Long-Term Effect of Modified Glass Ionomer Cement with Mimicked Biological Property of Recombinant Translationally Controlled Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
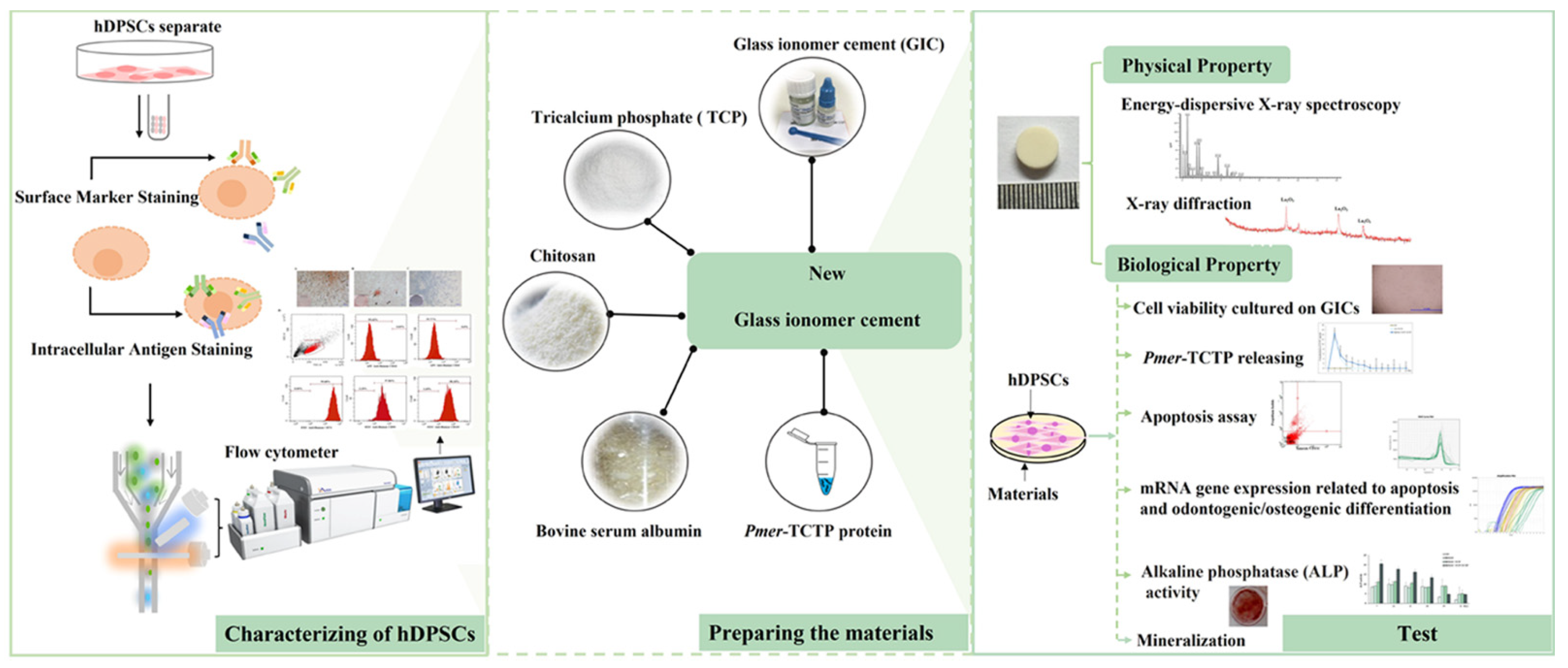
2.2. Cell Culture and Characterization of Human Dental Pulp Stem Cells (hDPSCs)
2.3. Expression and Purification of Pmer-TCTP Protein
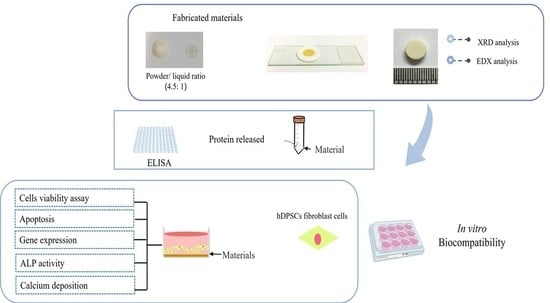
2.4. Preparation of Specimens
2.5. X-ray Diffraction (XRD)
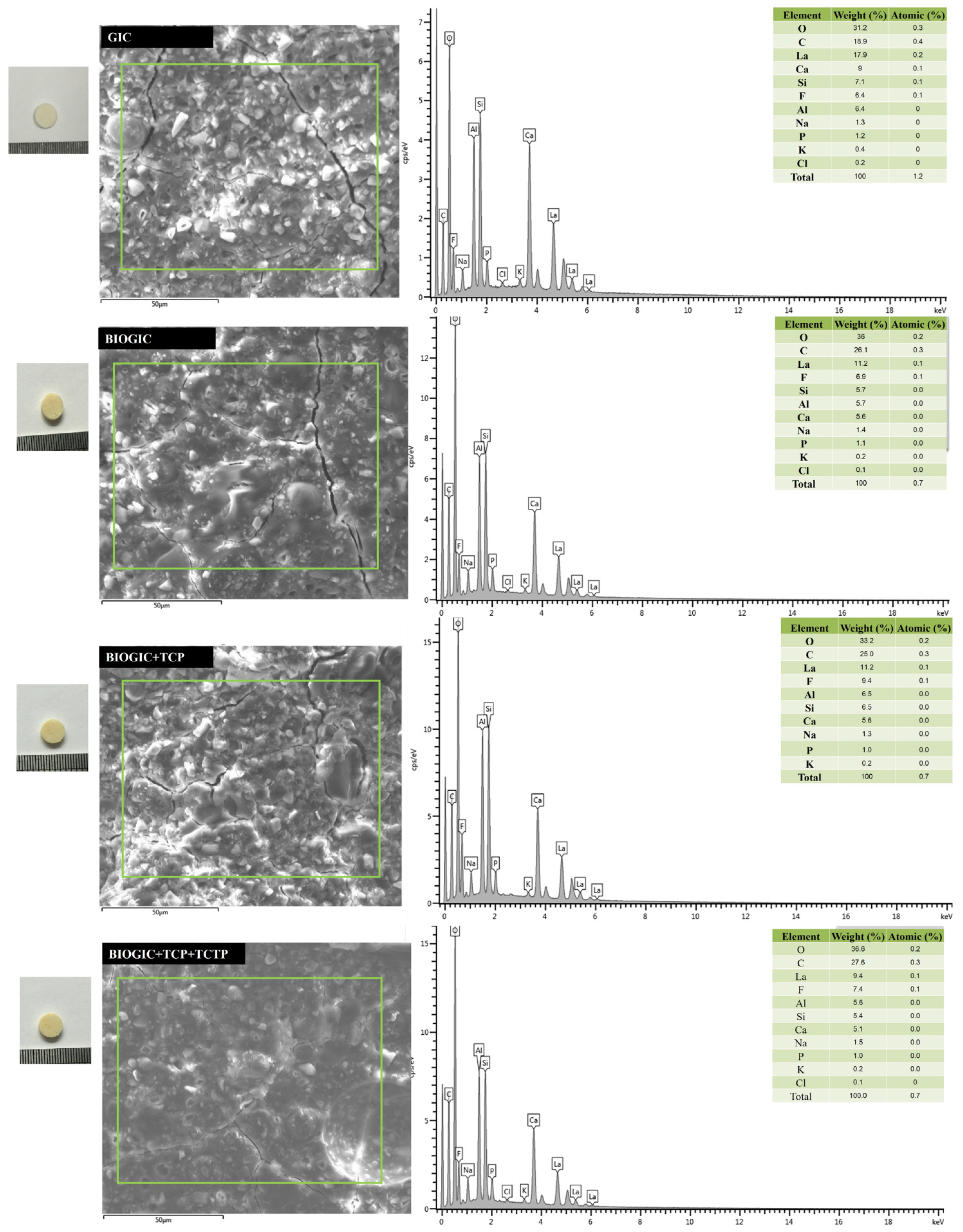
2.6. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Analysis (EDX)
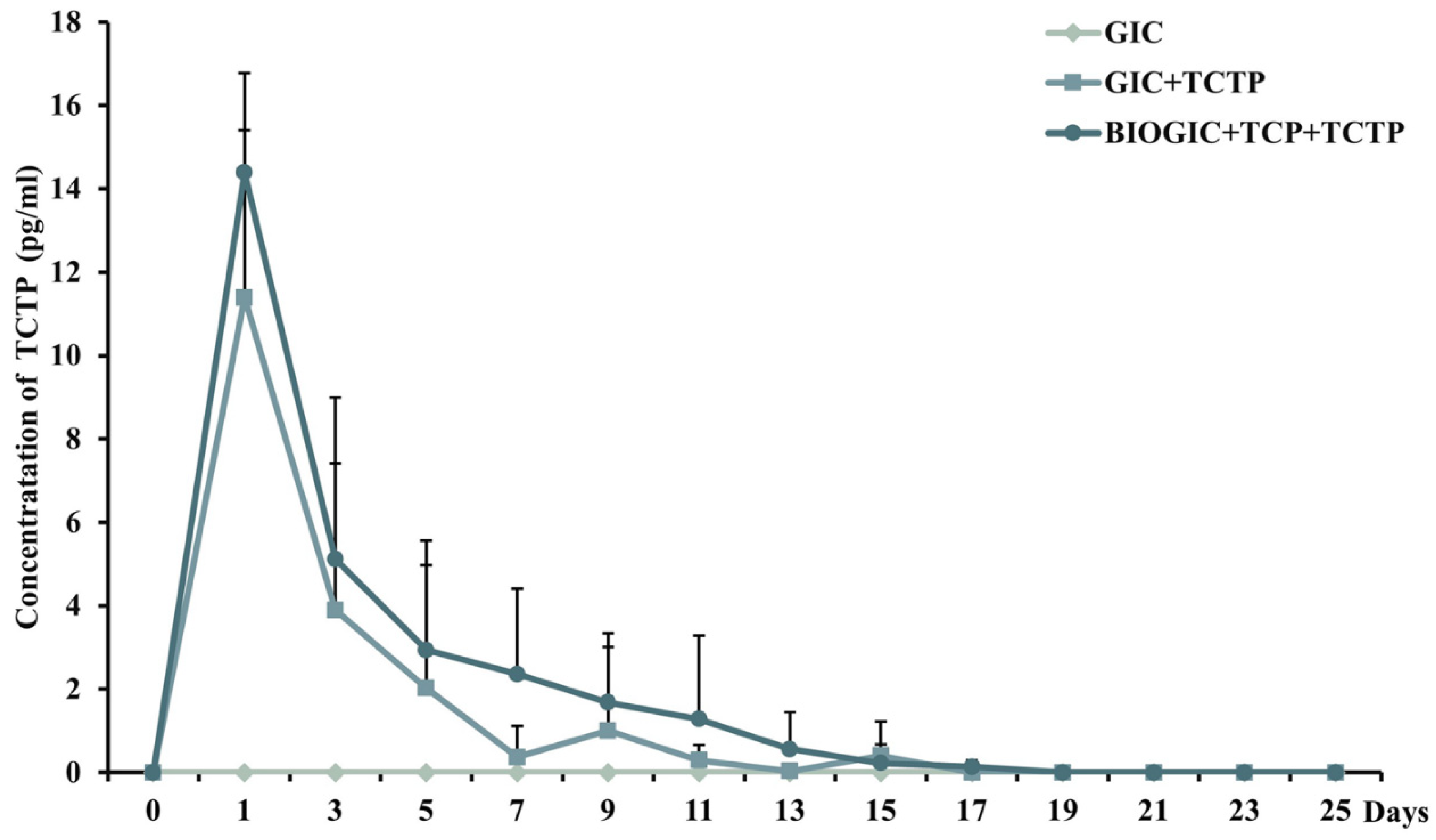
2.7. Determination of the Released Pmer-TCTP
2.8. Long-Term Cell Viability Assay
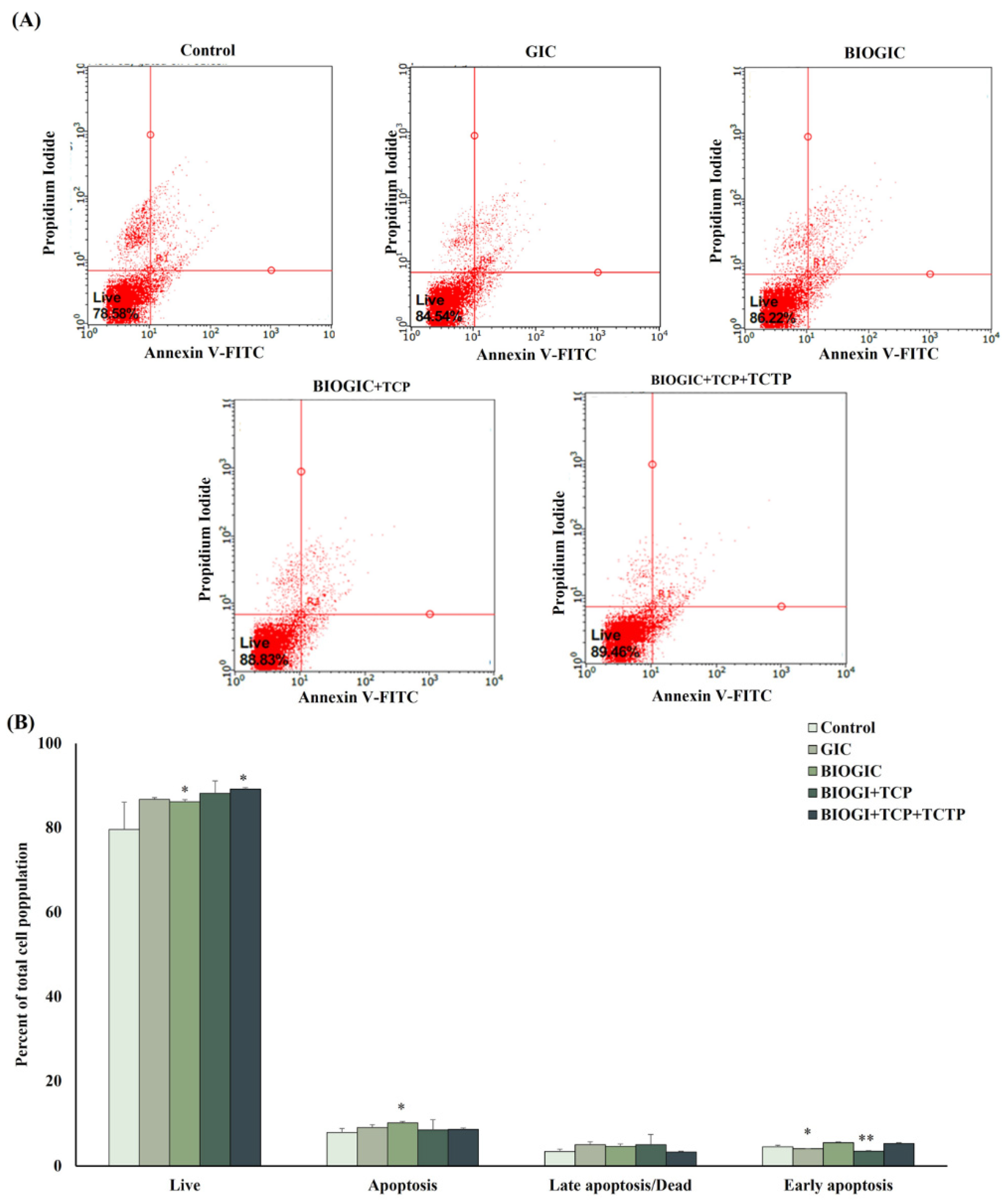
2.9. Apoptotic Assay
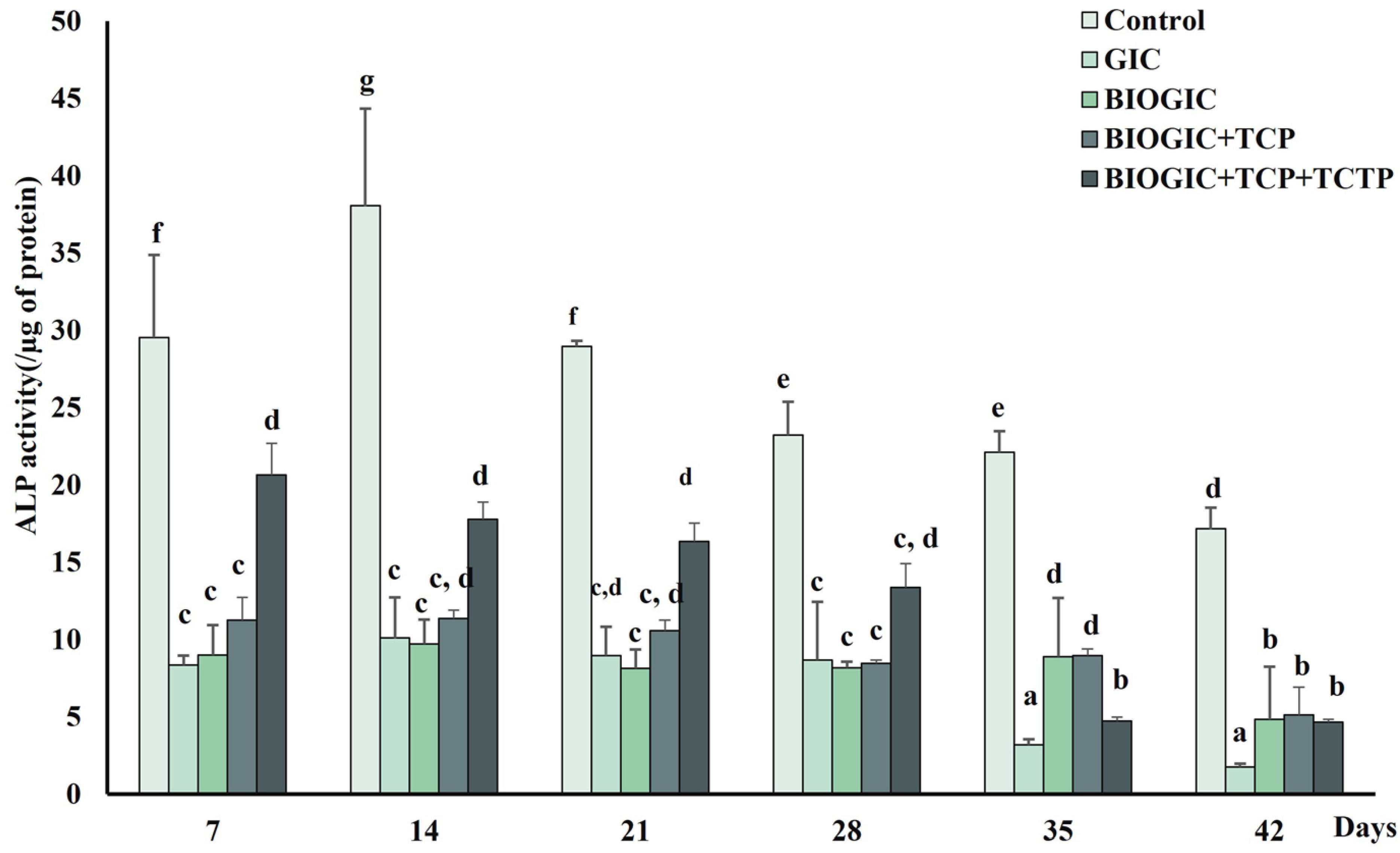
2.10. Alkaline Phosphatase (ALP) Activity Assay
2.11. Alizarin Red Staining (ARS)
2.12. Determination of Gene Expression by Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.13. Statistical Analysis
3. Results
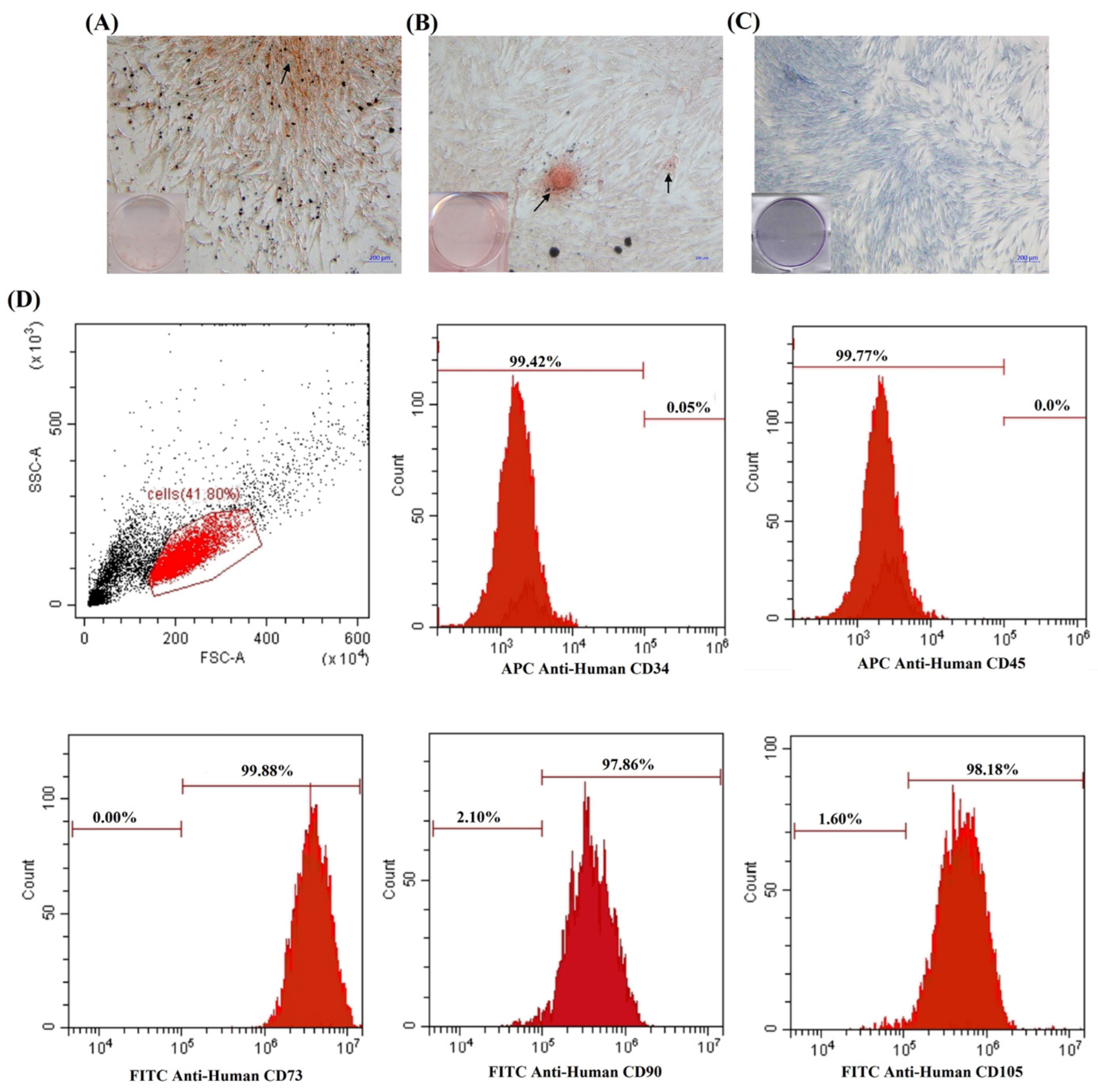
3.1. Characterization of hDPSCs
3.2. X-ray Diffractometer (XRD)
3.3. Energy-Dispersive X-ray Spectroscopy (EDX)
3.4. Pmer-TCTP Release
3.5. Long-Term In Vitro Effect of Different GICs on hDPSCs
3.6. Apoptosis Assay
3.7. Alkaline Phosphatase (ALP) Activity
3.8. Mineralization of hDPSCs
3.9. Apoptosis-Related Gene Expression by qRT-PCR
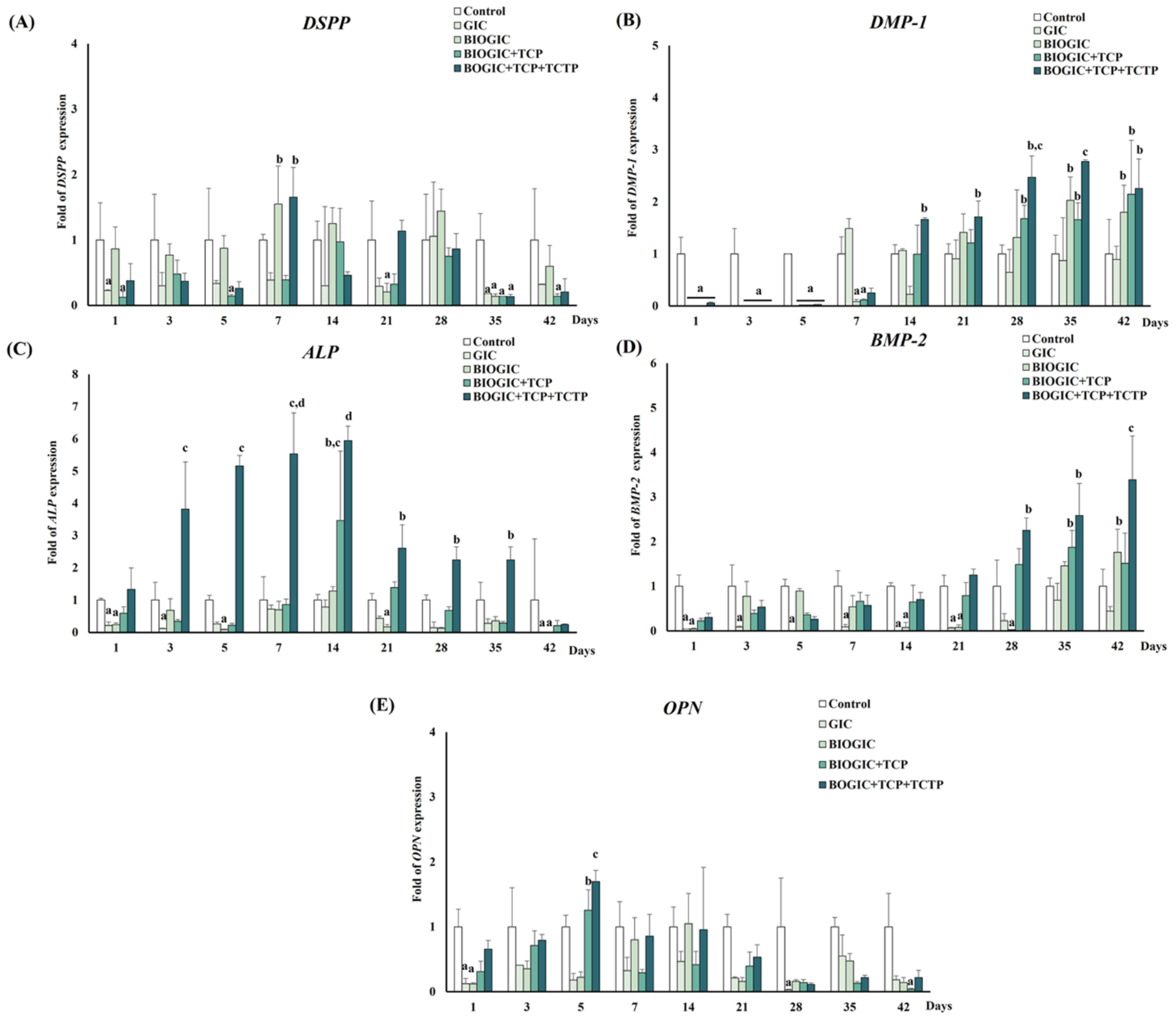
3.10. mRNA Gene Expression Related to Odontogenic/Osteogenic Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institution Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Schmalz, G. Endodontic regeneration: Hard shell, soft core. Odontology 2021, 109, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Neo, J.; Esguerra, R.J.; Fawzy, A.S. Characterization of antibacterial and adhesion properties of chitosan-modified glass ionomer cement. J. Biomater. Appl. 2015, 30, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, B. Analysis of anion and cation release from glass ionomer cements using ion chromatography. Fluoride 2020, 53, 447–456. [Google Scholar]
- Billington, R.; Williams, J.; Pearson, G. Ion processes in glass ionomer cements. J. Dent. 2006, 34, 544–555. [Google Scholar] [CrossRef]
- Silva, R.M.; Pereira, F.V.; Mota, F.A.; Watanabe, E.; Soares, S.M.; Santos, M.H. Dental glass ionomer cement reinforced by cellulose microfibers and cellulose nanocrystals. Mater. Sci. Eng. C 2016, 58, 389–395. [Google Scholar] [CrossRef]
- Mao, B.; Xie, Y.; Yang, H.; Yu, C.; Ma, P.; You, Z.; Tsauo, C.; Chen, Y.; Cheng, L.; Han, Q. Casein phosphopeptide-amorphous calcium phosphate modified glass ionomer cement attenuates demineralization and modulates biofilm composition in dental caries. Dent. Mater. J. 2021, 40, 84–93. [Google Scholar] [CrossRef]
- Lucas, M.E.; Arita, K.; Nishino, M. Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cement. Biomaterials 2003, 24, 3787–3794. [Google Scholar] [CrossRef]
- Limapornvanich, A.; Jitpukdeebodintra, S.; Hengtrakool, C.; Kedjarune-Leggat, U. Bovine serum albumin release from novel chitosan-fluoro-aluminosilicate glass ionomer cement: Stability and cytotoxicity studies. J. Dent. 2009, 37, 686–690. [Google Scholar] [CrossRef]
- Rakkiettiwong, N.; Hengtrakool, C.; Thammasitboon, K.; Kedjarune-Leggat, U. Effect of novel chitosan-fluoroaluminosilicate glass ionomer cement with added transforming growth factor beta-1 on pulp cells. J. Endod. 2011, 37, 367–371. [Google Scholar] [CrossRef]
- Celik, H.; Aslan Felek, S.; Islam, A.; Demirci, M.; Samim, E.; Oztuna, D. The impact of fixated glass ionomer cement and springy cortical bone incudostapedial joint reconstruction on hearing results. Acta Otolaryngol. 2009, 129, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Cole, I.; Dan, N.; Anker, A. Bone replacement in head and neck surgery: A biocompatible alternative. Aust. N. Z. J. Surg. 1996, 66, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Bijle, M.N.; Ekambaram, M.; Lo, E.C.; Yiu, C.K.Y. Antibacterial and mechanical properties of arginine-containing glass ionomer cements. Dent. Mater. 2020, 36, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Hengtrakool, C.; Pearson, G.; Wilson, M. Interaction between GIC and S. sanguis biofilms: Antibacterial properties and changes of surface hardness. J. Dent. 2006, 34, 588–597. [Google Scholar] [CrossRef]
- Bommer, U.A. The Translational Controlled Tumour Protein TCTP: Biological Functions and Regulation. Results Probl. Cell Differ. 2017, 64, 69–126. [Google Scholar] [CrossRef]
- Nagano-Ito, M.; Ichikawa, S. Biological effects of Mammalian translationally controlled tumor protein (TCTP) on cell death, proliferation, and tumorigenesis. Biochem. Res. Int. 2012, 2012, 204960. [Google Scholar] [CrossRef]
- Graidist, P.; Yazawa, M.; Tonganunt, M.; Nakatomi, A.; Lin, C.C.-J.; Chang, J.-Y.; Phongdara, A.; Fujise, K. Fortilin binds Ca2+ and blocks Ca2+-dependent apoptosis in vivo. Biochem. J. 2007, 408, 181–191. [Google Scholar] [CrossRef]
- Lucibello, M.; Gambacurta, A.; Zonfrillo, M.; Pierimarchi, P.; Serafino, A.; Rasi, G.; Rubartelli, A.; Garaci, E. TCTP is a critical survival factor that protects cancer cells from oxidative stress-induced cell-death. Exp. Cell Res. 2011, 317, 2479–2489. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Wanachottrakul, N.; Chotigeat, W.; Kedjarune-Leggat, U. Translationally controlled tumor protein against apoptosis from 2-hydroxy-ethyl methacrylate in human dental pulp cells. J. Mater. Sci. Mater. Med. 2011, 22, 1479. [Google Scholar] [CrossRef]
- Wanachottrakul, N.; Chotigeat, W.; Kedjarune-Leggat, U. Effect of novel chitosan-fluoroaluminosilicate resin modified glass ionomer cement supplemented with translationally controlled tumor protein on pulp cells. J. Mater. Sci. Mater. Med. 2014, 25, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Solderer, A.; Heumann, C.; Attin, T.; Schmidlin, P.R. Tricalcium phosphate (-containing) biomaterials in the treatment of periodontal infra-bony defects: A systematic review and meta-analysis. J. Dent. 2021, 114, 103812. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, H.; Liu, Y.; Fan, B.; Li, X.; Xiao, X.; Lan, P.; Li, M.; Geng, L.; Liu, D.; et al. Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci. Rep. 2016, 6, 23367. [Google Scholar] [CrossRef] [PubMed]
- Loongyai, W.; Phongdara, A.; Chotigeat, W. Cloning and expression of a TCTP homolog from the ovaries of banana prawn. Mar. Biol. 2007, 150, 455–462. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Wanichpakorn, S.; Kedjarune-Leggat, U. Translationally controlled tumor protein supplemented chitosan modified glass ionomer cement promotes osteoblast proliferation and function. Mater. Sci. Eng. C 2015, 54, 61–68. [Google Scholar] [CrossRef]
- Karaöz, E.; Demircan, P.C.; Sağlam, Ö.; Aksoy, A.; Kaymaz, F.; Duruksu, G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem. Cell Biol. 2011, 136, 455. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Chuang, P.-C.; Kuo, H.-K.; Chen, Y.-H.; Su, W.-H.; Wu, P.-C. Differentiation of stem cells from human exfoliated deciduous teeth toward a phenotype of corneal epithelium in vitro. Cornea 2015, 34, 1471–1477. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nuti, N.; Corallo, C.; Chan, B.; Ferrari, M.; Gerami-Naini, B. Multipotent differentiation of human dental pulp stem cells: A literature review. Stem Cell Rev. Rep. 2016, 12, 511–523. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan cross-linked poly (acrylic acid) hydrogels: Drug release control and mechanism. Colloids Surf. B 2017, 152, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, S.; Mehrnejad, F.; Lotfi-Sousefi, Z.; Yahyaei, M.; Naderi-Manesh, H. Design and synthesis of polyacrylic acid/deoxycholic acid-modified chitosan copolymer and a close inspection of human growth hormone-copolymer interactions: An experimental and computational study. Colloids Surf. B 2021, 206, 111956. [Google Scholar] [CrossRef]
- Saito, K.; Nakatomi, M.; Ohshima, H. Dynamics of bromodeoxyuridine label–retaining dental pulp cells during pulpal healing after cavity preparation in mice. J. Endod. 2013, 39, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, C.; Kimura, K.; Nakayama, T.; Toyoshima, K.; Terashita, M. Primary and secondary induction of apoptosis in odontoblasts after cavity preparation of rat molars. J. Dent. Res. 2001, 80, 1530–1534. [Google Scholar] [CrossRef]
- Kedjarune-Leggat, U.; Saetan, U.; Khongsaengkaeo, A.; Suwannarat, S.; Deachamag, P.; Wonglapsuwan, M.; Pornprasit, R.; Thongkamwitoon, W.; Phumklai, P.; Chaichanan, J.; et al. Biological activities of a recombinant fortilin from Fenneropenaeus merguiensis. PLoS ONE 2020, 15, e0239672. [Google Scholar] [CrossRef]
- Mao, K.; Zhou, F.; Cui, F.; Li, J.; Hou, X.; Li, P.; Du, M.; Liang, M.; Wang, Y. Preparation and properties of α-calcium sulphate hemihydrate and β-tricalcium phosphate bone substitute. Biomed. Mater. Eng. 2013, 23, 197–210. [Google Scholar] [CrossRef]
- Taktak, R.; Elghazel, A.; Bouaziz, J.; Charfi, S.; Keskes, H. Tricalcium phosphate-fluorapatite as bone tissue engineering: Evaluation of bioactivity and biocompatibility. Mater. Sci. Eng. C 2018, 86, 121–128. [Google Scholar] [CrossRef]
- Qin, C.; D’Souza, R.; Feng, J. Dentin matrix protein 1 (DMP1): New and important roles for biomineralization and phosphate homeostasis. J. Dent. Res. 2007, 86, 1134–1141. [Google Scholar] [CrossRef]
- Malik, Z.; Alexiou, M.; Hallgrimsson, B.; Economides, A.N.; Luder, H.U.; Graf, D. Bone Morphogenetic Protein 2 Coordinates Early Tooth Mineralization. J. Dent. Res. 2018, 97, 835–843. [Google Scholar] [CrossRef]
- Yang, G.; Yuan, G.; MacDougall, M.; Zhi, C.; Chen, S. BMP-2 induced Dspp transcription is mediated by Dlx3/Osx signaling pathway in odontoblasts. Sci. Rep. 2017, 7, 10775. [Google Scholar] [CrossRef]
- Saito, K.; Nakatomi, M.; Ida-Yonemochi, H.; Ohshima, H. Osteopontin is essential for type I collagen secretion in reparative dentin. J. Dent. Res. 2016, 95, 1034–1041. [Google Scholar] [CrossRef] [PubMed]




| Groups | Powder Compositions (% by Weight) |
|---|---|
| GIC | 100% of glass ionomer cement powder |
| BIOGIC | 80% of glass ionomer cement powder, 15% of chitosan, and 5% of bovine serum albumin |
| BIOGIC + TCP | 79.95% of glass ionomer cement powder, 15% of chitosan, 5% of bovine serum albumin, and 0.05% of tricalcium phosphate |
| BIOGIC + TCP + TCTP | 79.95% of glass ionomer cement powder, 15% chitosan, 5% of bovine serum albumin, 0.05% of tricalcium phosphate, and 1 µg Pmer-TCTP (added when mixing the powder and liquid components) |
| Gene | Primer Sequence (5′-3′) | GenBank Code |
|---|---|---|
| BAX | F: TGCTTCAGGGTTTCATCCAG R: GGCGGCAATCATCCTCTG | NC_000019 |
| TPT1 | F: AAATGTTAACAAATGTGGCAATTAT R: AACAATGCCTCCACTCCAAA | NM_003295.3 |
| Caspase-3 | F: AGAACTGGACTGTGGCATTGAG R: GCTTGTCGGCATACTGTTTCAG | NC_000004.12 |
| BCL-2 | F: TTTGAGTTCGGTGGGGTCAT R: TGACTTCACTTGTGGCCCAG | NM_000633 |
| DSPP | F: GGGATGTTGGCGATGCA R: CCAGCTACTTGAGGTCCATCTTC | NM_014208.3 |
| DMP-1 | F: GCAGAGTGATGACCCAGAG R: GCTCGCTTCTGTCATCTTCC | NM_004407.3 |
| ALP | F: CCACAAGCCCGTGACAGA R: GCGGCAGACTTTGGTTTC | NM_001127501 |
| BMP-2 | F: GCTTCCGCCTGTTTGTGTTTG R: AAGAGACATGTGAGGATTAGCAGGT | NM_007553 |
| OPN | F: ACACATATTGATGGCCGAAGGTGA R: TGTGAGGTGATGTCCTCGTCTGT | NM_00582.2 |
| GAPDH | F: GCACCGTCAAGGCTGAGAAC R: ATGGTGGTGAAGACGCCAGT | NM_001289745.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangsuwan, P.; Chotigeat, W.; Tannukit, S.; Kedjarune-Leggat, U. Long-Term Effect of Modified Glass Ionomer Cement with Mimicked Biological Property of Recombinant Translationally Controlled Protein. Polymers 2022, 14, 3341. https://doi.org/10.3390/polym14163341
Sangsuwan P, Chotigeat W, Tannukit S, Kedjarune-Leggat U. Long-Term Effect of Modified Glass Ionomer Cement with Mimicked Biological Property of Recombinant Translationally Controlled Protein. Polymers. 2022; 14(16):3341. https://doi.org/10.3390/polym14163341
Chicago/Turabian StyleSangsuwan, Prawichaya, Wilaiwan Chotigeat, Sissada Tannukit, and Ureporn Kedjarune-Leggat. 2022. "Long-Term Effect of Modified Glass Ionomer Cement with Mimicked Biological Property of Recombinant Translationally Controlled Protein" Polymers 14, no. 16: 3341. https://doi.org/10.3390/polym14163341
APA StyleSangsuwan, P., Chotigeat, W., Tannukit, S., & Kedjarune-Leggat, U. (2022). Long-Term Effect of Modified Glass Ionomer Cement with Mimicked Biological Property of Recombinant Translationally Controlled Protein. Polymers, 14(16), 3341. https://doi.org/10.3390/polym14163341