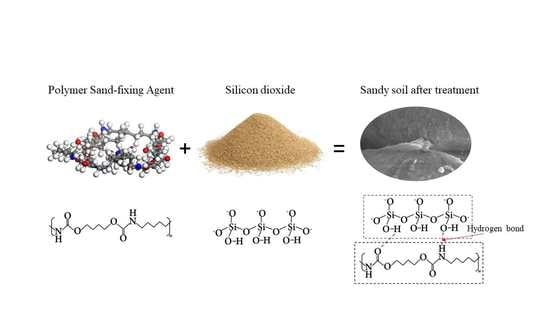
Molecular Dynamics Study on the Adsorption and Modification Mechanism of Polymeric Sand-Fixing Agent
Abstract
:1. Introduction
2. Research Contents and Methods
2.1. Material Selection
2.2. Molecule Models
2.3. Simulation Details
3. Results
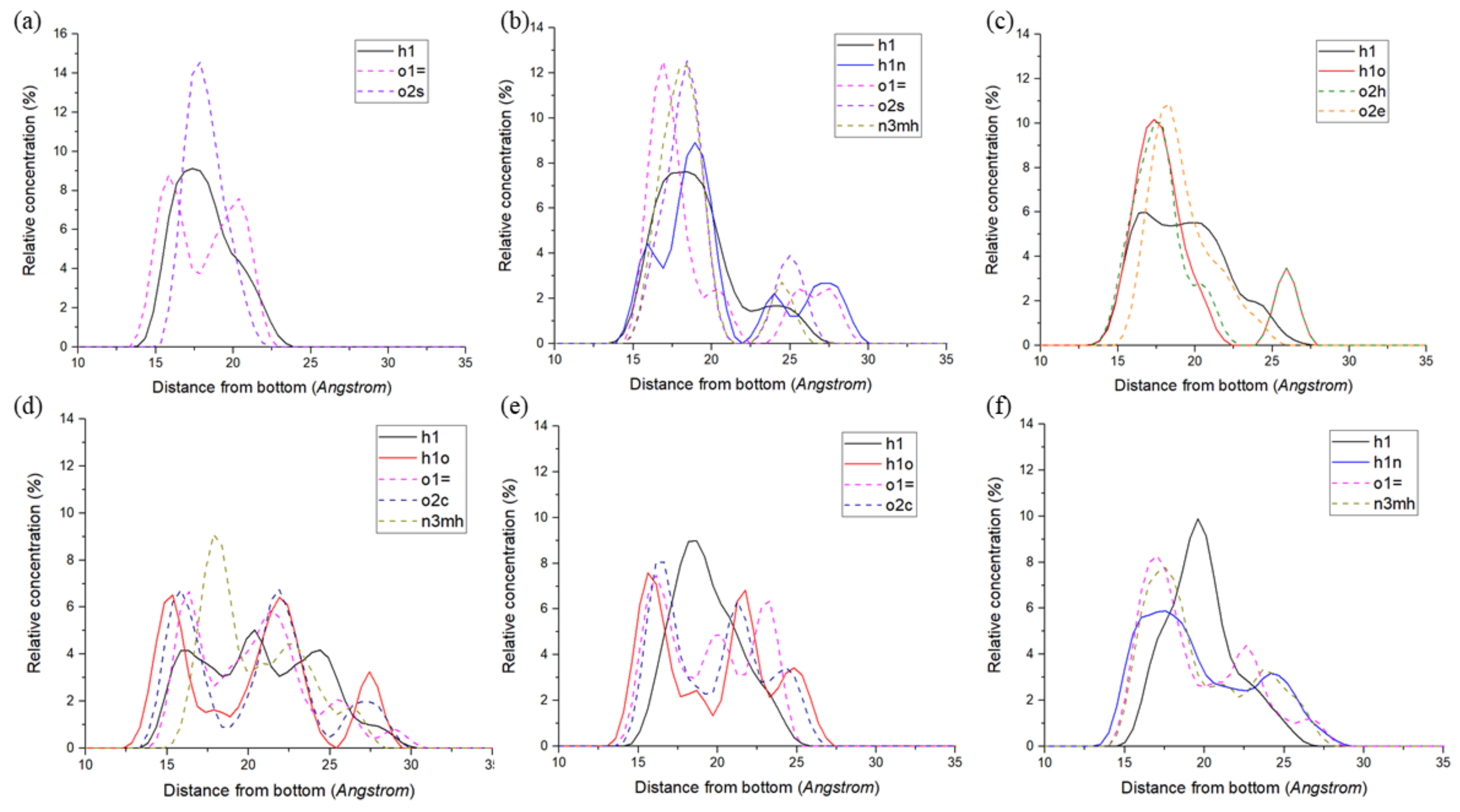
3.1. Relative Density Distribution of Atoms on Quartz Surface
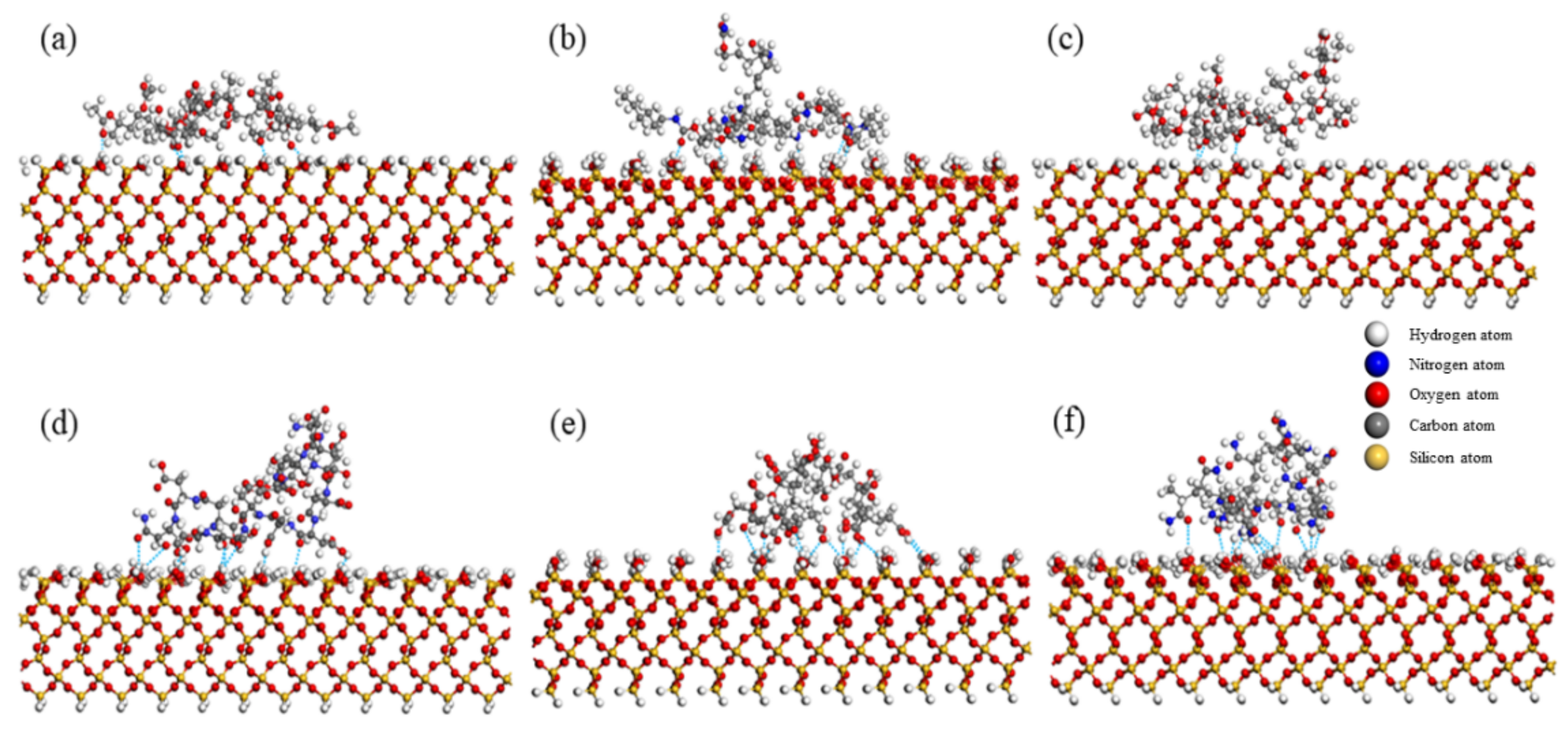
3.2. Number of Hydrogen Bonds Formed by Polymer on Quartz Surface
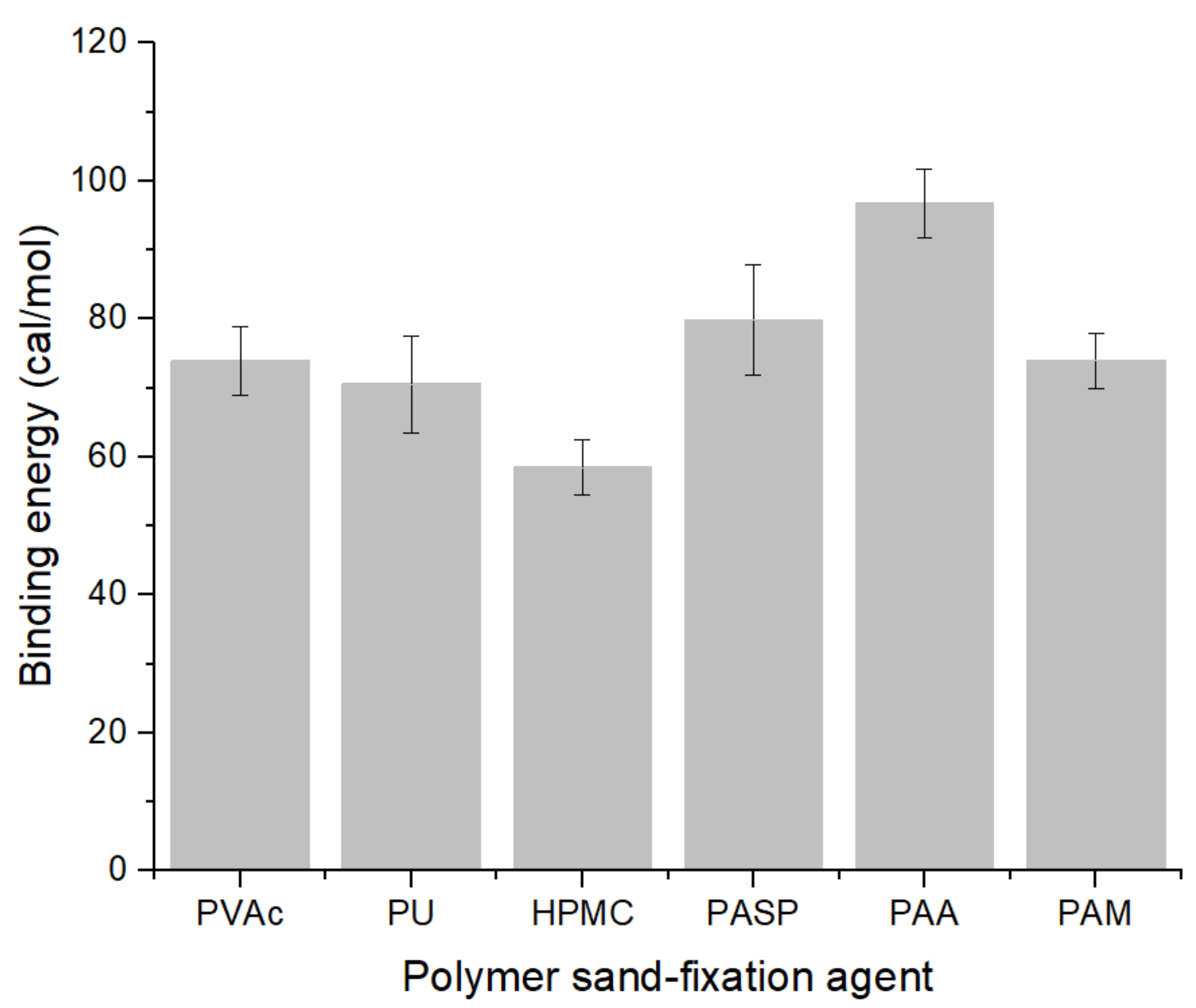
3.3. Binding Energy of Polymer on Quartz Surface
4. Discussion
4.1. Effect of Functional Groups on Hydrogen Bonds
4.2. Effect of Functional Groups on Binding Energy
4.3. Number of Hydrogen Bonds per Molecular Weight
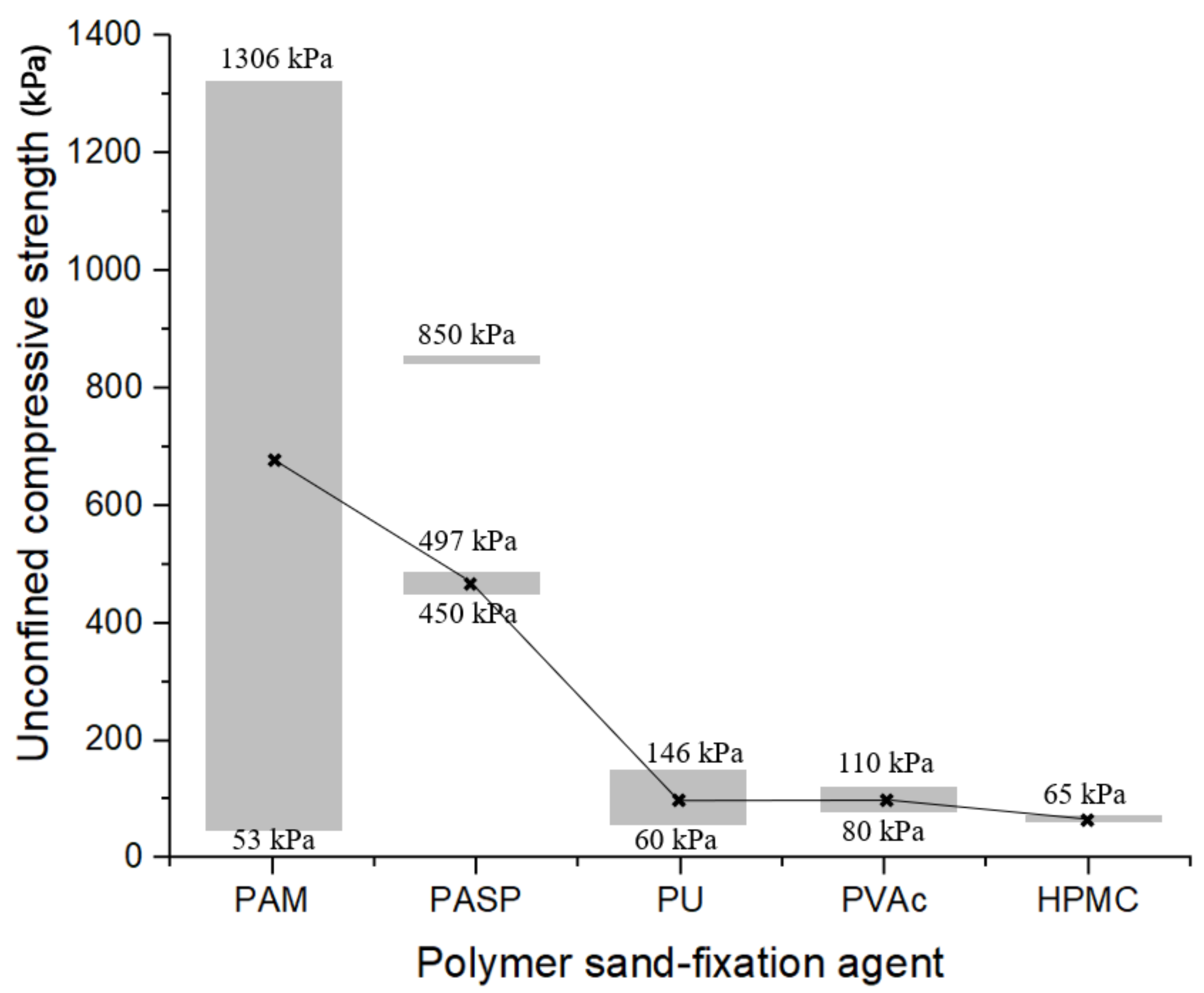
4.4. Mechanical Properties Analysis
5. Conclusions
- (1)
- The number of hydrogen bonds formed under the unit molecular weight is positively correlated with the compressive strength, and the number of hydrogen bonds can be used as one of the indicators to evaluate the improvement effect of sandy soil at molecular scale, which is helpful for the screening and development of new high-performance sand-fixing agents, which provides an accurate, efficient and inexpensive qualitative evaluation method for the curing effect of sand fixers and assists in the screening and development of new high-performance sand-fixers.
- (2)
- Functional groups play an important role in the adsorption process. When the adsorption surface is quartz, the carboxyl group (PAA: −96.74 cal/mol) has the highest binding energy. The binding energy of hydroxyl group is the lowest (HPMC: −58.43 cal/mol). When the molecular weight was close, the binding energy of ester group (PVAc: −73.96 cal/mol) was slightly higher than that of peptide bond (PU: −70.56 cal/mol). Under the premise of chain structure, the binding energy of carboxyl group (PAA: −96.74 cal/mol) was higher than that of amide group (PAM: −74.01 cal/mol).
- (3)
- This study was conducted under vacuum drying conditions, focusing on the effect of the material on sand particles after adsorption. However, the effect of water on adsorption cannot be ignored. In future research, more work will focus on the interaction between polymer molecules and quartz surface in aqueous solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albaladejo, J.; Martinez-Mena, M.; Roldan, A.; Castillo, V. Soil Degradation and Desertification Induced by Vegetation Removal in a Semiarid Environment. Soil Use Manag. 1998, 14, 1–5. [Google Scholar] [CrossRef]
- Zang, Y.X.; Gong, W.; Xie, H.; Liu, B.L.; Chen, H.L. Chemical Sand Stabilization: A Review of Material, Mechanism, and Problems. Environ. Technol. Rev. 2015, 4, 119–132. [Google Scholar] [CrossRef]
- Yuan, J.; Ye, C.; Luo, L.; Pei, X.; Yang, Q.; Chen, J.; Liao, B. Sand Fixation Property and Erosion Control through New Cellulose-Based Curing Agent on Sandy Slopes under Rainfall. Bull. Eng. Geol. Environ. 2020, 79, 4051–4061. [Google Scholar] [CrossRef]
- Dang, X.; Chen, H.; Shan, Z. Preparation and characterization of poly (Acrylic acid)—Corn starch blend for use as chemical sand-fixing materials. Mater. Res. Express 2017, 4, 075506. [Google Scholar] [CrossRef]
- Rizwan, M.; Rubina Gilani, S.; Iqbal Durani, A.; Naseem, S. Materials Diversity of Hydrogel: Synthesis, Polymerization Process and Soil Conditioning Properties in Agricultural Field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Jalili, S.; Akhavan, M. A Coarse-Grained Molecular Dynamics Simulation of a Sodium Dodecyl Sulfate Micelle in Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2009, 352, 99–102. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhan, W.; Yi, H.; Zhao, Y.; Song, S. Molecular Dynamics Simulations Study for the Effect of Cations Hydration on the Surface Tension of the Electrolyte Solutions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 80–84. [Google Scholar] [CrossRef]
- Ma, S.; Chen, P.; Xu, J.; Xiong, X. Molecular Dynamics Simulations of Key Physical Properties and Microstructure of Epoxy Resin Cured with Different Curing Agents. J. Mater. Sci. 2022, 57, 1123–1133. [Google Scholar] [CrossRef]
- Quezada, G.R.; Jeldres, R.I.; Fawell, P.D.; Toledo, P.G. Use of Molecular Dynamics to Study the Conformation of an Anionic Polyelectrolyte in Saline Medium and Its Adsorption on a Quartz Surface. Miner. Eng. 2018, 129, 102–105. [Google Scholar] [CrossRef]
- Anastassiou, A.; Mavrantzas, V.G. Molecular Structure and Work of Adhesion of Poly(n-Butyl Acrylate) and Poly(n-Butyl Acrylate-Co-Acrylic Acid) on α-Quartz, α-Ferric Oxide, and α-Ferrite from Detailed Molecular Dynamics Simulations. Macromolecules 2015, 48, 8262–8284. [Google Scholar] [CrossRef]
- Duan, H.; Liu, W.; Wang, X.; Liu, W.; Zhang, X. Effect of Secondary Amino on the Adsorption of N-Dodecylethylenediamine on Quartz Surface: A Molecular Dynamics Study. Powder Technol. 2019, 351, 46–53. [Google Scholar] [CrossRef]
- Lan, T.; Zeng, H.; Tang, T. Understanding Adsorption of Violanthrone-79 as a Model Asphaltene Compound on Quartz Surface Using Molecular Dynamics Simulations. J. Phys. Chem. C 2018, 122, 28787–28796. [Google Scholar] [CrossRef]
- Xiao, F.; Yan, B.Q.; Zou, X.Y.; Cao, X.Q.; Dong, L.; Lyu, X.J.; Li, L.; Qiu, J.; Chen, P.; Hu, S.G.; et al. Study on Ionic Liquid Modified Montmorillonite and Molecular Dynamics Simulation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124311. [Google Scholar] [CrossRef]
- Machida, M.; Fotoohi, B.; Amamo, Y.; Ohba, T.; Kanoh, H.; Mercier, L. Cadmium(II) Adsorption Using Functional Mesoporous Silica and Activated Carbon. J. Hazard. Mater. 2012, 221–222, 220–227. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Z.; Zhou, C.; Yang, X. Enhancement of Soil Ecological Self-Repair Using a Polymer Composite Material. Catena 2020, 188, 104443. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, C.; Liu, Z.; Sun, H.; Du, J.; Zhang, L. Improving Soil-Water Characteristics and Pore Structure of Silty Soil Using Nano-Aqueous Polymer Stabilisers. KSCE J. Civ. Eng. 2021, 25, 3298–3305. [Google Scholar] [CrossRef]
- Huang, W.; Du, J.; Sun, H.; Zhou, C.; Liu, Z.; Zhang, L. New Polymer Composites Improve Silty Clay Soil Microstructure: An Evaluation Using NMR. Land Degrad. Dev. 2021, 32, 3272–3281. [Google Scholar] [CrossRef]
- Homauoni, Z.J.; Yasrobi, S.S. Stabilization of Dune Sand with Poly(Methyl Methacrylate) and Polyvinyl Acetate Using Dry and Wet Processing. Geotech. Geol. Eng. 2011, 29, 571–579. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Kanungo, D.P.; Wei, J.; Bai, Y.; Li, D.; Song, Z.; Lu, Y. Study on the Brittleness Characteristics of Sand Reinforced with Polypropylene Fiber and Polyurethane Organic Polymer. Fibers Polym. 2019, 20, 620–632. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Li, X. Shear Strength and Compression Coefficient for Conditioned Sand Subjected to Earth Chamber Stress Levels. Adv. Mater. Sci. Eng. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Yang, J.; Wang, F.; Fang, L.; Tan, T. Synthesis, Characterization and Application of a Novel Chemical Sand-Fixing Agent-Poly(Aspartic Acid) and Its Composites. Environ. Pollut. 2007, 149, 125–130. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Cukrowicz, S.; Sitarz, M.; Kornaus, K.; Kaczmarska, K.; Bobrowski, A.; Gubernat, A.; Grabowska, B. Organobentonites Modified with Poly(Acrylic Acid) and Its Sodium Salt for Foundry Applications. Materials 2021, 14, 1947. [Google Scholar] [CrossRef]
- Yang, K.; Tang, Z. Effectiveness of Fly Ash and Polyacrylamide as a Sand-Fixing Agent for Wind Erosion Control. Water. Air. Soil Pollut. 2012, 223, 4065–4074. [Google Scholar] [CrossRef]
- Yan, L.; Yang, Y.; Jiang, H.; Zhang, B.; Zhang, H. The Adsorption of Methyl Methacrylate and Vinyl Acetate Polymers on α-Quartz Surface: A Molecular Dynamics Study. Chem. Phys. Lett. 2016, 643, 1–5. [Google Scholar] [CrossRef]
- Sun, H. Ab Initio Calculations and Force Field Development for Computer Simulation of Polysilanes. Macromolecules 1995, 28, 701–712. [Google Scholar] [CrossRef]
- Sun, H. Ab Initio Characterizations of Molecular Structures, Conformation Energies, and Hydrogen-Bonding Properties for Polyurethane Hard Segments. Macromolecules 1993, 26, 5924–5936. [Google Scholar] [CrossRef]
- Prathab, B.; Subramanian, V.; Aminabhavi, T.M. Molecular Dynamics Simulations to Investigate Polymer-Polymer and Polymer-Metal Oxide Interactions. Polymer 2007, 48, 409–416. [Google Scholar] [CrossRef]
- Semoto, T.; Tsuji, Y.; Yoshizawa, K. Molecular Understanding of the Adhesive Force between a Metal Oxide Surface and an Epoxy Resin: Effects of Surface Water. Bull. Chem. Soc. Jpn. 2012, 85, 672–678. [Google Scholar] [CrossRef]
- Liu, J.; Shi, B.; Jiang, H.; Huang, H.; Wang, G.; Kamai, T. Research on the Stabilization Treatment of Clay Slope Topsoil by Organic Polymer Soil Stabilizer. Eng. Geol. 2011, 117, 114–120. [Google Scholar] [CrossRef]
- Yang, J.; Cao, H.; Wang, F.; Tan, T. Application and Appreciation of Chemical Sand Fixing Agent-Poly (Aspartic Acid) and Its Composites. Environ. Pollut. 2007, 150, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, F.; Fang, L.; Tan, T. The Effects of Aging Tests on a Novel Chemical Sand-Fixing Agent-Polyaspartic Acid. Compos. Sci. Technol. 2007, 67, 2160–2164. [Google Scholar] [CrossRef]
- Ajalloeian, R.; Matinmanesh, H.; Abtahi, S.M.; Rowshanzamir, M. Effect of Polyvinyl Acetate Grout Injection on Geotechnical Properties of Fine Sand. Geomech. Geoengin. 2013, 8, 86–96. [Google Scholar] [CrossRef]
- Wang, Y.; Han, C.; Zhang, L.; Zhao, H. Synthesis of Hydroxypropyl Methyl Cellulose Grafted Methacrylate/Vinyl Acetate and Its Application as a New Sand-Fixing Agent. J. Beijing Univ. Chem. Technol. Nat. Sci. 2014, 41, 82–87. [Google Scholar]

| Material Type | Molecular Weight (g/mol) | Chemical Formula | Functional Group | Structural Formula | References |
|---|---|---|---|---|---|
| PVAc | 1292 | C60H92O30 |  Ester group |  | [18] |
| PU | 1292 | C60H112N10O20 | 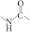 Peptide bond | 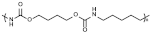 | [19] |
| HPMC | 1390 | C60H110O35 |  Hydroxyl | 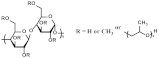 | [20] |
| PASP | 1734 | C60H70N16O45 |  Peptide bonds and Carboxyl | 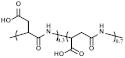 | [21,22] |
| PAA | 1442 | C60H82O40 |  Carboxyl | 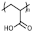 | [4,23] |
| PAM | 1422 | C60H102N20O20 |  Amide group |  | [3,24] |
| Atom Type | Description | Atom Type | Description |
|---|---|---|---|
| h1 | Hydrogen, nonpolar | O2s | Oxygen, SP3, in esters |
| h1n | Hydrogen, bonded to N, Cl | O2h | Oxygen, SP3, in alcohol |
| h1o | Hydrogen, bonded to O, F | O2e | Oxygen, SP3, in ethers |
| o1= | Oxygen, SP2, in carbonyl | O2c | Oxygen, SP3, in acid |
| O2 | Oxygen, SP3, generic | n3mh | Nitrogen, SP3, in amides with hydrogen |
| Type of Sand-Fixation Agent | PVAc | PU | HPMC | PASP | PAA | PAM |
|---|---|---|---|---|---|---|
| The number of hydrogen bonds (mol/g) | 0.00387 | 0.00387 | 0.00216 | 0.0058 | 0.00902 | 0.00844 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Geng, X.; Li, J.; Zhou, C.; Liu, Z. Molecular Dynamics Study on the Adsorption and Modification Mechanism of Polymeric Sand-Fixing Agent. Polymers 2022, 14, 3365. https://doi.org/10.3390/polym14163365
Huang W, Geng X, Li J, Zhou C, Liu Z. Molecular Dynamics Study on the Adsorption and Modification Mechanism of Polymeric Sand-Fixing Agent. Polymers. 2022; 14(16):3365. https://doi.org/10.3390/polym14163365
Chicago/Turabian StyleHuang, Wei, Xueyu Geng, Jing Li, Cuiying Zhou, and Zhen Liu. 2022. "Molecular Dynamics Study on the Adsorption and Modification Mechanism of Polymeric Sand-Fixing Agent" Polymers 14, no. 16: 3365. https://doi.org/10.3390/polym14163365
APA StyleHuang, W., Geng, X., Li, J., Zhou, C., & Liu, Z. (2022). Molecular Dynamics Study on the Adsorption and Modification Mechanism of Polymeric Sand-Fixing Agent. Polymers, 14(16), 3365. https://doi.org/10.3390/polym14163365