Synthesis of Polycarboxylate Viscosity Reducer and the Effect of Different Chain Lengths of Polyether on Viscosity Reduction of Heavy Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
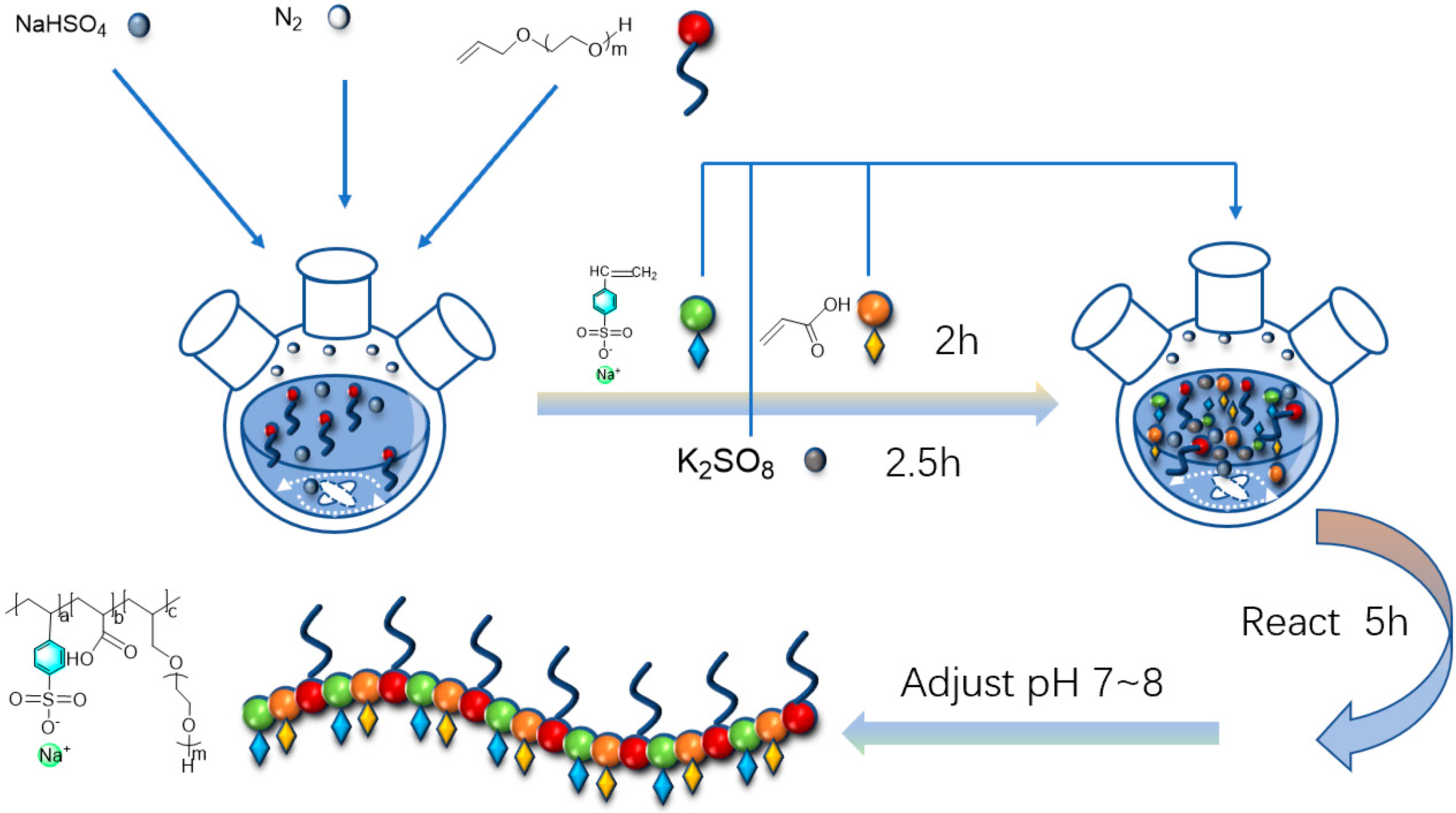
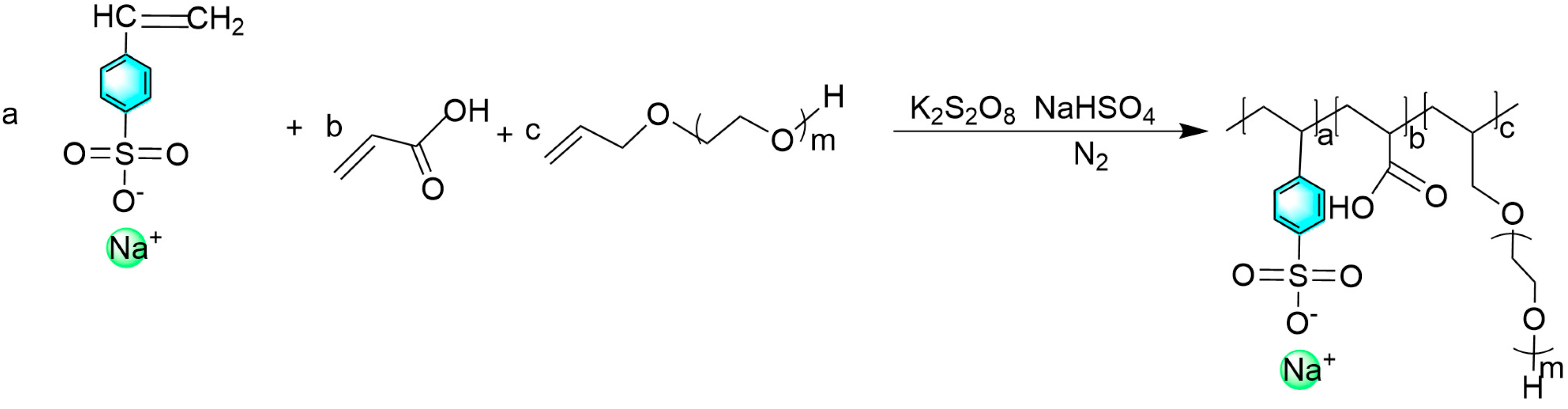
2.2. Synthesis of Polyether Carboxylic Acid–Sulfonate Polymeric Surfactants
2.3. Characterization
2.4. Determination of Heavy Oil Properties
2.5. Viscosity-Reducing Properties of Polyether Carboxylic Acid–Sulfonate Series
2.6. Surface Tension Measurement
2.7. Contact Angle Measurement
2.8. Emulsification Speed Measurement
2.9. Stability of Emulsions
2.10. Foaming Performance and Foam Stability
3. Results and Discussion
3.1. Characterization
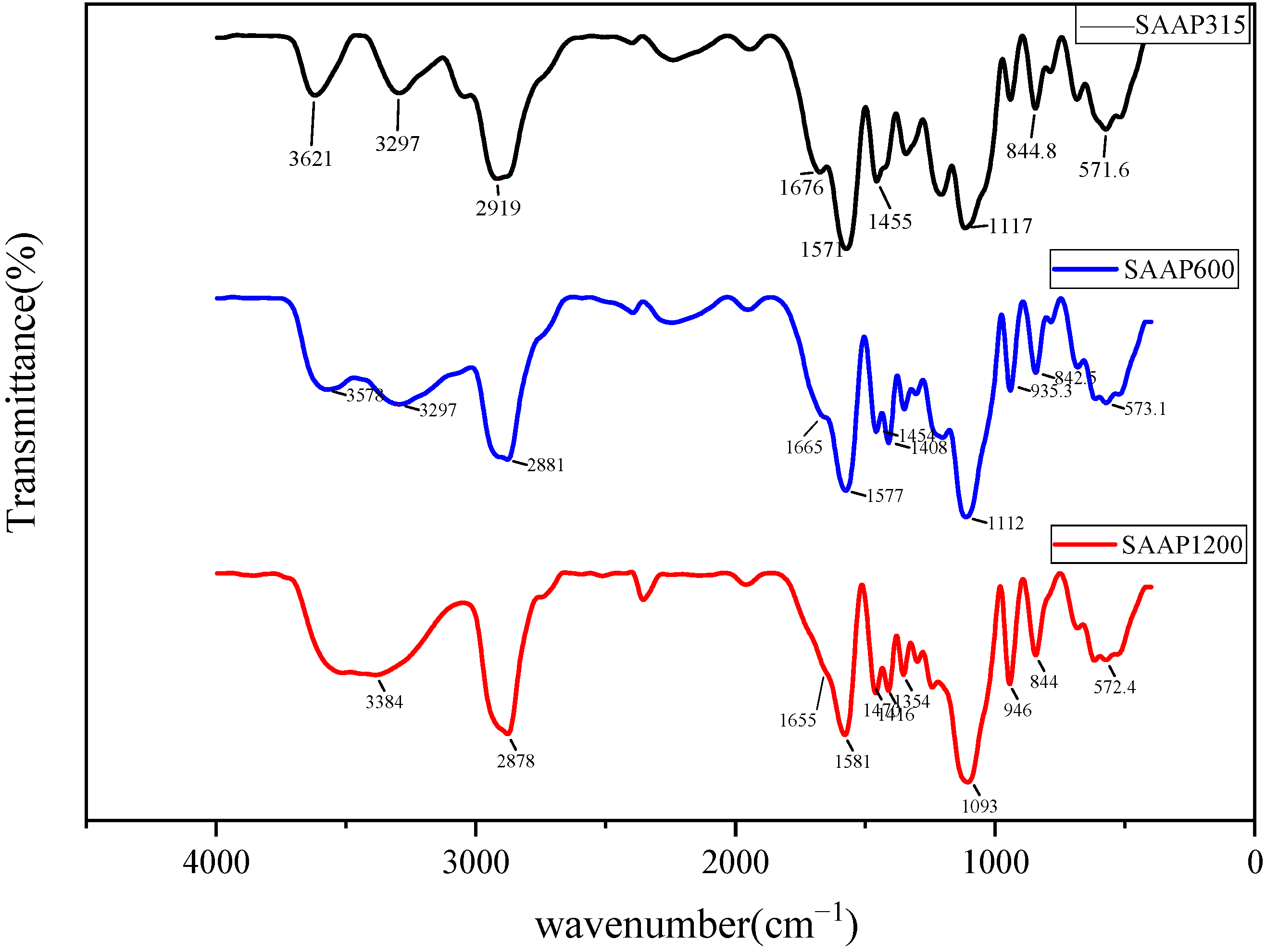
3.1.1. FT-IR Spectroscopy
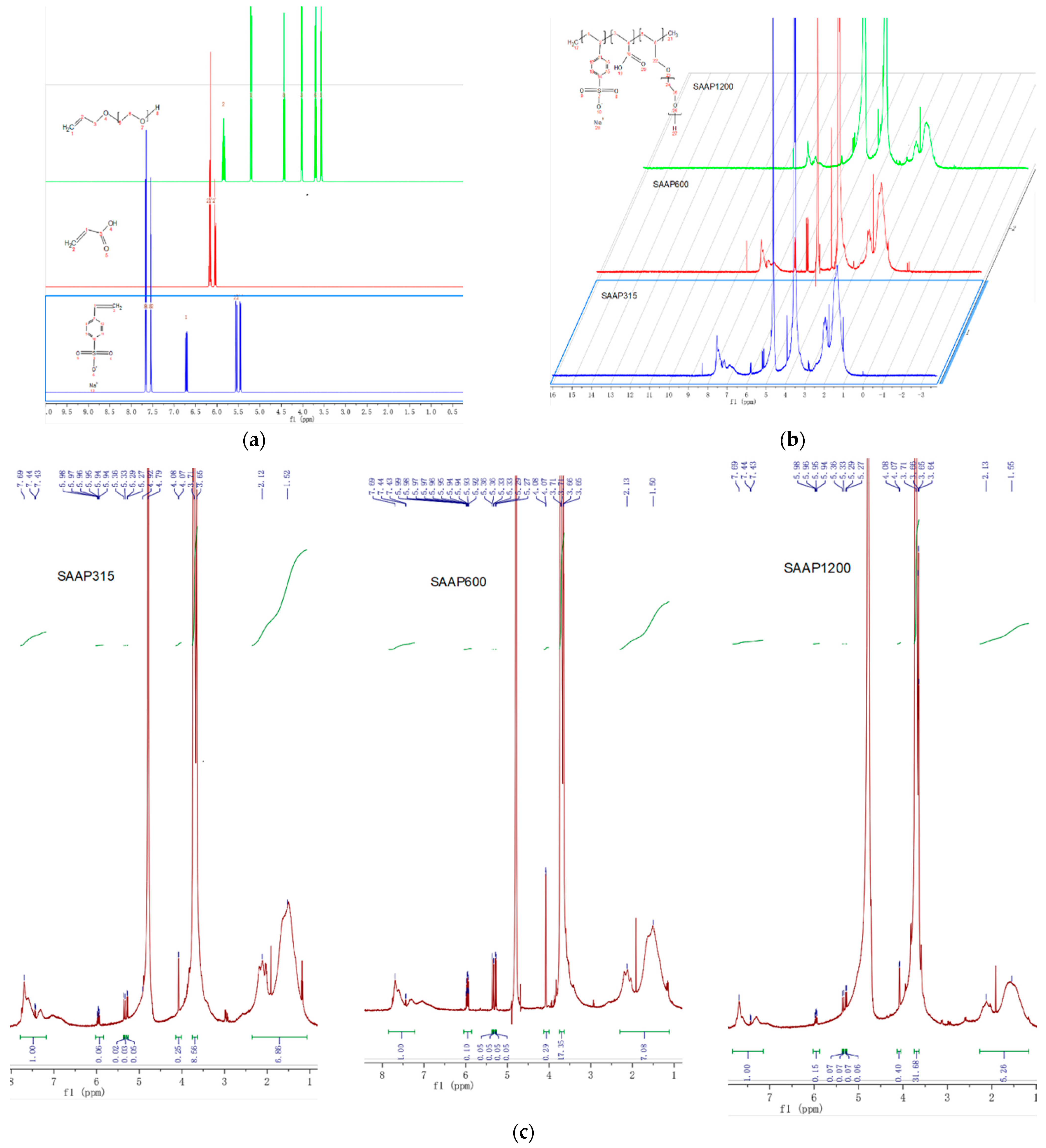
3.1.2. 1HNMR
3.1.3. Relative Molecular Mass Distribution
3.2. Determination of Heavy Oil Properties
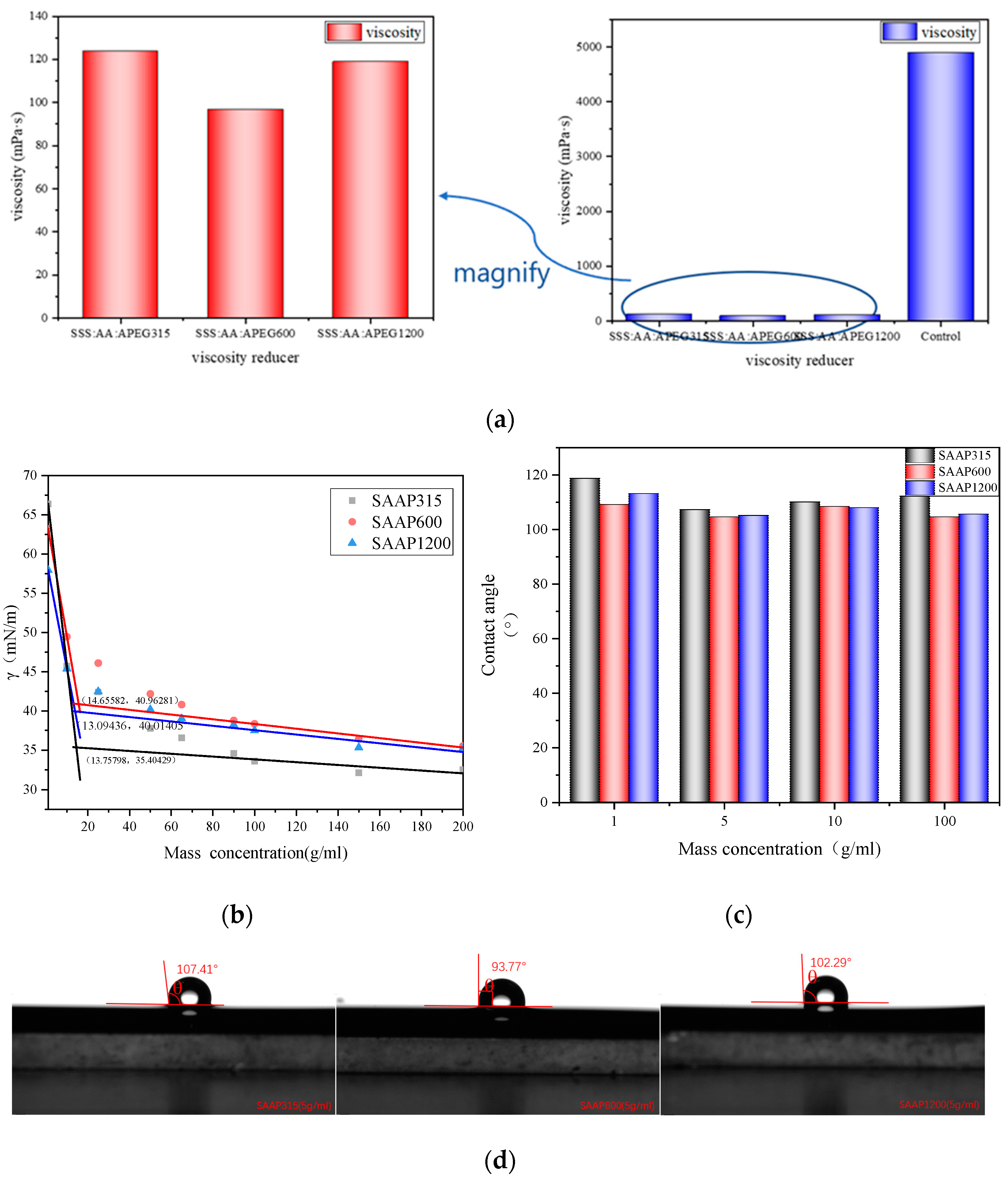
3.3. Viscosity-Reducing Properties of Polyether Carboxylic Acid–Sulfonate Viscosity Reducers
3.4. Surface Tension of Polyether Carboxylate–Sulfonate Viscosity Reducers
3.5. Contact Angle of Polyether Carboxylic Acid–Sulfonate Viscosity Reducers
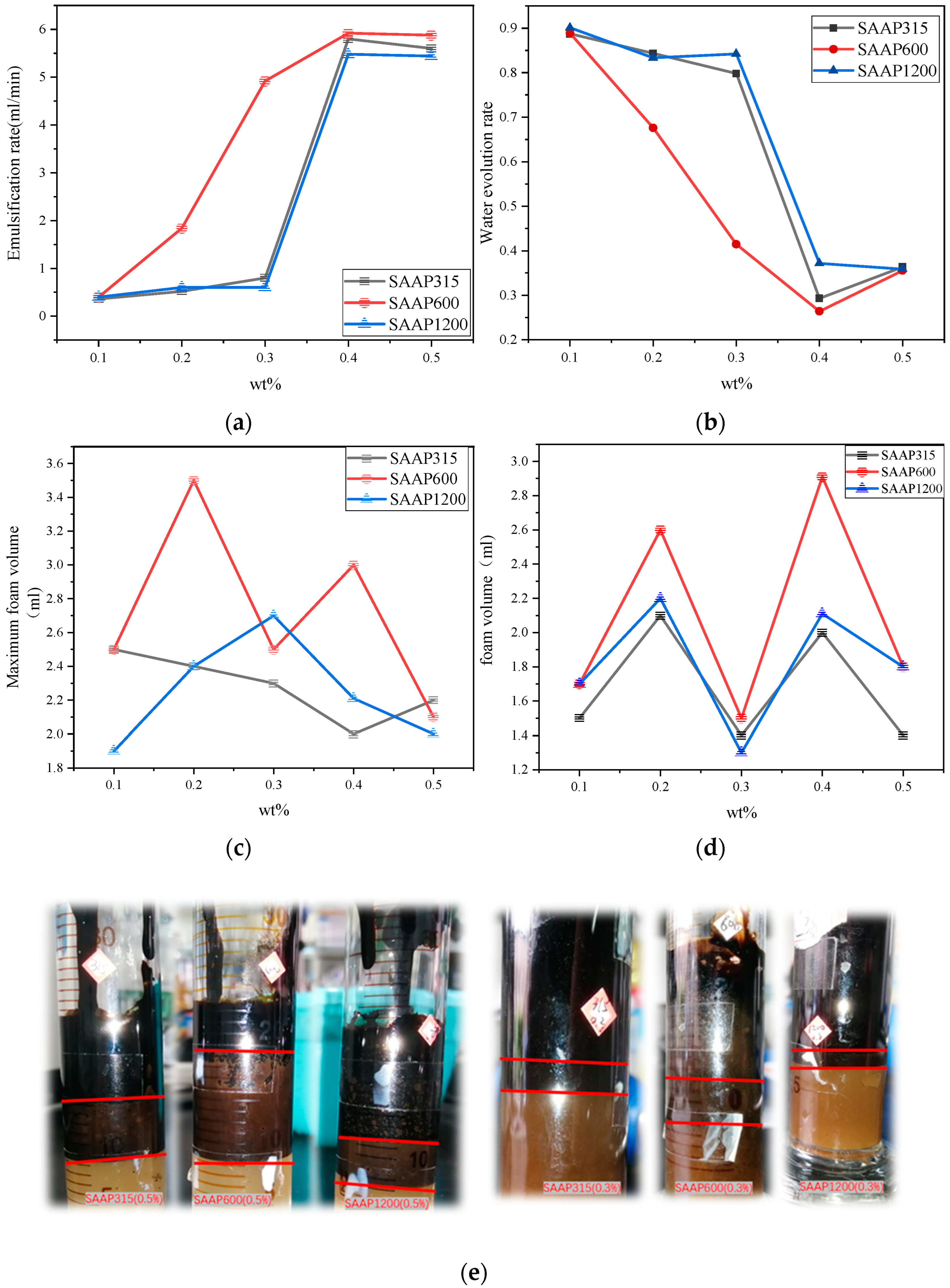
3.6. Emulsification Speed of Polyether Carboxylic Acid–Sulfonate Viscosity Reducers
3.7. Stability of Different Polyether Carboxylate–Sulfonate Viscosity-Reducing Emulsions
3.8. Foaming Performance and Foam Stability of Polyether Carboxylate–Sulfonate Viscosity Reducers
3.9. Optimization of Conditions for the Synthesis of Polyether Carboxylic Acid–Sulfonic Acid Viscosity Reducers
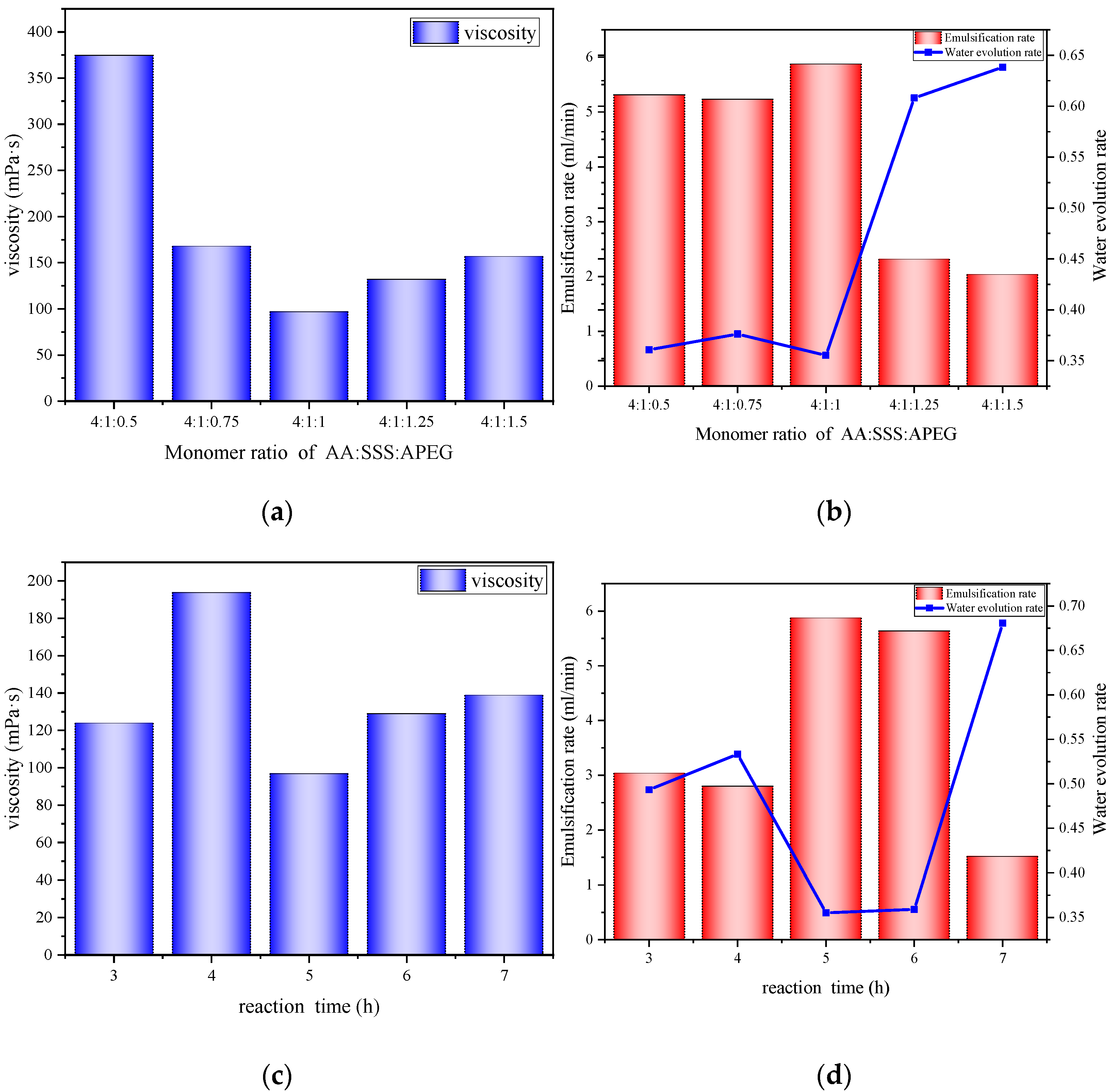
3.9.1. Effect of Monomer Molar Ratio
3.9.2. Effect of Reaction Time
3.9.3. Effect of Reaction Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malkin, A.Y.; Zadymova, N.M.; Skvortsova, Z.N.; Traskine, V.Y.; Kulichikhin, V.G. Formation of concentrated emulsions in heavy oil. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 343–349. [Google Scholar] [CrossRef]
- Sharma, P.; Kostarelos, K.; Salman, M. Optimization of closed-cycle oil recovery: A non-thermal process for bitumen and extra heavy oil recovery. RSC Adv. 2021, 11, 26554–26562. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Xu, Y.; Yang, Z.; Cai, B.; Wang, X.; Zhou, L.; Liu, H.; Xu, M.; Wang, L.; Li, S. Progress and development directions of stimulation techniques for ultra-deep oil and gas reservoirs. Pet. Explor. Dev. 2021, 48, 221–231. [Google Scholar] [CrossRef]
- Yen, T.F.; Erdman, J.G.; Pollack, S.S. Investigation of the Structure of Petroleum Asphaltenes by X-ray Diffraction. Anal. Chem. 1961, 33, 1587–1594. [Google Scholar] [CrossRef]
- Murgich, J. Intermolecular Forces in Aggregates of Asphaltenes and Resins. Pet. Sci. Technol. 2002, 20, 983–997. [Google Scholar] [CrossRef]
- Khadim, M.A.; Sarbar, M.A. Role of asphaltene and resin in oil field emulsions. J. Pet. Sci. Eng. 1999, 23, 213–221. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Liu, G. Review on new heavy oil viscosity reduction technologies. IOP Conf. Ser. Earth Environ. Sci. 2022, 983, 12059. [Google Scholar] [CrossRef]
- Wang, X. Application of Chemical Flooding in Increasing Yield of Tertiary Oil Recovery. IOP Conf. Ser. Earth Environ. Sci. 2021, 781, 22066. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Huang, X.; Cao, Y.; Zhu, C. Polymer Surfactants and Their Application in the Oilfield. Henan Sci. 2014, 32, 1425–1431. [Google Scholar] [CrossRef]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef]
- Qin, B.; Peng, P.; Jing, Z. The relationship between the composition and emulsification performance of the condensates wit -COOH, -SO3H, -(OCH2CHC)n-groups. Pet. Process. Petrochem. 2002, 33, 32–35. [Google Scholar] [CrossRef]
- Xiao, S.; Sun, Y.; Wang, S.; Wu, C.; Song, H. Study on polyether carboxylate surfactant as viscosity reducing agent for heavy oil recovery. Energy Chem. Ind. 2018, 39, 49–54. [Google Scholar]
- Bao, X.; Zhang, W.; Sha, O.; Li, Y. Synthesis and application of coconut acid polyether carboxylate anionic-nonionic surfactant for EOR. Appl. Chem. Ind. 2015, 44, 1230–1232. [Google Scholar] [CrossRef]
- Wu, Z.; Yue, X.; Cheng, T.; Yu, J.; Yang, H. Effect of viscosity and interfacial tension of surfactant–polymer flooding on oil recovery in high-temperature and high-salinity reservoirs. J. Pet. Explor. Prod. Technol. 2013, 4, 9–16. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, P.; Li, Y.; Li, J.; Zhang, G. Dispersion performance and mechanism of polycarboxylates bearing side chains of moderate length in coal-water slurries. Fuel 2017, 190, 221–228. [Google Scholar] [CrossRef]
- Gopi, N.; Sethumadhavan, A.D.N.; Wasan, D.T. Ethanol-Based Foam Stability As Probed by Foam Lamella Thinning. Ind. Eng. Chem. Res. 2003, 42, 2634–2638. [Google Scholar] [CrossRef]
- Hansen, L.D.; McCarlie, V.W. From Foam Rubber to Volcanoes: The Physical Chemistry of Foam Formation. J. Chem. Educ. 2004, 81, 1581–1584. [Google Scholar] [CrossRef]
- Wang, J.; Buckley, J.S. Asphaltene Stability in Crude Oil and Aromatic SolventssThe Influence of Oil Composition. Energy Fuels 2003, 17, 1445–1451. [Google Scholar] [CrossRef]
- Castellano, O.; Gimon, R.; Canelon, C.; Aray, Y.; Soscun, H. Molecular Interactions between Orinoco Belt Resins. Energy Fuels 2012, 26, 2711–2720. [Google Scholar] [CrossRef]
- de Lara, L.S.; Michelon, M.F.; Miranda, C.R. Molecular dynamics studies of fluid/oil interfaces for improved oil recovery processes. J. Phys. Chem. B 2012, 116, 14667–14676. [Google Scholar] [CrossRef]
- Makoto Kunieda, K.N.; Liang, Y.; Miranda, C.R.; Ueda, A.; Takahashi, S.; Okabe, H.; Atsuoka, T. Self-Accumulation of Aromatics at the Oil-Water Interface through Weak Hydrogen Bonding. J. Am. Chem. Soc. 2010, 132, 18281–18286. [Google Scholar] [CrossRef] [PubMed]
- Takanohashi, T.; Sato, S.; Tanaka, R. Structural Relaxation Behaviors of Three Different Asphaltenes Using MD Calculations. Pet. Sci. Technol. 2004, 22, 901–914. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, S. Application of molecular dynamics simulation in enhanced oil recovery. Sci. Sin. Chim. 2021, 51, 761–771. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Wen, J.; Gao, W.; Tian, Y.; Deng, Q.; Liu, Z. Functional characteristics and dominant enhanced oil recovery mechanism of polymeric surfactant. J. Mol. Liq. 2022, 354, 118921. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Aksenenko, E.V.; Makievski, A.V.; Nikolenko, M.V.; Javadi, A.; Schneck, E.; Miller, R. Particular Behavior of Surface Tension at the Interface between Aqueous Solution of Surfactant and Alkane. Langmuir 2019, 35, 15214–15220. [Google Scholar] [CrossRef]
- Mehrabianfar, P.; Bahraminejad, H.; Manshad, A.K. An introductory investigation of a polymeric surfactant from a new natural source in chemical enhanced oil recovery (CEOR). J. Pet. Sci. Eng. 2021, 198, 108172. [Google Scholar] [CrossRef]
- Esfandyari, H.; Shadizadeh, S.R.; Esmaeilzadeh, F.; Davarpanah, A. Implications of anionic and natural surfactants to measure wettability alteration in EOR processes. Fuel 2020, 278, 118392. [Google Scholar] [CrossRef]
- Rellegadla, S.; Jain, S.; Sangwai, J.S.; Lavania, M.; Lal, B.; Gieg, L.; Rajasekar, A.; Bera, A.; Agrawal, A. Wettability Alteration of the Oil-Wet Carbonate by Viscosity-Augmented Guar Galactomannan for Enhanced Oil Recovery. ACS Appl. Polym. Mater. 2021, 3, 1983–1994. [Google Scholar] [CrossRef]
- Marie, E.; Rotureau, E.; Dellacherie, E.; Durand, A. From polymeric surfactants to colloidal systems. Colloids Surf. A Physicochem. Eng. Asp. 2007, 308, 25–32. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Wu, Y.; Xie, L.; Yang, H.; Zhang, B. Review of Influence for Stability of Heavy Oil-in-Water Emulsions. J. Liaoning Shiyou Univ. 2019, 39, 11–18. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Liu, Z.; Liu, R.; Liu, W.; Zhang, H. Effects of molecular structure of polymeric surfactant on its physico-chemical properties, percolation and enhanced oil recovery. J. Ind. Eng. Chem. 2021, 101, 165–177. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Shang, Y. Measurement techniques of form performance and influence factors of foam stability. China Min. Mag. 2014, 23, 230–234. [Google Scholar]
- Liu, X.; Chen, Z.; Cui, Z. Foaming systems for foam flooding with both high foaming performance and ultralow oil/water interfacial tension. J. Mol. Liq. 2022, 355, 118920. [Google Scholar] [CrossRef]

| Viscosity Depressant | Mn | MW |
|---|---|---|
| SAAP315 | 8816 | 59,714 |
| SAAP600 | 10,518 | 55,695 |
| SAAP1200 | 10,103 | 46,576 |
| Main Components | Content |
|---|---|
| Pentadecane, 2,6,10,14-tetramethyl- | 0.064342 |
| Stigmastane | 0.086701 |
| beta.-iso-Methyl ionone | 0.038009 |
| Coprostane | 0.036826 |
| Cholestane | 0.130264 |
| Octadecane | 0.0516781 |
| Heptadecane | 0.0583817 |
| Tetracosane | 0.0403763 |
| Cholestan-3-one, 4,4-dimethyl-, (5.alpha.)- | 0.0367927 |
| Dodecane, 2,6,10-trimethyl- | 0.0109083 |
| Undecane | 0.006227 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, R.; Tang, Y.; Zhu, J.; Sun, Y.; Zhang, G. Synthesis of Polycarboxylate Viscosity Reducer and the Effect of Different Chain Lengths of Polyether on Viscosity Reduction of Heavy Oil. Polymers 2022, 14, 3367. https://doi.org/10.3390/polym14163367
Wang J, Liu R, Tang Y, Zhu J, Sun Y, Zhang G. Synthesis of Polycarboxylate Viscosity Reducer and the Effect of Different Chain Lengths of Polyether on Viscosity Reduction of Heavy Oil. Polymers. 2022; 14(16):3367. https://doi.org/10.3390/polym14163367
Chicago/Turabian StyleWang, Junqi, Ruiqing Liu, Yiwen Tang, Junfeng Zhu, Yonghui Sun, and Guanghua Zhang. 2022. "Synthesis of Polycarboxylate Viscosity Reducer and the Effect of Different Chain Lengths of Polyether on Viscosity Reduction of Heavy Oil" Polymers 14, no. 16: 3367. https://doi.org/10.3390/polym14163367
APA StyleWang, J., Liu, R., Tang, Y., Zhu, J., Sun, Y., & Zhang, G. (2022). Synthesis of Polycarboxylate Viscosity Reducer and the Effect of Different Chain Lengths of Polyether on Viscosity Reduction of Heavy Oil. Polymers, 14(16), 3367. https://doi.org/10.3390/polym14163367







