Advanced Dual−Function Hollow Copper−Sulfide−Based Polyimide Composite Window Film Combining Near−Infrared Thermal Shielding and Organic Pollutants’ Photodegradation
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabrication of Cu NP Composite PI
2.2. Preparation of Cu2−xS NP Composite PI
2.3. Photocatalytic Test
2.4. Characterization
3. Results and Discussion
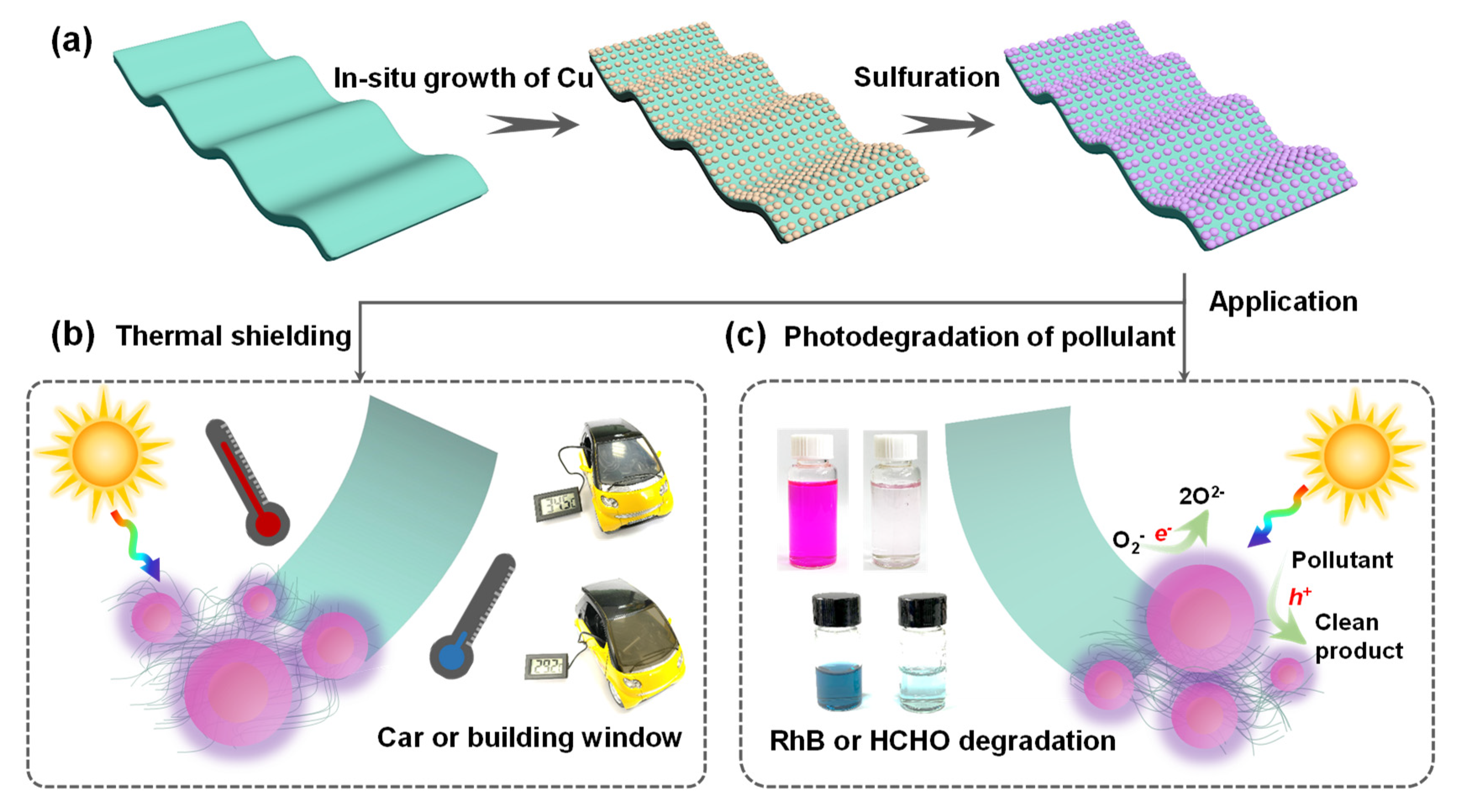
3.1. In Situ Growth of Cu2−xS NPs on PI Films
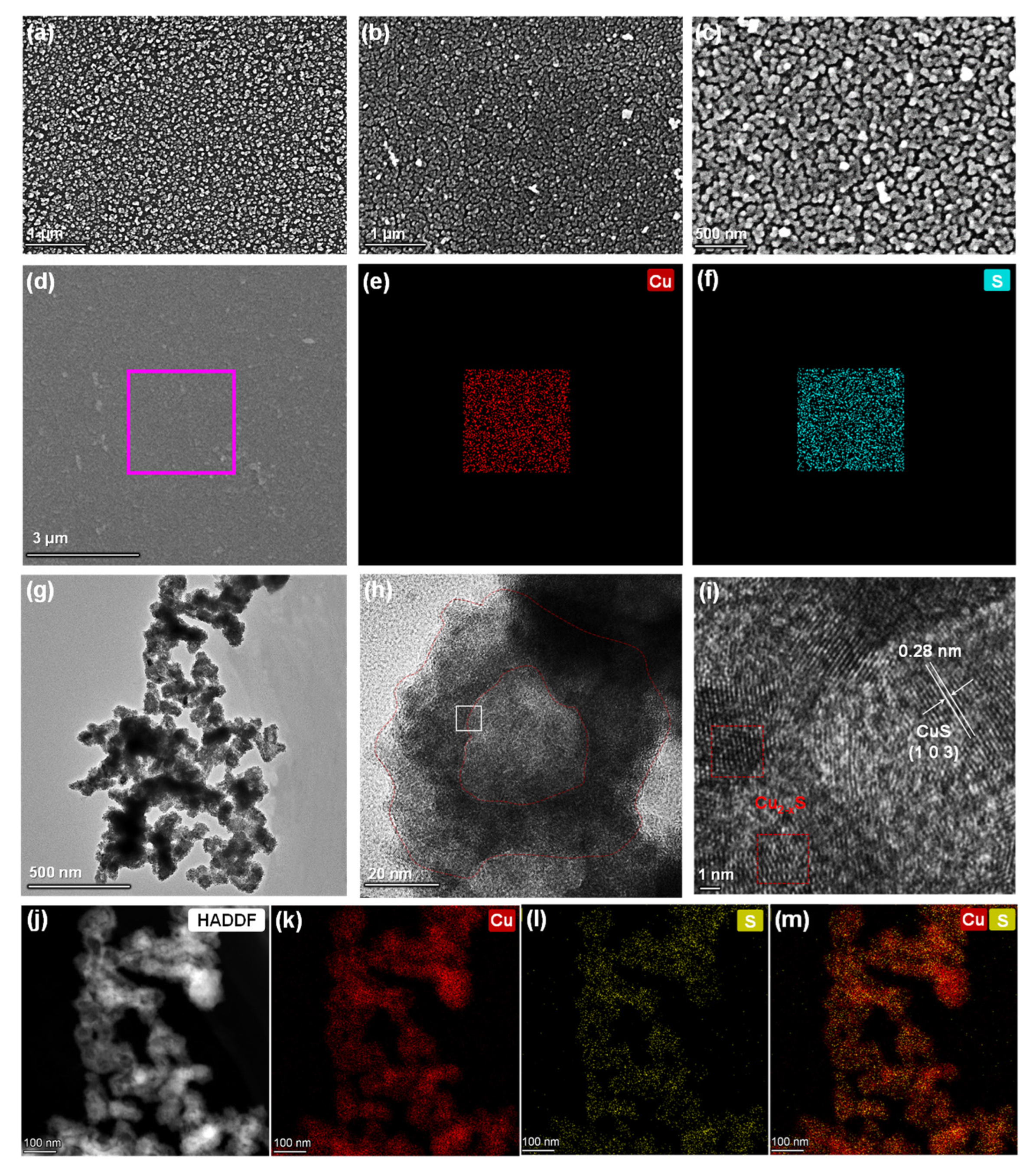
3.2. Morphology and Structure Observations of Cu2−xS/PI Film
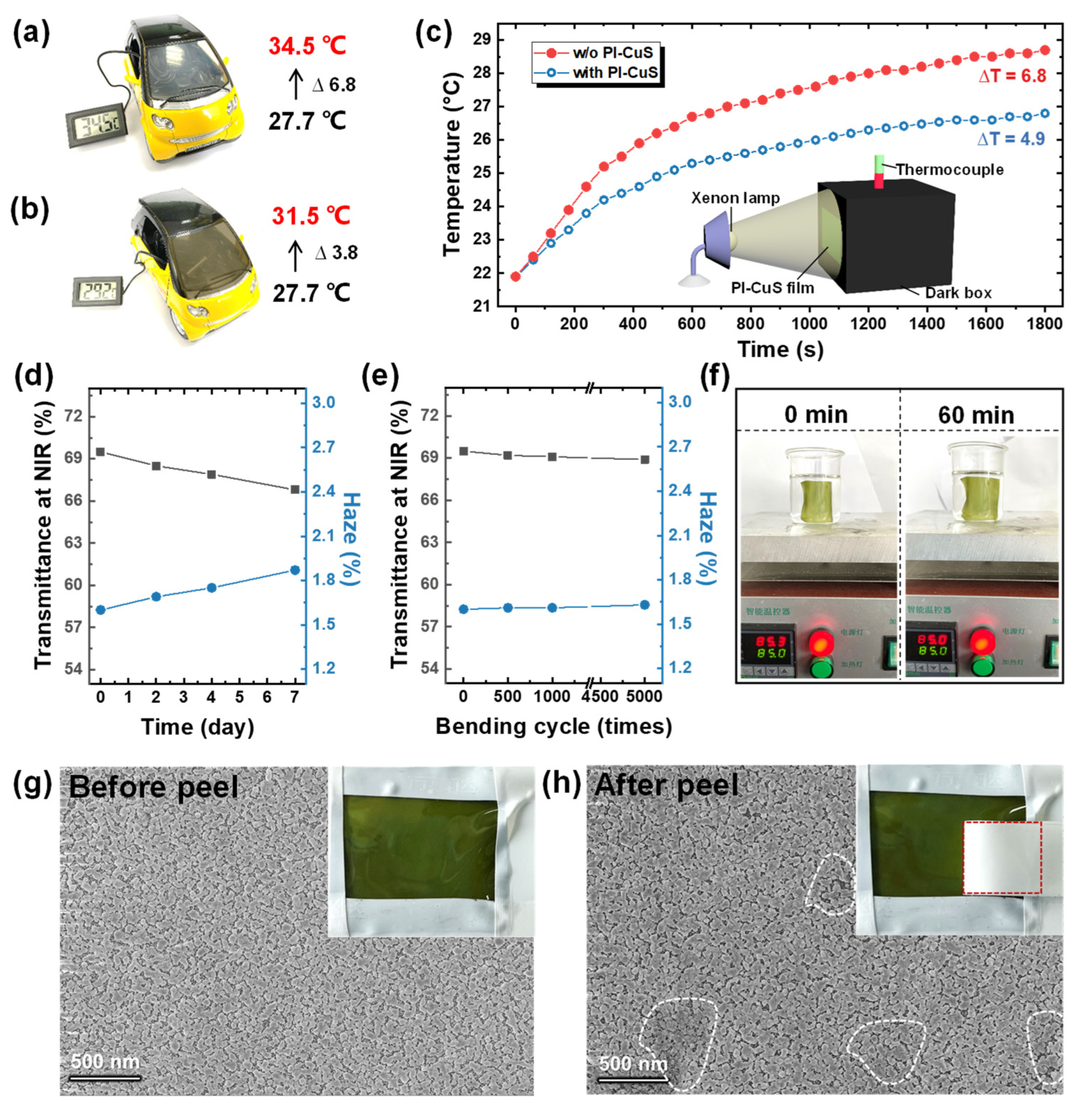
3.3. Thermal−Shielding Performance Evaluation
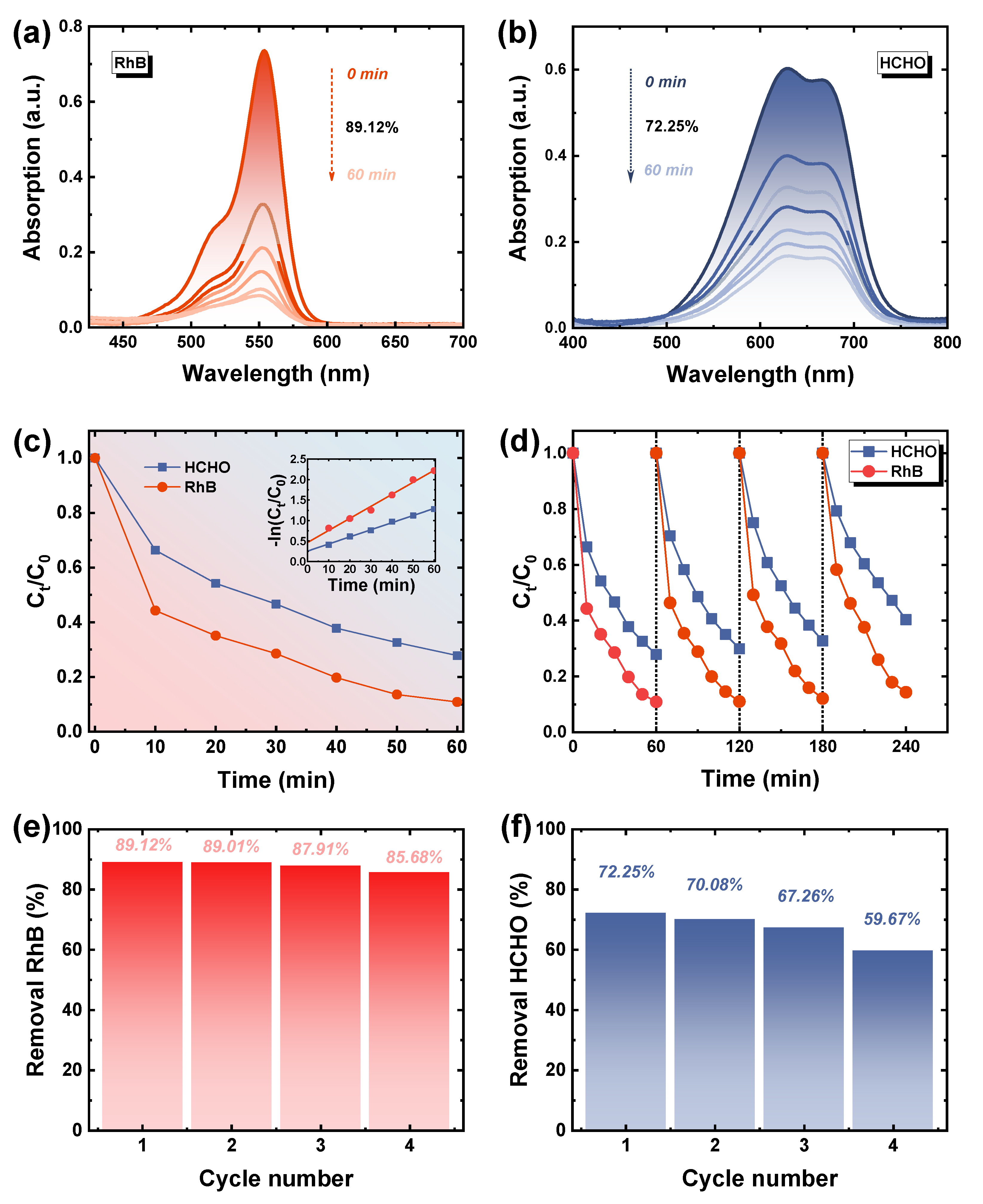
3.4. HCHO Removal Using Cu2−xS/PI Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahidi, A.; Sciarretta, A. Energy saving potentials of connected and automated vehicles. Transport. Res. C−Emerg. 2018, 95, 822–843. [Google Scholar] [CrossRef]
- Asefi, G.; Habibollahzade, A.; Ma, T.; Houshfar, E.; Wang, R. Thermal management of building−integrated photovoltaic/thermal systems: A comprehensive review. Sol. Energy 2021, 216, 188–210. [Google Scholar] [CrossRef]
- Aburas, M.; Soebarto, V.; Williamson, T.; Liang, R.; Ebendorff−Heidepriem, H.; Wu, Y. Thermochromic smart window technologies for building application: A review. Appl. Energy 2019, 255, 113522. [Google Scholar] [CrossRef]
- Smalyukh, I.I. Thermal management by engineering the alignment of nanocellulose. Adv. Mater. 2021, 33, 2001228. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Koch, C.; Wu, Y. Building information modelling based building energy modelling: A review. Appl. Energy 2019, 238, 320–343. [Google Scholar] [CrossRef]
- Park, K.; Jin, S.; Kim, G. Transparent window film with embedded nano−shades for thermoregulation. Consstr. Build. Mater. 2021, 269, 121280. [Google Scholar] [CrossRef]
- Lambin, P.; Liubimau, A.; Bychanok, D.; Vitale, L.; Kuzhir, P. Thermal and electromagnetic properties of polymer holey structures produced by additive manufacturing. Polymers 2020, 12, 2892. [Google Scholar] [CrossRef]
- Song, M.; Jiang, J.; Qin, H.; Ren, X.; Jiang, F. Flexible and super thermal insulating cellulose nanofibril/emulsion composite aerogel with quasi−closed pores. ACS Appl. Mater. Interfaces 2020, 12, 45363–45372. [Google Scholar] [CrossRef]
- Connelly, K.; Wu, Y.; Chen, J.; Lei, Y. Design and development of a reflective membrane for a novel Building Integrated Concentrating Photovoltaic (BICPV) ‘Smart Window’ system. Appl. Energy 2016, 182, 331–339. [Google Scholar] [CrossRef]
- Center, R.R.D. Reference Solar Spectral Irradiance: ASTM G−173; National Renewable Energy Laboratory: Golden, CO, USA, 2009; Volume 21, p. 2018. [Google Scholar]
- Xiang, B.; Zhang, J. A new member of solar heat−reflective pigments: BaTiO3 and its effect on the cooling properties of ASA (acrylonitrile−styrene−acrylate copolymer). Sol. Energy Mater. Sol. Cells 2018, 180, 67–75. [Google Scholar] [CrossRef]
- Qi, Y.; Xiang, B.; Zhang, J. Effect of titanium dioxide (TiO2) with different crystal forms and surface modifications on cooling property and surface wettability of cool roofing materials. Sol. Energy Mater. Sol. Cells 2017, 172, 34–43. [Google Scholar] [CrossRef]
- Ding, C.; Han, A.; Ye, M.; Zhang, Y.; Yao, L.; Yang, J. Synthesis and characterization of a series of new green solar heat−reflective pigments: Cr−doped BiPO4 and its effect on the aging resistance of PMMA (Poly (methyl methacrylate)). Sol. Energy Mater. Sol. Cells 2019, 191, 427–436. [Google Scholar] [CrossRef]
- Radhika, S.P.; Sreeram, K.J.; Unni Nair, B. Mo−doped cerium gadolinium oxide as environmentally sustainable yellow pigments. ACS Sustain. Chem. Eng. 2014, 2, 1251–1256. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Tang, H.; Zhou, Z.; Zhang, D.; Ye, L.; Zhao, D. Recent advances in the development of radiative sky cooling inspired from solar thermal harvesting. Nano Energy 2021, 81, 105611. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Liu, Y.; Wu, W.; Xu, S. Synthesis of Sb−doped SnO2 (ATO) hollow microspheres and its application in photo−thermal shielding coating. Prog. Org. Coat. 2019, 136, 105229. [Google Scholar] [CrossRef]
- Khanyile, B.; Madiba, I.; Mtshali, C.; Mabakachaba, B.; Moloi, S.; Nkosi, M.; Maaza, M. Effect of the bottom layer thickness on the structural and optical phase transition properties of V2O5/V/V2O5 thin films. Mater. Today 2022, 53, 454–461. [Google Scholar] [CrossRef]
- Wen, R.−T.; Granqvist, C.G.; Niklasson, G.A. Eliminating degradation and uncovering ion−trapping dynamics in electrochromic WO3 thin films. Nat. Mater. 2015, 14, 996–1001. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Z.; Ye, H.; Xu, X.; Wu, X.; Wang, J. Performance analyses of building energy on phase transition processes of VO2 windows with an improved model. Appl. Energy 2015, 159, 502–508. [Google Scholar] [CrossRef]
- Sun, H.; Xie, Z.; Ju, C.; Hu, X.; Yuan, D.; Zhao, W.; Shui, L.; Zhou, G. Dye−doped electrically smart windows based on polymer−stabilized liquid crystal. Polymers 2019, 11, 694. [Google Scholar] [CrossRef]
- Sousa−Castillo, A.; Ameneiro−Prieto, Ó.; Comesaña−Hermo, M.; Yu, R.; Vila−Fungueiriño, J.M.; Pérez−Lorenzo, M.; Rivadulla, F.; García de Abajo, F.J.; Correa−Duarte, M.A. Hybrid plasmonic nanoresonators as efficient solar heat shields. Nano Energy 2017, 37, 118–125. [Google Scholar] [CrossRef]
- Yang, S.−G.; Liu, J.−C.; Yang, Y.; Fang, Q.; Rong, C.; Gan, J.−P. Experimental investigation of rupture and dispersion characteristics of float glass subjected to vented explosion loads of methane−air mixtures. Int. J. Impact Eng. 2020, 144, 103651. [Google Scholar] [CrossRef]
- Longcore, T.; Rich, C. Ecological light pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Harima, T.; Nagahama, T. Evaluation methods for retroreflectors and quantitative analysis of near−infrared upward reflective solar control window film—Part I: Theory and evaluation methods. Sol. Energy 2017, 148, 177–192. [Google Scholar] [CrossRef]
- Chao, L.; Bao, L.; Wei, W.; Tegus, O. A review of recent advances in synthesis, characterization and NIR shielding property of nanocrystalline rare−earth hexaborides and tungsten bronzes. Sol. Energy 2019, 190, 10–27. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Silas, K.; Ghani, W.A.W.A.K.; Choong, T.S.; Rashid, U. Carbonaceous materials modified catalysts for simultaneous SO2/NOx removal from flue gas: A review. Catal. Rev. 2019, 61, 134–161. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.−H.; Deep, A. Photocatalytic mineralization of hydrogen sulfide as a dual−phase technique for hydrogen production and environmental remediation. Appl. Catal. B−Environ. 2019, 259, 118025. [Google Scholar] [CrossRef]
- Tang, X.; Bai, Y.; Duong, A.; Smith, M.T.; Li, L.; Zhang, L. Formaldehyde in China: Production, consumption, exposure levels, and health effects. Environ. Int. 2009, 35, 1210–1224. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, J.; Si, Y. Gradient porous structured MnO2−nonwoven composite: A binder−free polymeric air filter for effective room−temperature formaldehyde removal. Polymers 2022, 14, 2504. [Google Scholar] [CrossRef]
- Ye, J.; Zhou, M.; Le, Y.; Cheng, B.; Yu, J. Three−dimensional carbon foam supported MnO2/Pt for rapid capture and catalytic oxidation of formaldehyde at room temperature. Appl. Catal. B−Environ. 2020, 267, 118689. [Google Scholar] [CrossRef]
- Hu, F.; Peng, Y.; Chen, J.; Liu, S.; Song, H.; Li, J. Low content of CoOx supported on nanocrystalline CeO2 for toluene combustion: The importance of interfaces between active sites and supports. Appl. Catal. B−Environ. 2019, 240, 329–336. [Google Scholar] [CrossRef]
- Huang, Y.; Long, B.; Tang, M.; Rui, Z.; Balogun, M.−S.; Tong, Y.; Ji, H. Bifunctional catalytic material: An ultrastable and high−performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation. Appl. Catal. B−Environ. 2016, 181, 779–787. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, L.; Jiang, D. Surface oxygen vacancies on Co3O4 mediated catalytic formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2016, 6, 3845–3853. [Google Scholar] [CrossRef]
- Ye, J.; Cheng, B.; Yu, J.; Ho, W.; Wageh, S.; Al−Ghamdi, A.A. Hierarchical Co3O4−NiO hollow dodecahedron−supported Pt for room−temperature catalytic formaldehyde decomposition. Chem. Eng. J. 2022, 430, 132715. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Zhang, C.; Chen, X.; Liu, C.; Weng, W.; Shan, W.; He, H. A simple strategy to improve Pd dispersion and enhance Pd/TiO2 catalytic activity for formaldehyde oxidation: The roles of surface defects. Appl. Catal. B−Environ. 2021, 282, 119540. [Google Scholar] [CrossRef]
- Zhang, S.; Zhuo, Y.; Ezugwu, C.I.; Wang, C.−C.; Li, C.; Liu, S. Synergetic molecular oxygen activation and catalytic oxidation of formaldehyde over defective MIL−88B (Fe) nanorods at room temperature. Environ. Sci. Technol. 2021, 55, 8341–8350. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Gu, B.; Wei, X.; Xu, G.; Wang, X.; Swihart, M.T.; Yong, K.−T. Recent advances in copper sulphide−based nanoheterostructures. Chem. Soc. Rev. 2019, 48, 4950–4965. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibanez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound copper chalcogenide nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef]
- Liu, X.; Swihart, M.T. Heavily−doped colloidal semiconductor and metal oxide nanocrystals: An emerging new class of plasmonic nanomaterials. Chem. Soc. Rev. 2014, 43, 3908–3920. [Google Scholar] [CrossRef]
- Fenton, J.L.; Steimle, B.C.; Schaak, R.E. Exploiting crystallographic regioselectivity to engineer asymmetric three−component colloidal nanoparticle isomers using partial cation exchange reactions. J. Am. Chem. Soc. 2018, 140, 6771–6775. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Huang, T. Novel energy efficient window coatings based on in doped CuS nanocrystals with enhanced NIR shielding performance. Sol. Energy 2021, 220, 1–7. [Google Scholar] [CrossRef]
- Kwon, Y.−T.; Ryu, S.H.; Shin, J.W.; Yeo, W.−H.; Choa, Y.−H. Electrospun CuS/PVP nanowires and superior near−infrared filtration efficiency for thermal shielding applications. ACS Appl. Mater. Interfaces 2019, 11, 6575–6580. [Google Scholar] [CrossRef]
- Kwon, Y.−T.; Lim, G.−D.; Kim, S.; Ryu, S.H.; Hwang, T.−Y.; Park, K.−R.; Choa, Y.−H. Near−infrared absorbance properties of Cu2− xS/SiO2 nanoparticles and their PDMS−based composites. J. Mater. Chem. C 2018, 6, 754–760. [Google Scholar] [CrossRef]
- Leng, C.; Zhang, X.; Xu, F.; Yuan, Y.; Pei, H.; Sun, Z.; Li, L.; Bao, Z. Engineering gold nanorod–copper sulfide heterostructures with enhanced photothermal conversion efficiency and photostability. Small 2018, 14, 1703077. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Luo, B.; Chen, G.; Cai, J.; Jiang, Q.; Gu, B.; Wang, X. CuS@Corn stalk/chitin composite hydrogel for photodegradation and antibacterial. Polymers 2019, 11, 1393. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Wang, X.; Peng, Q.; Lin, R.; Wang, Y.; Shen, R.; Cao, X.; Zhang, L.; Zhou, G. Synergetic integration of Cu1.94S–ZnxCd1–xS heteronanorods for enhanced visible−light−driven photocatalytic hydrogen production. J. Am. Chem. Soc. 2016, 138, 4286–4289. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L. Fabrication of CuS/BiVO4 (0 4 0) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Xin, X.; Song, Y.; Guo, S.; Zhang, Y.; Wang, B.; Yu, J.; Li, X. In−situ growth of high−content 1T phase MoS2 confined in the CuS nanoframe for efficient photocatalytic hydrogen evolution. Appl. Catal. B−Environ. 2020, 269, 118773. [Google Scholar] [CrossRef]
- Liaw, D.−J.; Wang, K.−L.; Huang, Y.−C.; Lee, K.−R.; Lai, J.−Y.; Ha, C.−S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Liu, X.; Hu, L.; Wang, R.; Li, J.; Gu, H.; Liu, S.; Zhou, Y.; Tu, G. Flexible perovskite solar cells via surface−confined silver nanoparticles on transparent polyimide substrates. Polymers 2019, 11, 427. [Google Scholar] [CrossRef]
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yu, S.−H.; Chen, S.; Liu, G.; Liu, B. Large scale synthesis of uniform CuS nanotubes in ethylene glycol by a sacrificial templating method under mild conditions. J. Mater. Chem. 2006, 16, 3326–3331. [Google Scholar] [CrossRef]
- Clausen, J.; Christiansen, A.B.; Garnaes, J.; Mortensen, N.A.; Kristensen, A. Color effects from scattering on random surface structures in dielectrics. Opt. Express 2012, 20, 4376–4381. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Liu, J.; Shi, F.; Fan, C.; Chen, B.; Zhang, H.; Yu, L.; Liu, S.−H. Greatly improved heat−shielding performance of KxWO3 by trace Pt doping for energy−saving window glass applications. Sol. Energy Mater. Sol. Cells 2018, 174, 342–350. [Google Scholar] [CrossRef]
- Yibi, Y.; Chen, J.; Xue, J.; Song, J.; Zeng, H. Enhancement of adjustable localized surface plasmon resonance in ZnO nanocrystals via a dual doping approach. Sci. Bull. 2017, 62, 693–699. [Google Scholar] [CrossRef]
- Kim, M.R.; Hafez, H.A.; Chai, X.; Besteiro, L.V.; Tan, L.; Ozaki, T.; Govorov, A.O.; Izquierdo, R.; Ma, D. Covellite CuS nanocrystals: Realizing rapid microwave−assisted synthesis in air and unravelling the disappearance of their plasmon resonance after coupling with carbon nanotubes. Nanoscale 2016, 8, 12946–12957. [Google Scholar] [CrossRef]
- Comin, A.; Manna, L. New materials for tunable plasmonic colloidal nanocrystals. Chem. Soc. Rev. 2014, 43, 3957–3975. [Google Scholar] [CrossRef]
- Ghosh, S.; Saha, M.; De, S.K. Tunable surface plasmon resonance and enhanced electrical conductivity of in doped ZnO colloidal nanocrystals. Nanoscale 2014, 6, 7039–7051. [Google Scholar] [CrossRef]
- Maiti, P.S.; Ganai, A.K.; Bar−Ziv, R.; Enyashin, A.N.; Houben, L.; Bar Sadan, M. Cu2–xS–MoS2 nano−octahedra at the atomic scale: Using a template to activate the basal plane of MoS2 for hydrogen production. Chem. Mater. 2018, 30, 4489–4492. [Google Scholar] [CrossRef]
- Cao, H.; Qian, X.; Wang, C.; Ma, X.; Yin, J.; Zhu, Z. High symmetric 18−facet polyhedron nanocrystals of Cu7S4 with a hollow nanocage. J. Am. Chem. Soc. 2005, 127, 16024–16025. [Google Scholar] [CrossRef]
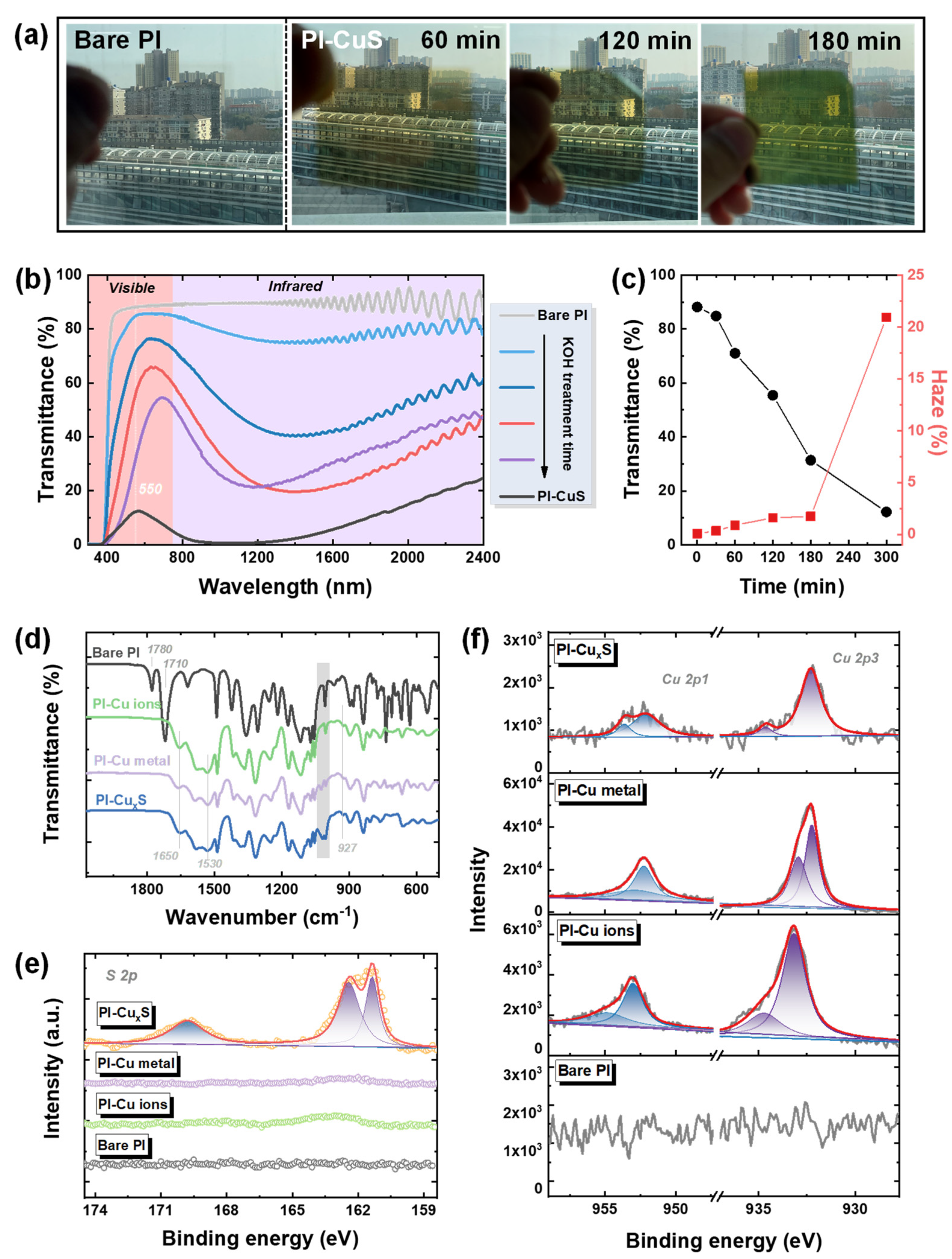
| Treatment Time (min) | 0 | 30 | 60 | 120 | 180 | 300 | |
|---|---|---|---|---|---|---|---|
| Transmittance (@ 550 nm, %) | 88.19 | 84.80 | 71.02 | 56.44 | 31.35 | 12.22 | |
| Average Transmittance (400–780 nm, %) | 86.99 | 81.27 | 65.31 | 50.01 | 35.08 | 7.98 | |
| Haze (%) | 0.08 | 0.34 | 0.89 | 1.60 | 1.74 | 20.92 | |
| Average shielding rate in the NIR region (780–2400 nm) | 10.43 | 21.63 | 49.94 | 69.45 | 66.40 | 91.57 | |
| Average shielding rate in the UV region (300–400 nm) | 100 | 100 | 100 | 100 | 100 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ma, J.; Shen, J.; Zhao, J.; Lu, C.; Tu, G. Advanced Dual−Function Hollow Copper−Sulfide−Based Polyimide Composite Window Film Combining Near−Infrared Thermal Shielding and Organic Pollutants’ Photodegradation. Polymers 2022, 14, 3382. https://doi.org/10.3390/polym14163382
Liu X, Ma J, Shen J, Zhao J, Lu C, Tu G. Advanced Dual−Function Hollow Copper−Sulfide−Based Polyimide Composite Window Film Combining Near−Infrared Thermal Shielding and Organic Pollutants’ Photodegradation. Polymers. 2022; 14(16):3382. https://doi.org/10.3390/polym14163382
Chicago/Turabian StyleLiu, Xiangfu, Jinming Ma, Jiulin Shen, Jianqiao Zhao, Chengxu Lu, and Guoli Tu. 2022. "Advanced Dual−Function Hollow Copper−Sulfide−Based Polyimide Composite Window Film Combining Near−Infrared Thermal Shielding and Organic Pollutants’ Photodegradation" Polymers 14, no. 16: 3382. https://doi.org/10.3390/polym14163382
APA StyleLiu, X., Ma, J., Shen, J., Zhao, J., Lu, C., & Tu, G. (2022). Advanced Dual−Function Hollow Copper−Sulfide−Based Polyimide Composite Window Film Combining Near−Infrared Thermal Shielding and Organic Pollutants’ Photodegradation. Polymers, 14(16), 3382. https://doi.org/10.3390/polym14163382






