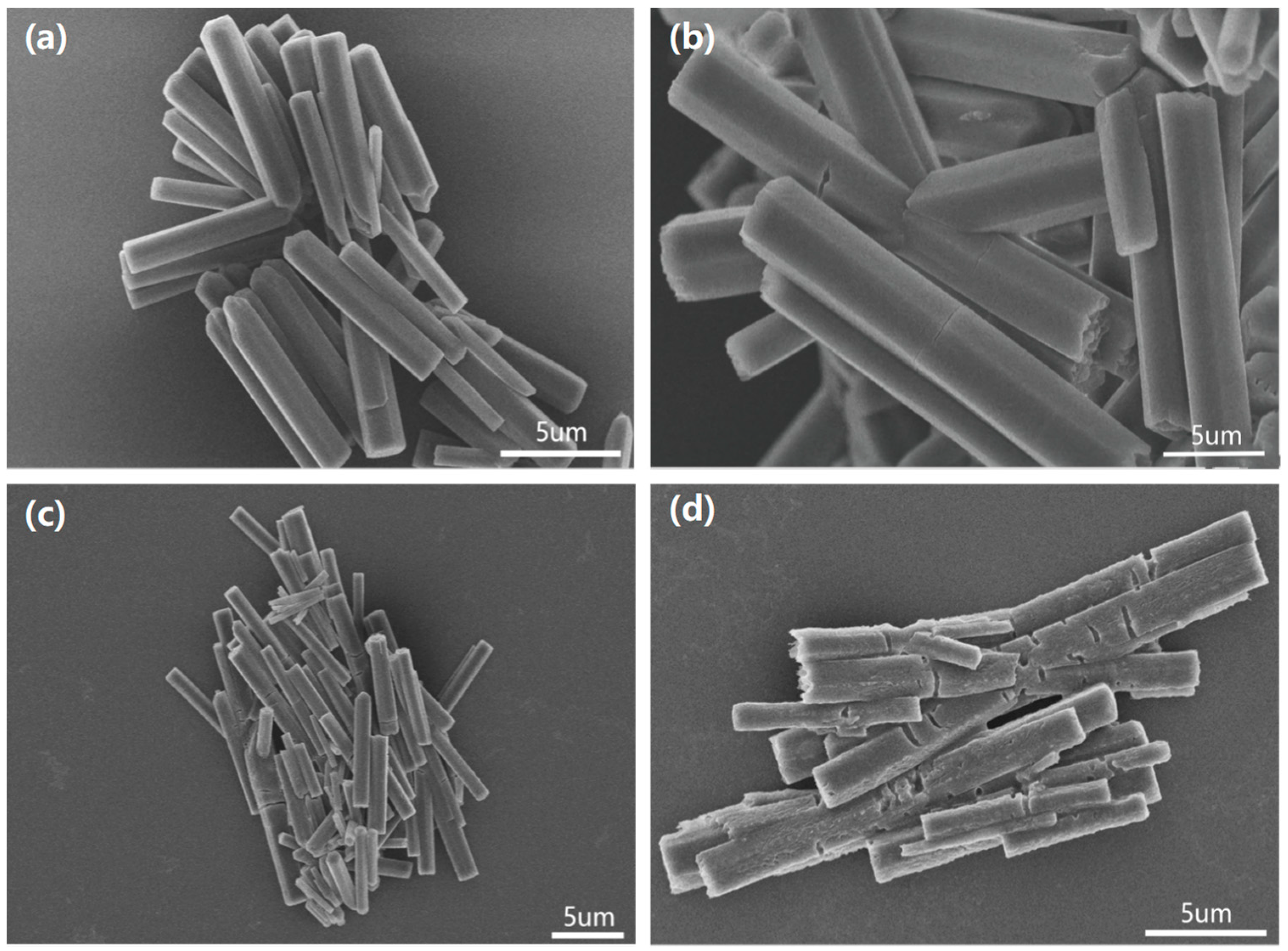
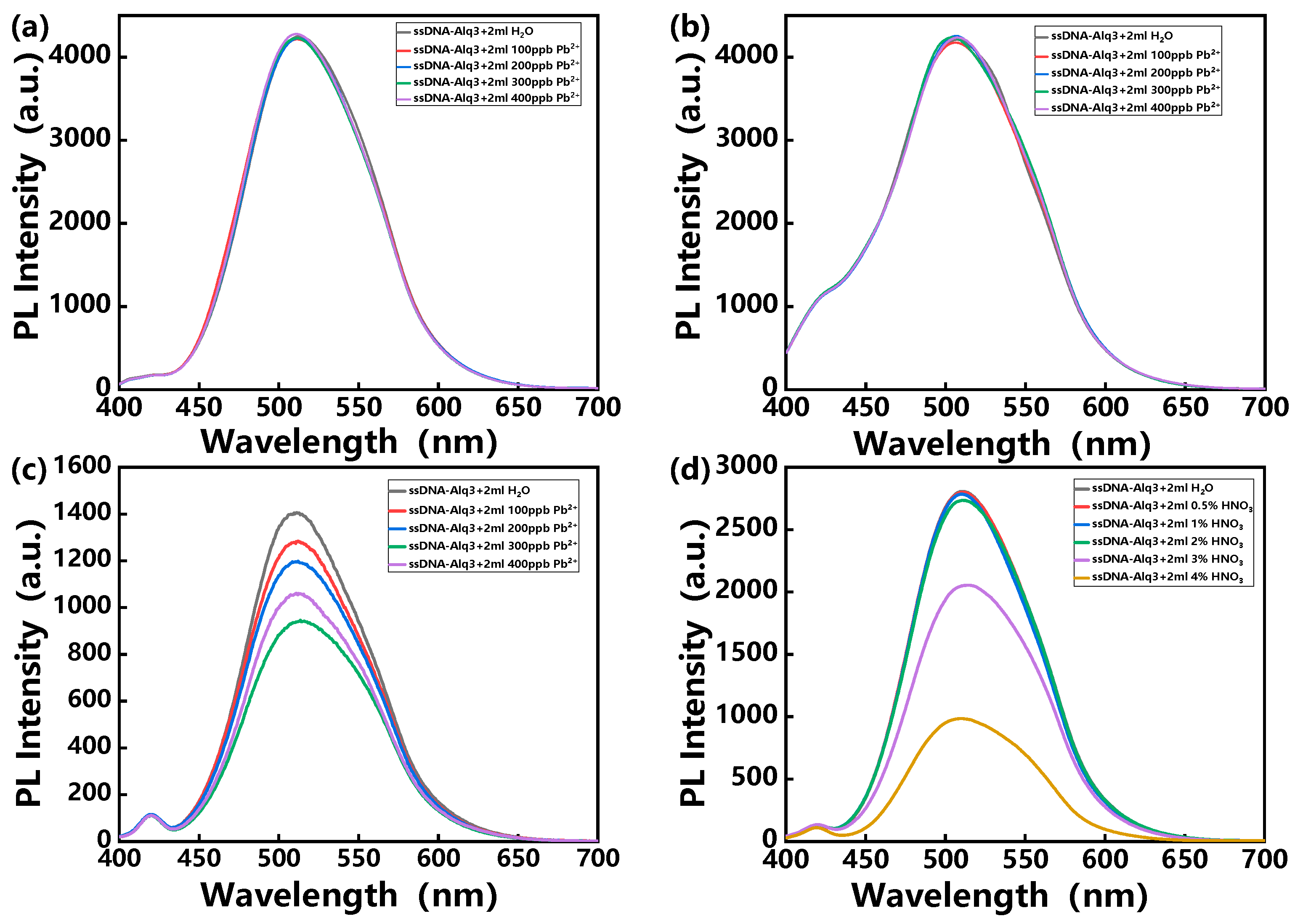
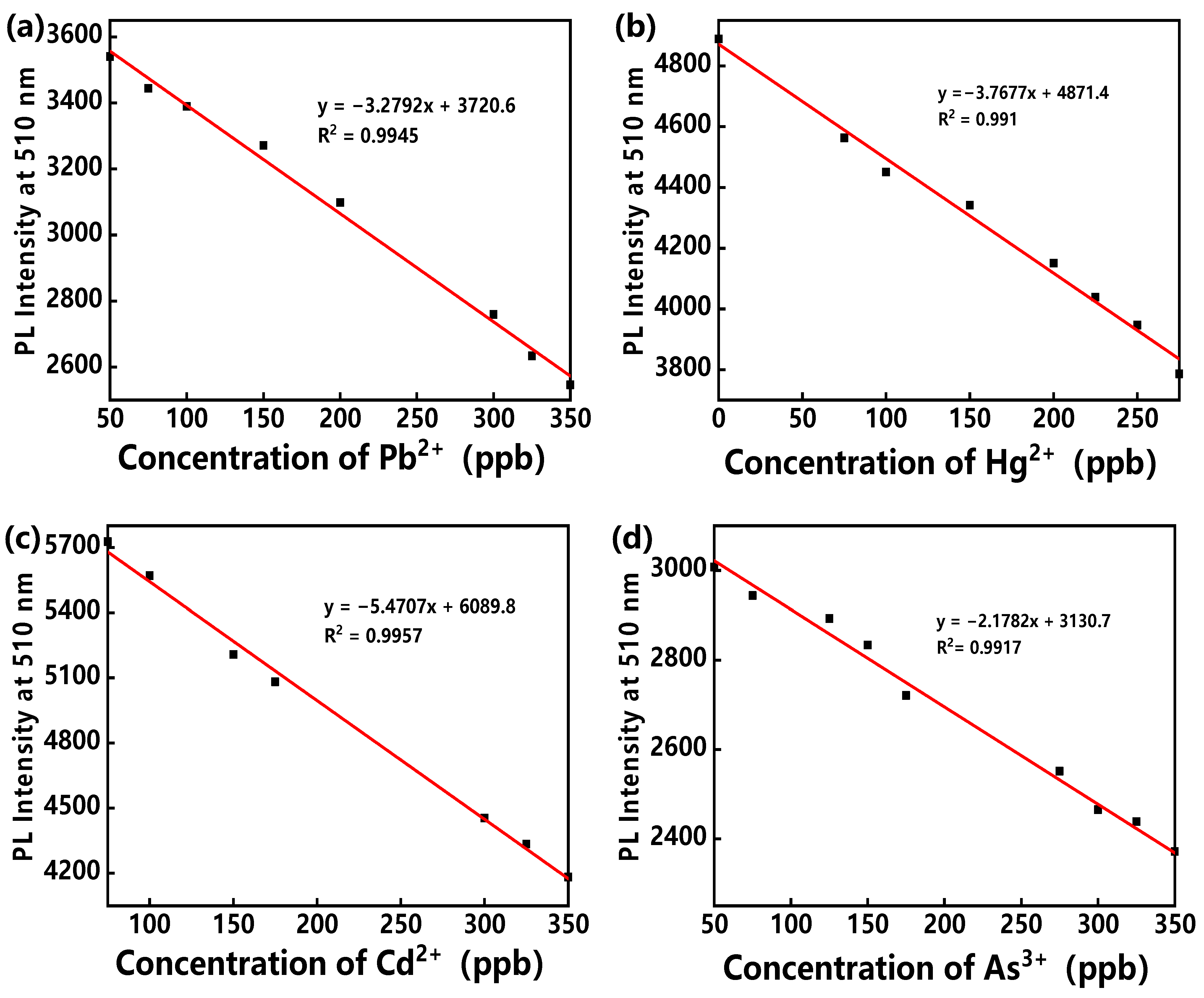
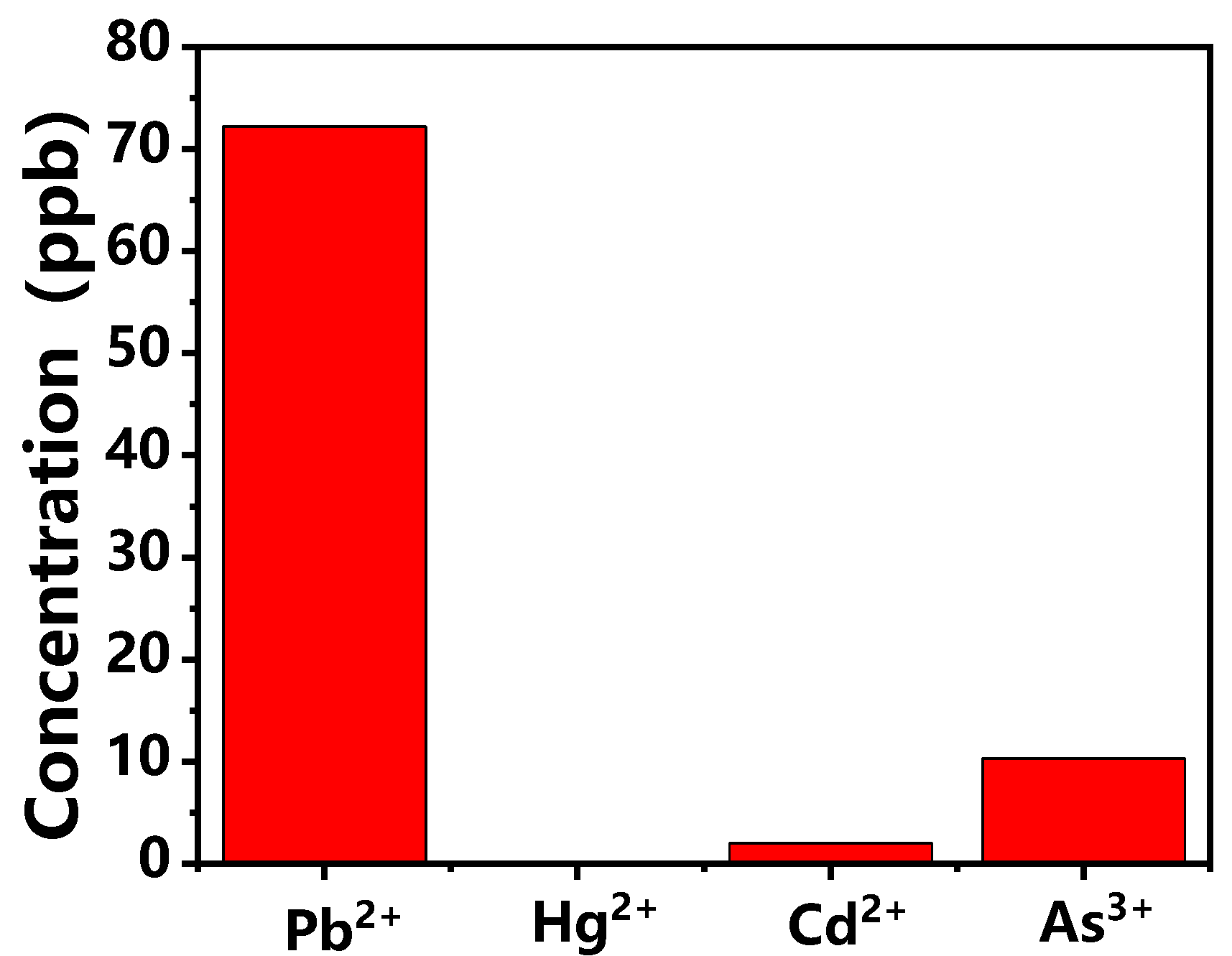
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.L.; Zhai, J.F.; Tian, J.Q.; Luo, Y.L.; Sun, X.P. Carbon nanoparticle for highly sensitive and selective fluorescent detection of mercury (II) ion in aqueous solution. Biosens. Bioelectron. 2011, 26, 4656–4660. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, K.N.; Liu, Q.S.; Xie, M.X. Visual and fluorescent detection of mercury ions by using a dually emissive ratiometric nanohybrid containing carbon dots and CdTe quantum dots. Microchim. Acta 2017, 184, 1199–1206. [Google Scholar] [CrossRef]
- Roxby, D.N.; Rivy, H.; Gong, C.; Gong, X.; Yuan, Z.; Chang, G.E.; Chen, Y.C. Microalgae living sensor for metal ion detection with nanocavity-enhanced photoelectrochemistry. Biosens. Bioelectron. 2020, 165, 112420. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Zhan, Y.; Singh, V.; Hung, T.V.; Nam, N.D. A novel highly efficient and ultrasensitive electrochemical detection of toxic mercury (II) Ions in canned tuna fish and tap water based on a copper metal-organic framework. J. Hazard. Mater. 2020, 399, 123042. [Google Scholar] [CrossRef]
- Hu, J.; Li, Z.; Zhai, C.; Zeng, L.; Zhu, M. Photo-assisted simultaneous electrochemical detection of multiple heavy metal ions with a metal-free carbon black anchored graphitic carbon nitride sensor. Anal. Chim. Acta 2021, 1183, 338951. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Cui, X.; Wang, F.; Ji, H.; Du, X.; Lu, X. GaOOH-modified metal-organic frameworks UiO-66-NH2: Selective and sensitive sensing four heavy-metal ions in real wastewater by electrochemical method. Talanta 2021, 234, 122679. [Google Scholar] [CrossRef]
- Gupta, P.; Rahm, C.E.; Jiang, D.; Gupta, V.K.; Heineman, W.R.; Justin, G.; Alvarez, N.T. Parts per trillion detection of heavy metals in as-is tap water using carbon nanotube microelectrodes. Anal. Chim. Acta 2021, 1155, 338353. [Google Scholar] [CrossRef]
- Kim, E.B.; Imran, M.; Lee, E.H.; Akhtar, M.S.; Ameen, S. Multiple ions detection by field-effect transistor sensors based on ZnO@GO and ZnO@rGO nanomaterials: Application to trace detection of Cr (III) and Cu (II). Chemosphere 2022, 286, 131695. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, J.X.; Wang, J.W.; Liu, Y.; Wang, L.C.; Weerasooriya, R.; Wu, Y.C. Doping ZIF-67 with transition metals results in bimetallic centers for electrochemical detection of Hg(II). Electrochim. Acta 2021, 387, 138539. [Google Scholar] [CrossRef]
- Li, G.; Belwal, T.; Luo, Z.; Li, Y.; Li, L.; Xu, Y.; Lin, X. Direct detection of Pb2+ and Cd2+ in juice and beverage samples using PDMS modified nanochannels electrochemical sensors. Food Chem. 2021, 386, 129632. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Nga, D.T.N.; Thu, V.T.; Piro, B.; Truong, T.N.P.; Yen, P.T.H.; Le, G.H.; Hung, L.Q.; Vu, T.A.; Ha, V.T.T. Novel nanoscale Yb-MOF used as highly efficient electrode for simultaneous detection of heavy metal ions. J. Mater. Sci. 2021, 56, 8172–8185. [Google Scholar] [CrossRef]
- Liu, S.; Ni, L.; Chen, W.; Wang, J.; Ma, F. Analysis of lead forms and transition in agricultural soil by nano-fluorescence method. J. Hazard. Mater. 2020, 389, 121469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.M.; Bao, B.; Sun, T.; Xue, B. Depolymerized phosphorus-doped polymeric carbon nitride: A mercury (II) ion fluorescent probe. Ceram. Int. 2021, 47, 24115–24120. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, M.; Wang, D.; Chen, K.; Lin, P.; Ge, Y.; Liu, W.; Wu, J. Highly selective fluorescence probe with peptide backbone for imaging mercury ions in living cells based on aggregation-induced emission effect. J. Hazard. Mater. 2021, 415, 125712. [Google Scholar] [CrossRef]
- Sharma, R.; Haldar, U.; Turabee, M.H.; Lee, H. Recyclable macromolecular thermogels for Hg(II) detection and separation via sol-gel transition in complex aqueous environments. J. Hazard. Mater. 2021, 410, 124625. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, Z.; Fang, H.; Zhang, Q.; Zhou, Q.; Chen, Z.; Yang, H.; Wang, F. Au sputtered paper chromatography tandem Raman platform for sensitive detection of heavy metal ions. ACS Sens. 2020, 5, 1455–1464. [Google Scholar] [CrossRef]
- Anusuya, T.; Kumar, V.; Kumar, V. Hydrophilic graphene quantum dots as turn-off fluorescent nanoprobes for toxic heavy metal ions detection in aqueous media. Chemosphere 2021, 282, 131019. [Google Scholar] [CrossRef]
- Kamal, S.; Yang, C.K. Silver enriched silver phosphate microcubes as an efficient recyclable SERS substrate for the detection of heavy metal ions. J. Colloid Interface Sci. 2022, 605, 173–181. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q.; Qi, L.; Lin, J.M.; Yu, L. Liquid crystal-based sensing platform for detection of Pb2+ assisted by DNAzyme and rolling circle amplification. J. Hazard. Mater. 2020, 400, 123218. [Google Scholar] [CrossRef]
- Yu, Y.; Naik, S.S.; Oh, Y.; Theerthagiri, J.; Lee, S.; Choi, M.Y. Lignin-mediated green synthesis of functionalized gold nanoparticles via pulsed laser technique for selective colorimetric detection of lead ions in aqueous media. J. Hazard. Mater. 2021, 420, 126585. [Google Scholar] [CrossRef]
- Berlina, A.N.; Komova, N.S.; Zherdev, A.V.; Dzantiev, B.B. Combination of phenylboronic acid and oligocytosine for selective and specific detection of lead(ii) by lateral flow test strip. Anal. Chim. Acta 2021, 1155, 338318. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Pang, J.; Zhang, F.; Chai, F. Silver nanoparticles for colorimetric detection and discrimination of mercury ions in lake water. ChemistrySelect 2021, 6, 6077–6082. [Google Scholar] [CrossRef]
- Zeng, H.; Tang, Y.; Zou, W.; Wang, C.; Tao, H.; Wu, Y. V6O13 Nanobelts for simultaneous detection of Cd(II) and Pb(II) in water. ACS Appl. Nano Mater. 2021, 4, 4654–4664. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Zhang, Y.; Zhou, X.; Hu, Z.; Liao, X.; Sheng, B.; Yuan, K.; Wu, X.; Cai, H.; et al. Colorimetric and SERS dual-mode sensing of mercury (II) based on controllable etching of Au@Ag core/shell nanoparticles. Sens. Actuators B Chem. 2021, 330, 129364. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Housaindokht, M.R.; Verdian, A. A low-cost paper-based aptasensor for simultaneous trace-level monitoring of mercury (II) and silver (I) ions. Anal. Biochem. 2020, 597, 113689. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, P.; Xiong, W.; Qi, B.; Shi, R.; Xiang, D.; Zhai, K. Simultaneous detection of mercury (II), lead (II) and silver (I) based on fluorescently labelled aptamer probes and graphene oxide. Environ. Technol. 2021, 42, 3065–3072. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Jang, C.H. Label-free liquid crystal-based detection of As(III) ions using ssDNA as a recognition probe. Microchem. J. 2020, 156, 104834. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, M.; Chen, Z.; Deng, Z.; Liu, N.; Fan, J.; Zhang, W. A fluorescence resonance energy transfer probe based on DNA-modified upconversion and gold nanoparticles for detection of lead ions. Front. Chem. 2020, 8, 238. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, T.; Hu, Q.; Zhong, L.; Wang, X.; Wan, H.; Wang, P. In-situ detection of cadmium with aptamer functionalized gold nanoparticles based on smartphone-based colorimetric system. Talanta 2020, 208, 120231. [Google Scholar] [CrossRef]
- Chen, T.; Wang, H.; Wang, Z.; Tan, M. Construction of time-resolved luminescence nanoprobe and its application in as (III) detection. Nanomaterials 2020, 10, 551. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jun, H.; Kim, J. Mercury biosensors: Polydiacetylene–liposome microarrays for selective and sensitive mercury (II) detection. Adv. Mater. 2009, 21, 367. [Google Scholar]
- Li, D.; Wieckowska, A.; Willner, I. Optical analysis of Hg2+ ions by oligonucleotide-gold-nanoparticle hybrids and DNA-based machines. Angew. Chem. Int. Ed. 2008, 47, 3927. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Park, J.H.; Back, S.H.; Feng, Y.; Cui, C.; Jin, L.Y.; Ahn, D.J. Mercury ion-DNA specificity triggers a distinctive photoluminescence depression in organic semiconductor probes guided with a thymine-rich oligonucleotide sequence. Nanoscale 2018, 10, 17540–17545. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Park, D.H.; Ahn, D.J. Organic semiconductor-DNA hybrid assemblies. Adv. Mater. 2020, 32, 2002213. [Google Scholar] [CrossRef]
- Cui, C.; Park, D.H.; Kim, J.; Joo, J.; Ahn, D.J. Oligonucleotide assisted light-emitting Alq3 microrods: Energy transfer effect with fluorescent dyes. Chem. Commun. 2013, 49, 5360–5362. [Google Scholar] [CrossRef]
- Mcnaught, A.D.; Wilkinson, A. IUPAC (1997) Compendium of Chemical Terminology: The Gold Book, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; p. 839. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Feng, Y.; Yi, T.; Piao, Y.; Park, D.H.; Cui, L.; Cui, C. Heavy Metal Ions Trigger a Fluorescent Quenching in DNA–Organic Semiconductor Hybrid Assemblies. Polymers 2022, 14, 3591. https://doi.org/10.3390/polym14173591
Li X, Feng Y, Yi T, Piao Y, Park DH, Cui L, Cui C. Heavy Metal Ions Trigger a Fluorescent Quenching in DNA–Organic Semiconductor Hybrid Assemblies. Polymers. 2022; 14(17):3591. https://doi.org/10.3390/polym14173591
Chicago/Turabian StyleLi, Xianyang, Yuhui Feng, Tao Yi, Yan Piao, Dong Hyuk Park, Longzhen Cui, and Chunzhi Cui. 2022. "Heavy Metal Ions Trigger a Fluorescent Quenching in DNA–Organic Semiconductor Hybrid Assemblies" Polymers 14, no. 17: 3591. https://doi.org/10.3390/polym14173591
APA StyleLi, X., Feng, Y., Yi, T., Piao, Y., Park, D. H., Cui, L., & Cui, C. (2022). Heavy Metal Ions Trigger a Fluorescent Quenching in DNA–Organic Semiconductor Hybrid Assemblies. Polymers, 14(17), 3591. https://doi.org/10.3390/polym14173591






