A Tumor Accelerator Based on Multicomponent Bone Scaffolds and Cancer Cell Homing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Gelatin Hydroxyapatite Glutaraldehyde (GHG) Scaffold Preparation
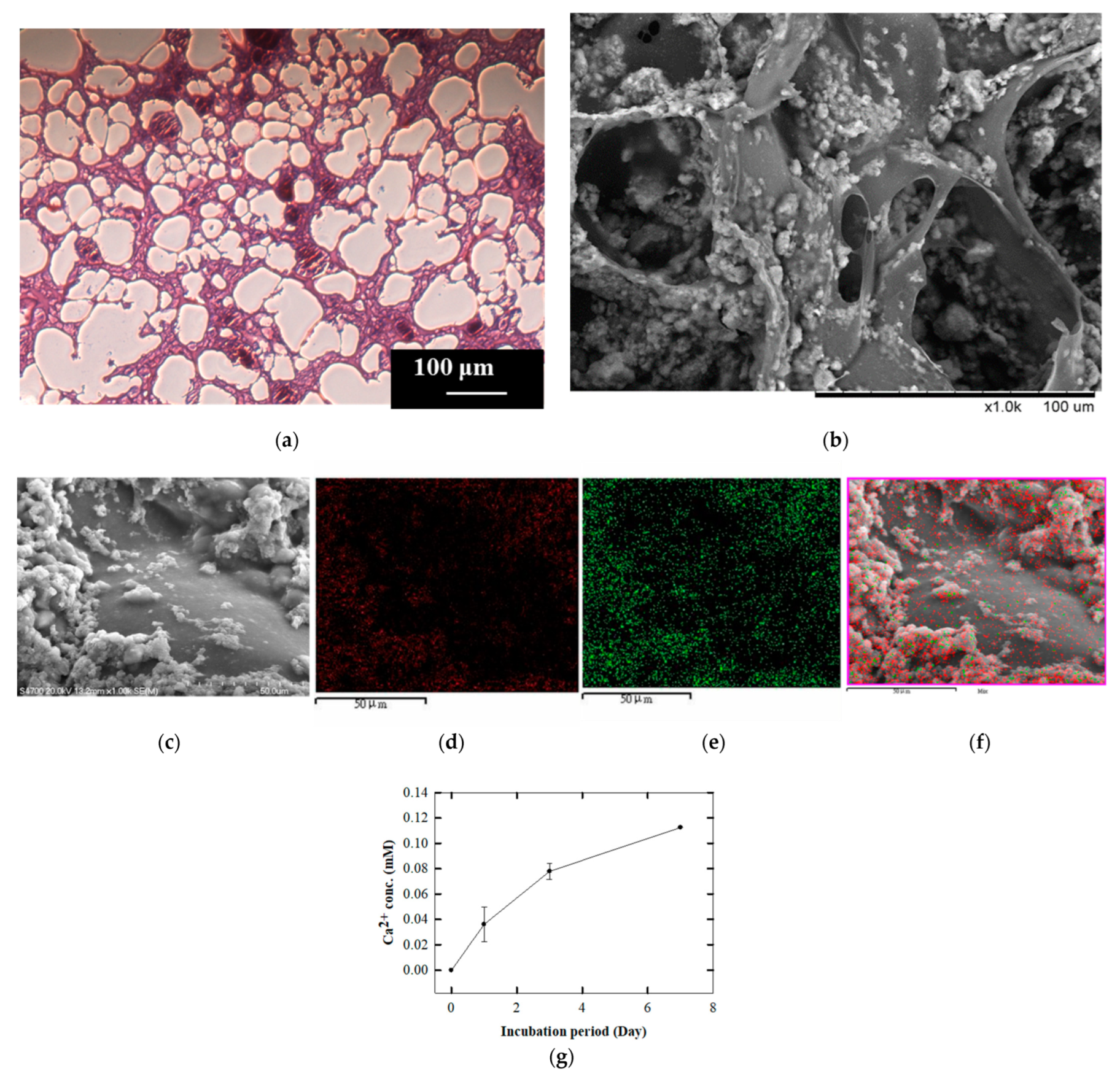
2.3. Calcium Release Assay in GHG Scaffold
2.4. EGF Immobilization
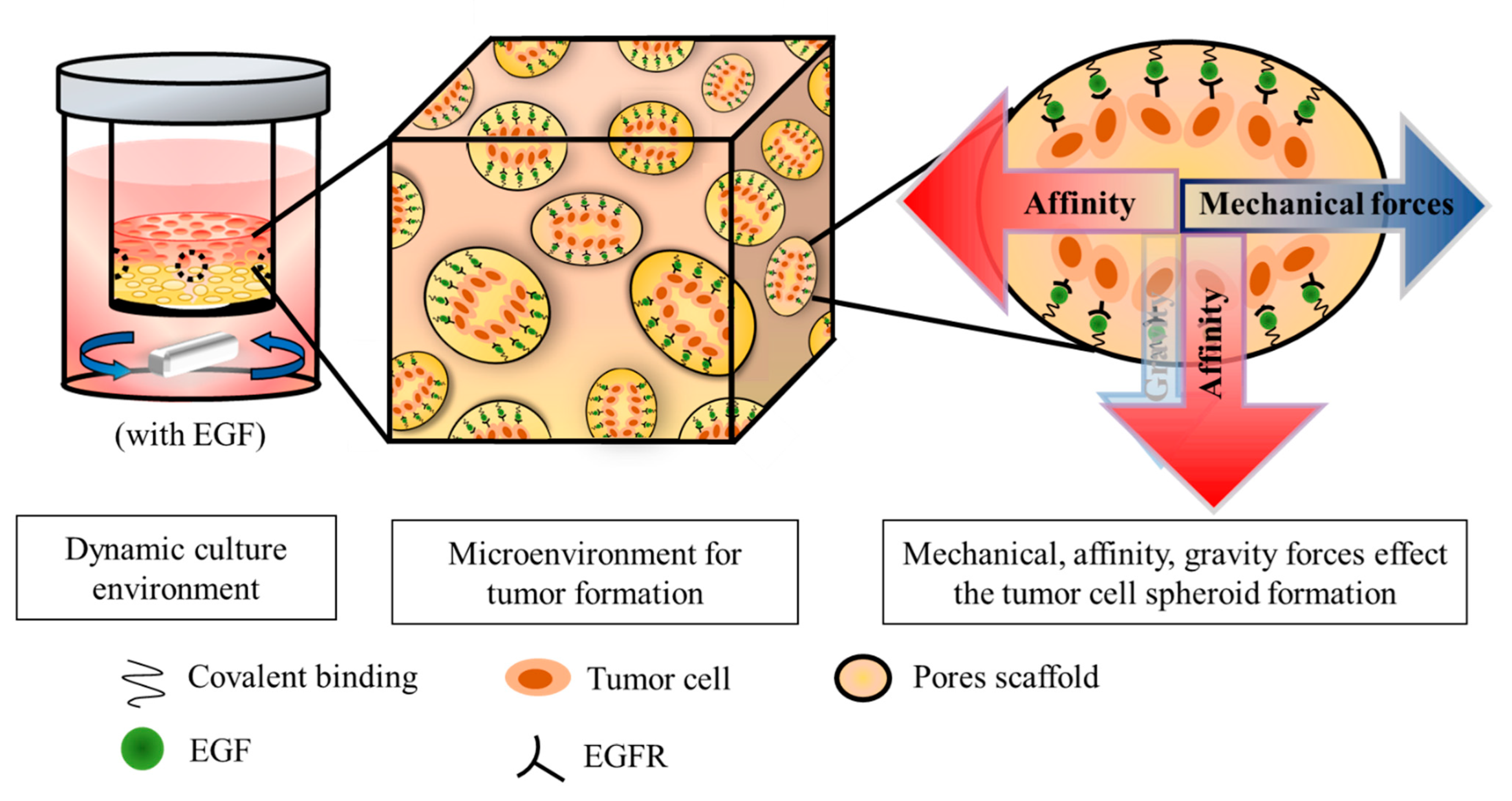
2.5. Dynamic Culture of Tumor Cells on Scaffold
2.6. MDA-MB-231 Proliferation Analysis
2.7. Effects of Scaffold Components and EGF on Cell Growth
2.8. Effects of EGF on the Proliferation of MDA-MB-231 in Dynamic Culture
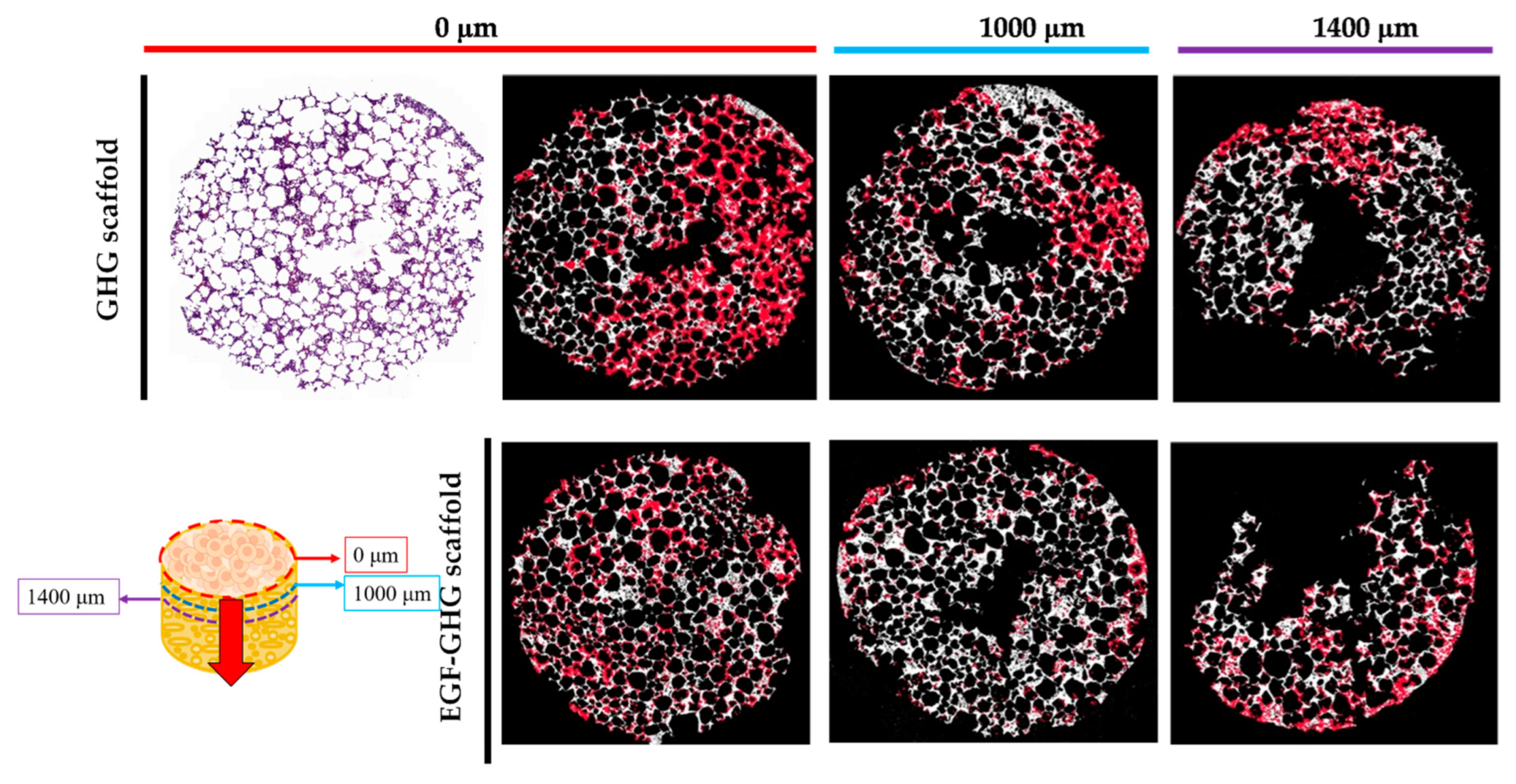
2.9. Effects of EGF on the Growth and Distribution of MDA-MB-231 on Scaffold
3. Results
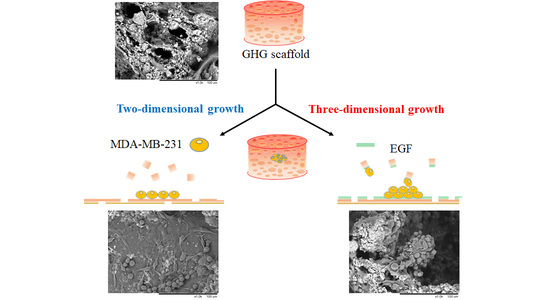
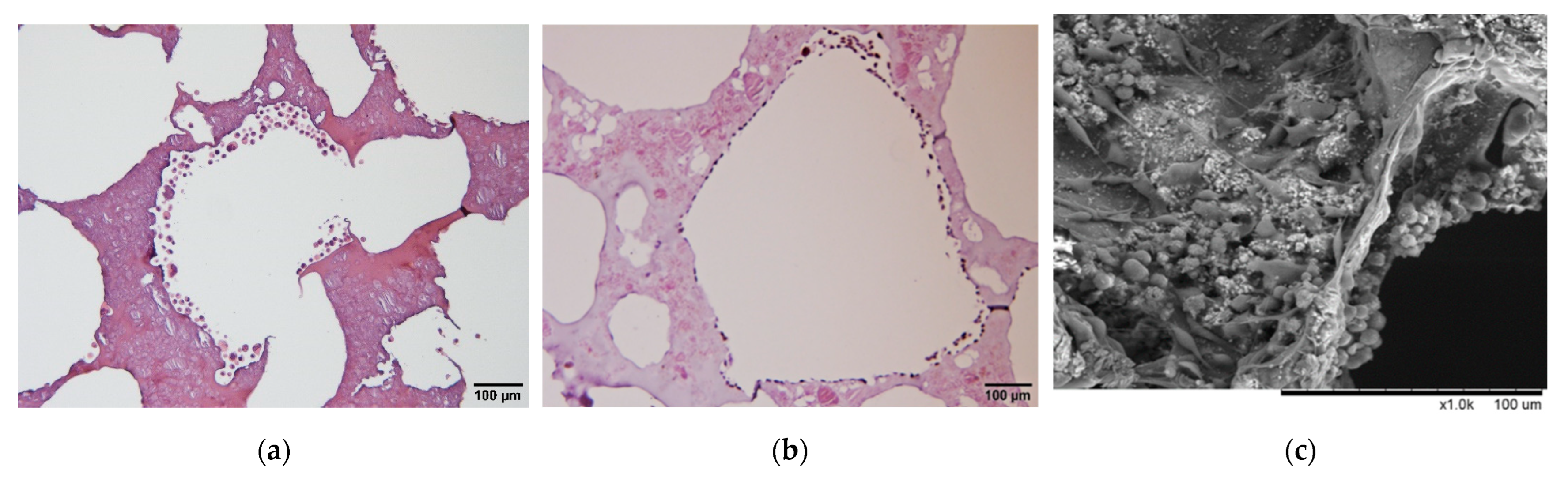
3.1. Preparation of Gelatin Hydroxyapatite Glutaraldehyde (GHG) Scaffold
3.2. Analysis of Micro-Characteristics of GHG Scaffold
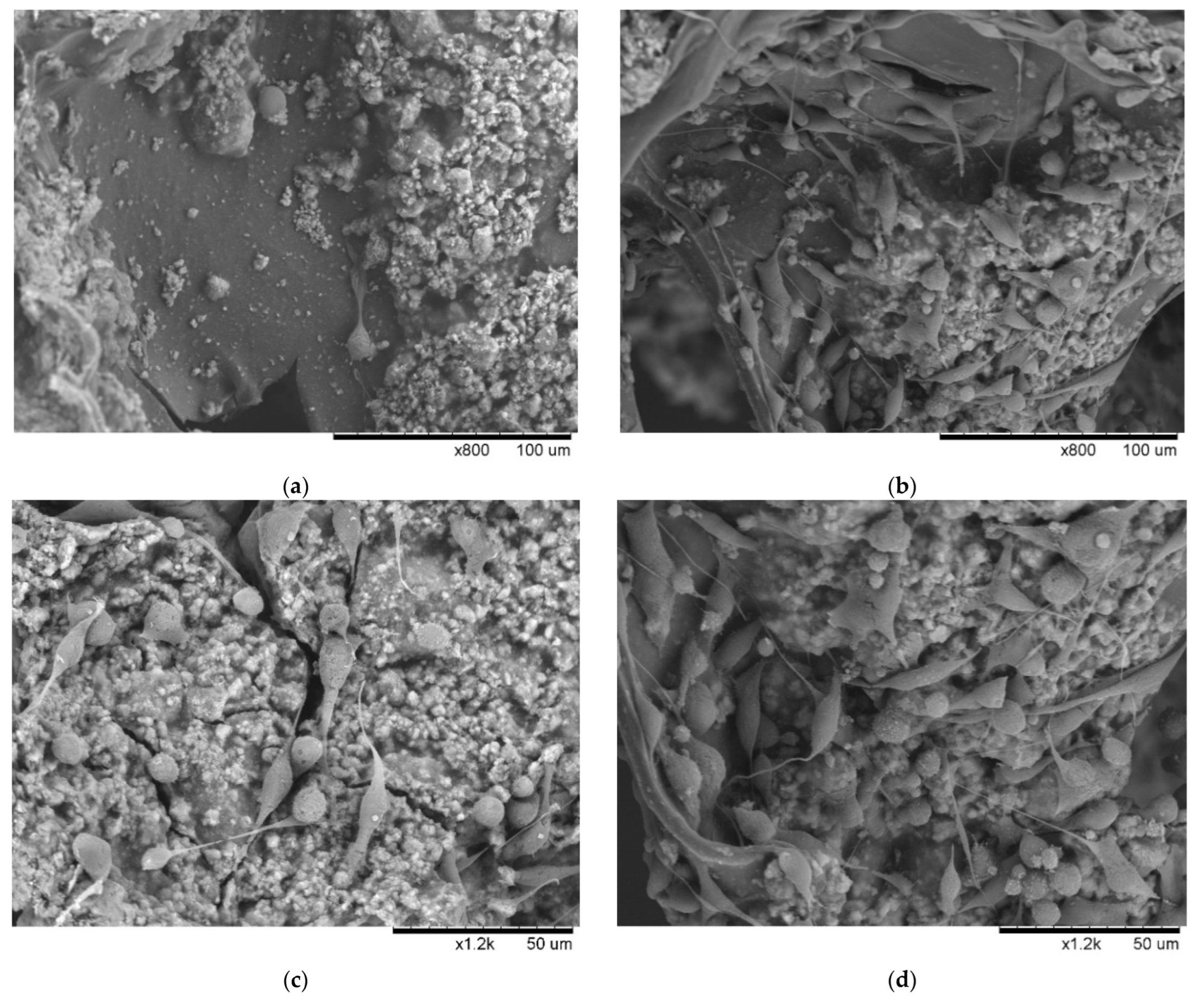
3.3. Effects of Scaffold Components and EGF on Cell Growth
3.4. Effects of EGF on the Proliferation of MDA-MB-231 in Dynamic Culture Based on Composite Matrix
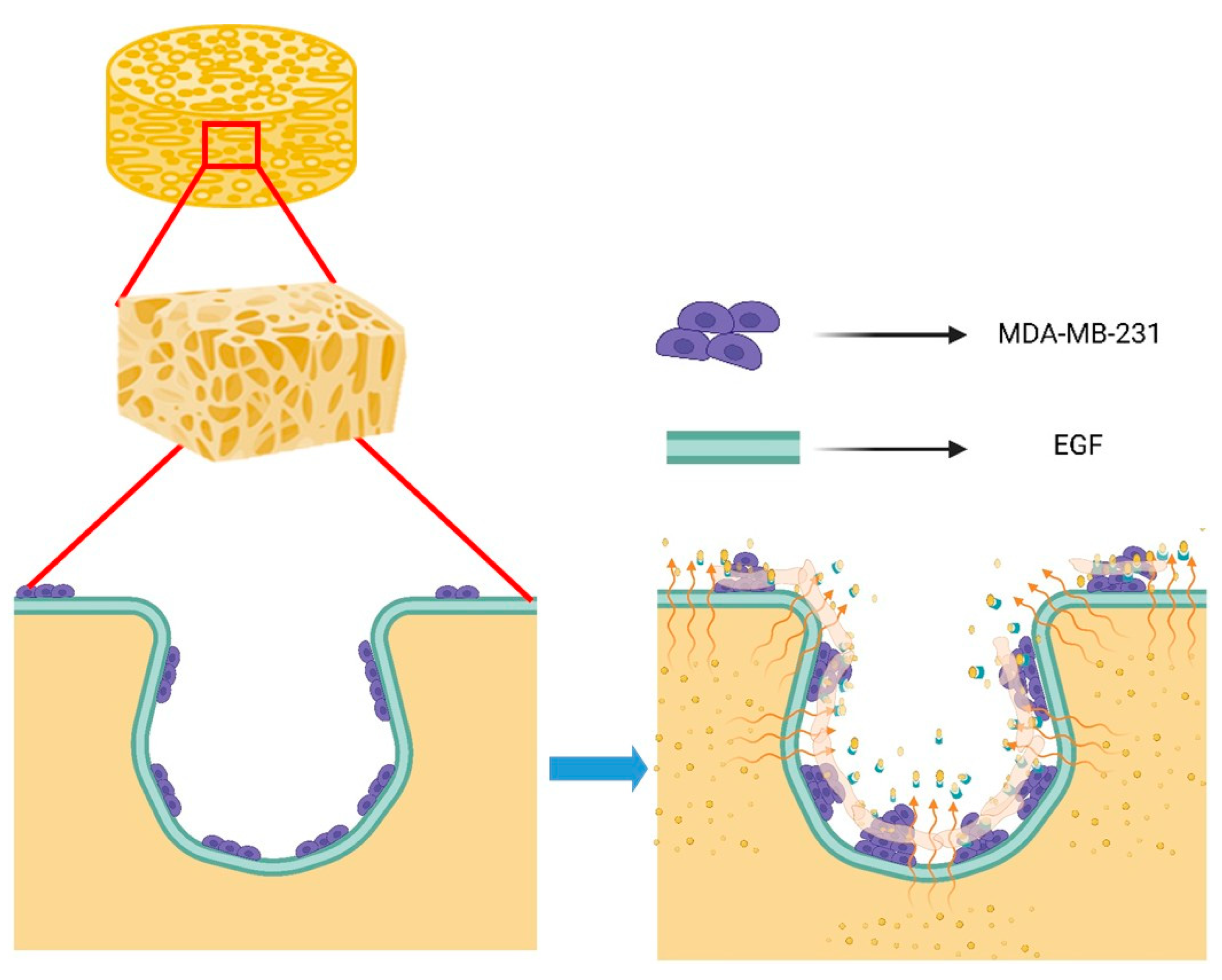
3.5. Contribution of EGF to the Behavior of MDA-MB-231 on Scaffold Based on a Multi-Component Matrix
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liverani, C.; De Vita, A.; Minardi, S.; Kang, Y.; Mercatali, L.; Amadori, D.; Bongiovanni, A.; La Manna, F.; Ibrahim, T.; Tasciotti, E. A biomimetic 3D model of hypoxia-driven cancer progression. Sci. Rep. 2019, 9, 12263. [Google Scholar] [CrossRef]
- Pape, J.; Stamati, K.; Al Hosni, R.; Uchegbu, I.F.; Schatzlein, A.G.; Loizidou, M.; Emberton, M.; Cheema, U. Tissue-Engineering the Fibrous Pancreatic Tumour Stroma Capsule in 3D Tumouroids to Demonstrate Paclitaxel Response. Int. J. Mol. Sci. 2021, 22, 4289. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Kuo, P.L. Bone Metastasis from Renal Cell Carcinoma. Int. J. Mol. Sci. 2016, 17, 987. [Google Scholar] [CrossRef]
- Al Husaini, H.; Wheatley-Price, P.; Clemons, M.; Shepherd, F.A. Prevention and management of bone metastases in lung cancer: A review. J. Thorac. Oncol. 2009, 4, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Pape, J.; Emberton, M.; Cheema, U. 3D Cancer Models: The Need for a Complex Stroma, Compartmentalization and Stiffness. Front. Bioeng. Biotechnol. 2021, 9, 660502. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Okoro, O.V.; Nie, L.; Petri, D.F.S.; Shavandi, A. Protein-Based 3D Biofabrication of Biomaterials. Bioengineering 2021, 8, 48. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Khoneisser, J.; Huang, P.C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef]
- Han, Y.; Wei, Q.; Chang, P.; Hu, K.; Okoro, O.V.; Shavandi, A.; Nie, L. Three-Dimensional Printing of Hydroxyapatite Composites for Biomedical Application. Crystals 2021, 11, 353. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Tian, X.; Cui, G.; Zhao, Y.; Yang, Q.; Yu, S.; Xing, G.; Zhang, B. Segmental bone regeneration using an rhBMP-2-loaded gelatin/nanohydroxyapatite/fibrin scaffold in a rabbit model. Biomaterials 2009, 30, 6276–6285. [Google Scholar] [CrossRef]
- Doyle, A.D.; Carvajal, N.; Jin, A.; Matsumoto, K.; Yamada, K.M. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 2015, 6, 8720. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, T.; Tengattini, S.; Rezwan, R.; Chiesa, E.; Temporini, C.; Dorati, R.; Massolini, G.; Conti, B.; Ubiali, D.; Terreni, M. Design of epidermal growth factor immobilization on 3D biocompatible scaffolds to promote tissue repair and regeneration. Sci. Rep. 2021, 11, 2629. [Google Scholar] [CrossRef] [PubMed]
- Masters, K.S. Covalent growth factor immobilization strategies for tissue repair and regeneration. Macromol. Biosci. 2011, 11, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E.A.; Templeman, K.L.; Thule, P.M.; Garcia, A.J. Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization. Drug Deliv. Transl. Res. 2015, 5, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, S.J.; Liu, G.; Kievit, F.M.; Lewis, A.M.; Wu, J.D.; Zhang, M. 3D porous chitosan-alginate scaffolds: A new matrix for studying prostate cancer cell-lymphocyte interactions in vitro. Adv. Healthc. Mater. 2012, 1, 590–599. [Google Scholar] [CrossRef]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Thibaudeau, L.; Taubenberger, A.V.; Holzapfel, B.M.; Quent, V.M.; Fuehrmann, T.; Hesami, P.; Brown, T.D.; Dalton, P.D.; Power, C.A.; Hollier, B.G.; et al. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis. Model. Mech. 2014, 7, 299–309. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, J.; Wang, Z.Y. A switch role of Src in the biphasic EGF signaling of ER-negative breast cancer cells. PLoS ONE 2012, 7, e41613. [Google Scholar] [CrossRef]
- Kar, S.; Molla, M.S.; Katti, D.R.; Katti, K.S. Tissue-engineered nanoclay-based 3D in vitro breast cancer model for studying breast cancer metastasis to bone. J. Tissue Eng. Regen. Med. 2019, 13, 119–130. [Google Scholar] [CrossRef]
- Xu, X.; Gurski, L.A.; Zhang, C.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials 2012, 33, 9049–9060. [Google Scholar] [CrossRef]
- Malanchi, I.; Santamaria-Martinez, A.; Susanto, E.; Peng, H.; Lehr, H.A.; Delaloye, J.F.; Huelsken, J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011, 481, 85–89. [Google Scholar] [CrossRef]
- Von Moos, R.; Haynes, I. Where Do Bone-Targeted Agents RANK in Breast Cancer Treatment? J. Clin. Med. 2013, 2, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, T.; Massague, J. Extracellular matrix players in metastatic niches. EMBO J. 2012, 31, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Geetha Bai, R.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Christofori, G. Rebuilding cancer metastasis in the mouse. Mol. Oncol. 2013, 7, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.C.; Ma, T.Y.; Li, C.H.; Chi, C.Y.; Su, C.M.; Huang, C.L.; Wang, Y.H.; Lee, T.M. A study of Drynaria fortunei in modulation of BMP-2 signalling by bone tissue engineering. Turk. J. Med. Sci. 2020, 50, 1444–1453. [Google Scholar] [CrossRef]
- Hokazono, E.; Osawa, S.; Nakano, T.; Kawamoto, Y.; Oguchi, Y.; Hotta, T.; Kayamori, Y.; Kang, D.; Cho, Y.; Shiba, K.; et al. Development of a new measurement method for serum calcium with chlorophosphonazo-III. Ann. Clin. Biochem. 2009, 46, 296–301. [Google Scholar] [CrossRef]
- Tseng, C.L.; Wang, T.W.; Dong, G.C.; Yueh-Hsiu Wu, S.; Young, T.H.; Shieh, M.J.; Lou, P.J.; Lin, F.H. Development of gelatin nanoparticles with biotinylated EGF conjugation for lung cancer targeting. Biomaterials 2007, 28, 3996–4005. [Google Scholar] [CrossRef]
- Evrova, O.; Kellenberger, D.; Scalera, C.; Calcagni, M.; Giovanoli, P.; Vogel, V.; Buschmann, J. Impact of UV sterilization and short term storage on the in vitro release kinetics and bioactivity of biomolecules from electrospun scaffolds. Sci. Rep. 2019, 9, 15117. [Google Scholar] [CrossRef]
- Travlos, G.S. Normal structure, function, and histology of the bone marrow. Toxicol. Pathol. 2006, 34, 548–565. [Google Scholar] [CrossRef]
- Chen, X.; Wu, D.; Xu, J.; Yan, T.; Chen, Q. Gelatin/Gelatin-modified nano hydroxyapatite composite scaffolds with hollow channel arrays prepared by extrusion molding for bone tissue engineering. Mater. Res. Express 2021, 8, 025006. [Google Scholar] [CrossRef]
- Liu, X.; Smith, L.A.; Hu, J.; Ma, P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009, 30, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Belgheisi, G.; Haghbin-Nazarpak, M.; Omidi, M.; Khojasteh, A.; Solati-Hashjin, M. Bone tissue engineering gelatin-hydroxyapatite/graphene oxide scaffolds with the ability to release vitamin D: Fabrication, characterization, and in vitro study. J. Mater. Sci. Mater. Med. 2020, 31, 97. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lai, Y.H.; Chen, Y.W.; Yao, C.H.; Chen, K.Y. Immobilization of bone morphogenetic protein-2 to gelatin/avidin-modified hydroxyapatite composite scaffolds for bone regeneration. J. Biomater. Appl. 2019, 33, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, R.F.; Mueser, T.C.; Zaleta-Rivera, K.; Carnes, K.A.; Gonzalez-Valdez, J.; Parkhurst, L.J. Detailed characterization of the solution kinetics and thermodynamics of biotin, biocytin and HABA binding to avidin and streptavidin. PLoS ONE 2019, 14, e0204194. [Google Scholar] [CrossRef]
- Ivers, L.P.; Cummings, B.; Owolabi, F.; Welzel, K.; Klinger, R.; Saitoh, S.; O’Connor, D.; Fujita, Y.; Scholz, D.; Itasaki, N. Dynamic and influential interaction of cancer cells with normal epithelial cells in 3D culture. Cancer Cell Int. 2014, 14, 108. [Google Scholar] [CrossRef]
- Rijal, G.; Li, W. 3D scaffolds in breast cancer research. Biomaterials 2016, 81, 135–156. [Google Scholar] [CrossRef]
- Manupati, K.; Dhoke, N.R.; Debnath, T.; Yeeravalli, R.; Guguloth, K.; Saeidpour, S.; De, U.C.; Debnath, S.; Das, A. Inhibiting epidermal growth factor receptor signalling potentiates mesenchymal-epithelial transition of breast cancer stem cells and their responsiveness to anticancer drugs. FEBS J. 2017, 284, 1830–1854. [Google Scholar] [CrossRef]
- Sommaggio, R.; Cappuzzello, E.; Dalla Pieta, A.; Tosi, A.; Palmerini, P.; Carpanese, D.; Nicole, L.; Rosato, A. Adoptive cell therapy of triple negative breast cancer with redirected cytokine-induced killer cells. Oncoimmunology 2020, 9, 1777046. [Google Scholar] [CrossRef]
- Kim, W.; Takyar, F.M.; Swan, K.; Jeong, J.; VanHouten, J.; Sullivan, C.; Dann, P.; Yu, H.; Fiaschi-Taesch, N.; Chang, W.; et al. Calcium-Sensing Receptor Promotes Breast Cancer by Stimulating Intracrine Actions of Parathyroid Hormone-Related Protein. Cancer Res. 2016, 76, 5348–5360. [Google Scholar] [CrossRef]
- Kozlow, W.; Guise, T.A. Breast cancer metastasis to bone: Mechanisms of osteolysis and implications for therapy. J. Mammary Gland Biol. Neoplasia 2005, 10, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Godet, I.; Yang, Y.; Salman, S.; Lu, H.; Lyu, Y.; Zuo, Q.; Wang, Y.; Zhu, Y.; Chen, C.; et al. Hypoxia-inducible factor-dependent ADAM12 expression mediates breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 2021, 118, e2020490118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Muralidharan, A.; Saateh, A.; Ding, Z.; Ten Dijke, P.; Boukany, P.E. A Programmable Multifunctional 3D Cancer Cell Invasion Micro Platform. Small 2022, 18, e2107757. [Google Scholar] [CrossRef]
- Catterall, R.; Kurdieh, R.; McCaffrey, L. Studying Cell Polarity Dynamics During Cancer Initiation Using Inducible 3D Organotypic Cultures. Methods Mol. Biol. 2022, 2438, 455–466. [Google Scholar] [PubMed]



| GHG Scaffold | |||
|---|---|---|---|
| Time (Day) | Ca2+ Conc. (mM) | Absent EGF | 6.61 EGF (nM) |
| 0 | 0 | 100.0 ± 13.01 | |
| 3 | 0.0824 ± 0.0064 | 119.2 ± 3.82 | 150.0 ± 14.14 |
| 7 | 0.1126 ± 0.0002 | 147.2 ± 16.12 | 170.0 ± 4.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-J.; Chou, P.-K.; Sher, Z.-Y.; Chen, Y.-R.; Chen, T.-Y.; Dong, G.-C. A Tumor Accelerator Based on Multicomponent Bone Scaffolds and Cancer Cell Homing. Polymers 2022, 14, 3340. https://doi.org/10.3390/polym14163340
Huang C-J, Chou P-K, Sher Z-Y, Chen Y-R, Chen T-Y, Dong G-C. A Tumor Accelerator Based on Multicomponent Bone Scaffolds and Cancer Cell Homing. Polymers. 2022; 14(16):3340. https://doi.org/10.3390/polym14163340
Chicago/Turabian StyleHuang, Chen-Ji, Pei-Kuan Chou, Zong-Yi Sher, You-Rong Chen, Tan-Yueh Chen, and Guo-Chung Dong. 2022. "A Tumor Accelerator Based on Multicomponent Bone Scaffolds and Cancer Cell Homing" Polymers 14, no. 16: 3340. https://doi.org/10.3390/polym14163340
APA StyleHuang, C.-J., Chou, P.-K., Sher, Z.-Y., Chen, Y.-R., Chen, T.-Y., & Dong, G.-C. (2022). A Tumor Accelerator Based on Multicomponent Bone Scaffolds and Cancer Cell Homing. Polymers, 14(16), 3340. https://doi.org/10.3390/polym14163340