Assessment of the Performance of Cationic Cellulose Derivatives as Calcium Carbonate Flocculant for Papermaking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cellulose Cationization
2.3. Cationic Cellulose Characterization
2.4. LDS Flocculation Tests
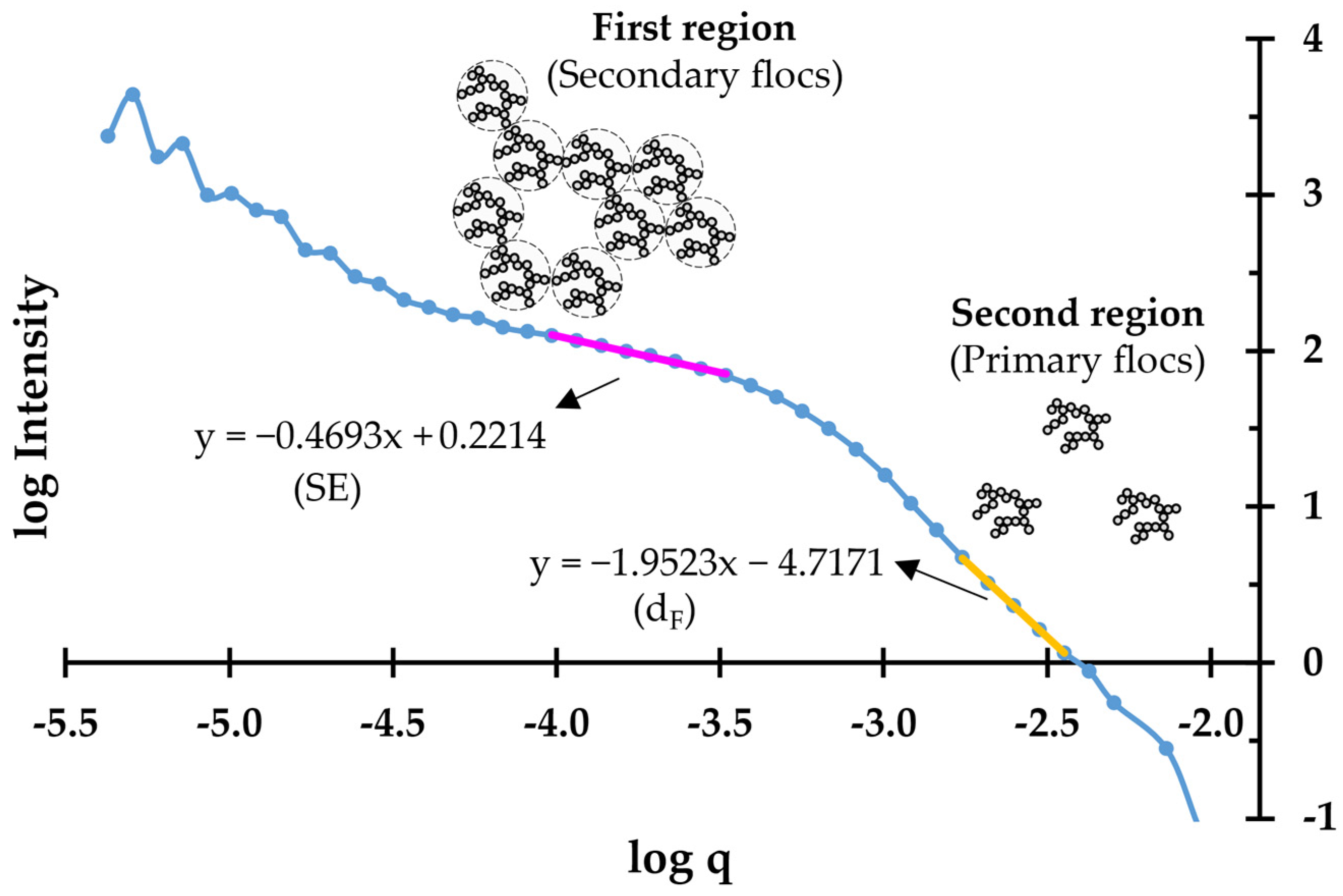
2.5. Mass Fractal Dimension of the PCC Flocs
2.6. Drainability and Retention Tests
3. Results and Discussion
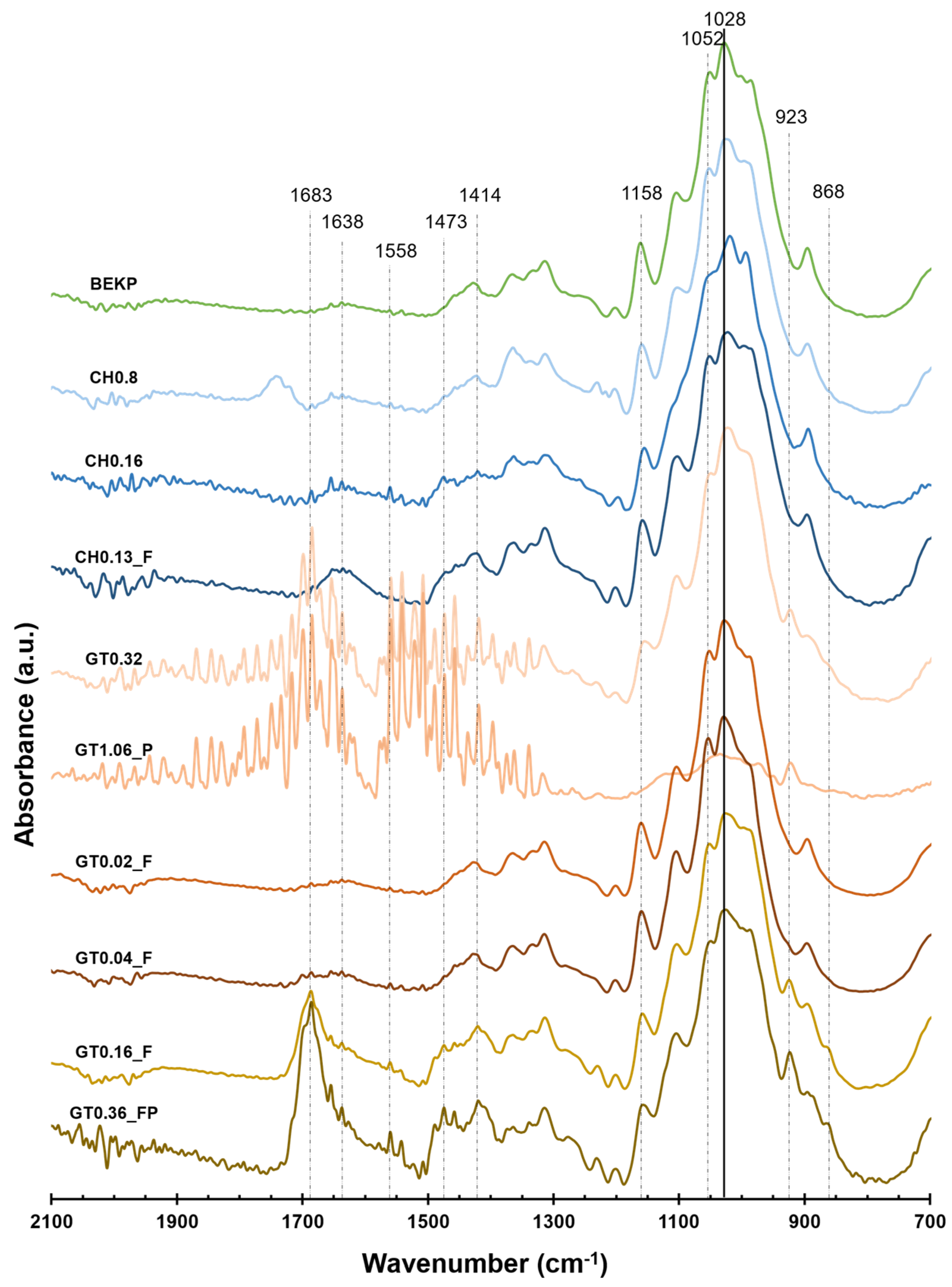
3.1. Chemical Analysis
3.2. Morphological Analysis
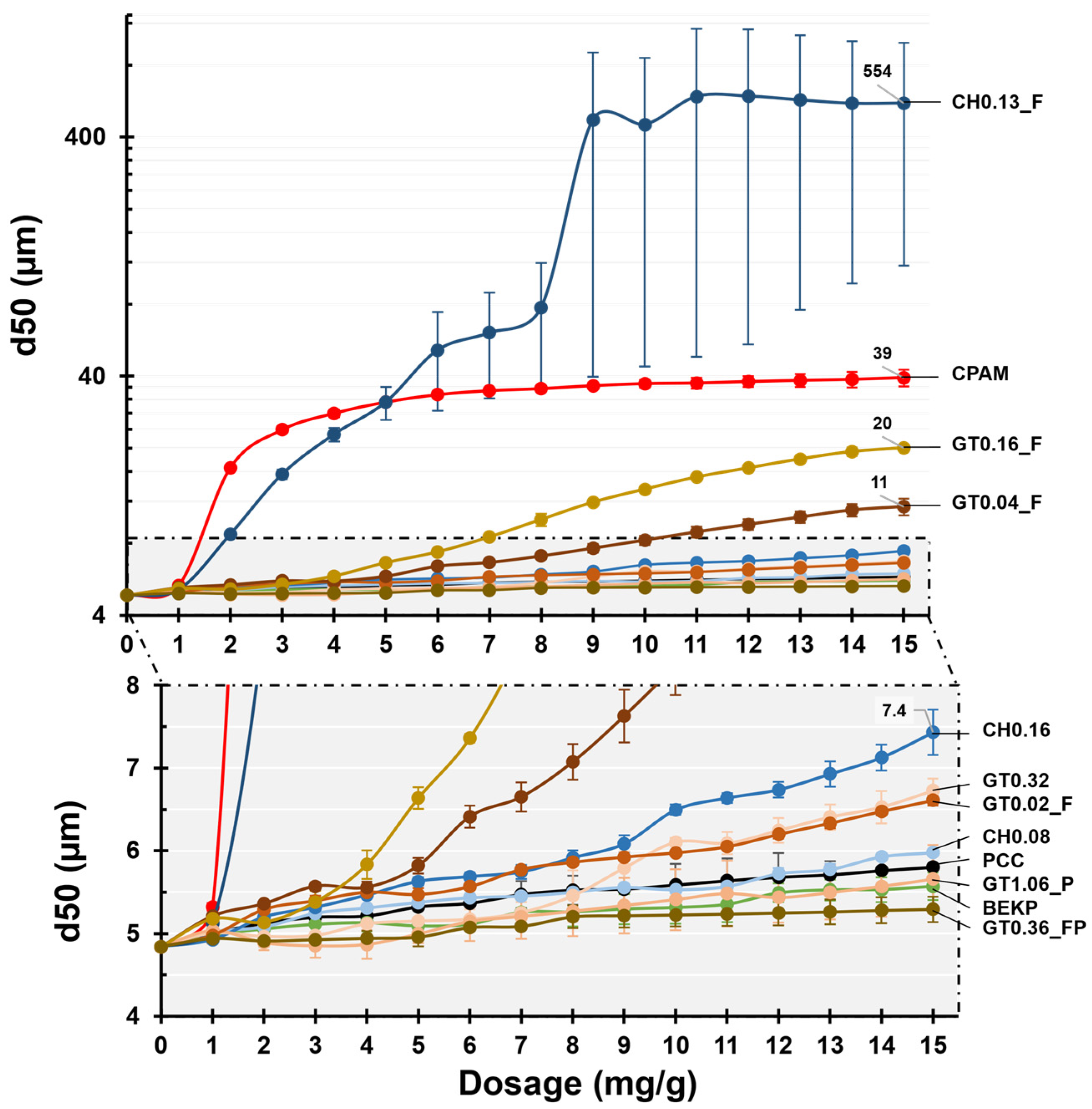
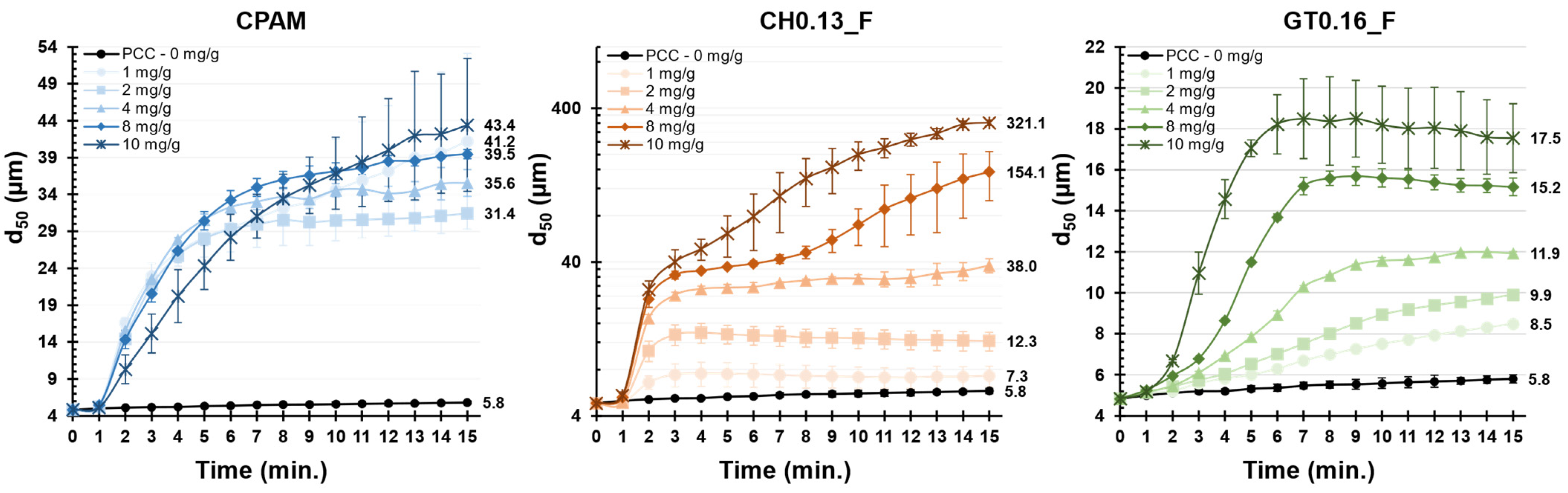
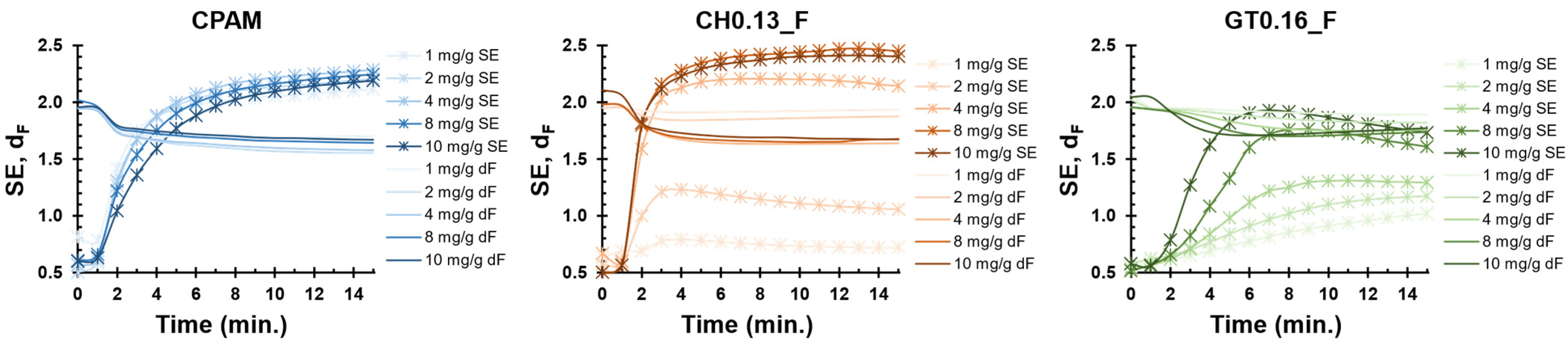
3.3. Performance in the Flocculation Tests
3.4. Mass Fractal Dimension of the PCC Flocs
3.5. Drainability and Filler Retention
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, J.; Song, Z.; Qian, X.; Liu, W. Modification of Papermaking Grade Fillers: A Brief Review. BioResources 2009, 4, 1190–1209. [Google Scholar]
- Thorn, I.; Au, C.O. (Eds.) Applications of Wet-End Paper Chemistry, 2nd ed.; Springer: Dordrecht, The Netherlands; London, UK, 2009; ISBN 978-1-4020-6037-3. [Google Scholar]
- Holik, H. (Ed.) Handbook of Paper and Board; Wiley-VCH: Weinheim, Germany, 2006; ISBN 978-3-527-30997-9. [Google Scholar]
- Biermann, C.J. Handbook of Pulping and Papermaking (Second Edition); Academic Press: San Diego, CA, USA, 1996; ISBN 978-0-08-053368-1. [Google Scholar]
- Hubbe, M.A.; Nanko, H.; McNeal, M.R. Retention Aid Polymer Interactions with Cellulosic Surfaces and Suspensions: A Review. BioResources 2009, 4, 850–906. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Gill, R.A. Fillers for Papermaking: A Review of Their Properties, Usage Practices, and Their Mechanistic Role. BioResources 2016, 11, 2886–2963. [Google Scholar] [CrossRef]
- Roberts, J.C. The Chemistry of Paper; RSC paperbacks; Royal Society of Chemistry: London, UK, 1996; ISBN 978-0-85404-518-1. [Google Scholar]
- Antunes, E.; Garcia, F.A.P.; Ferreira, P.; Blanco, A.; Negro, C.; Rasteiro, M.G. Use of New Branched Cationic Polyacrylamides to Improve Retention and Drainage in Papermaking. Ind. Eng. Chem. Res. 2008, 47, 9370–9375. [Google Scholar] [CrossRef]
- Joshi, S.J.; Abed, R.M.M. Biodegradation of Polyacrylamide and Its Derivatives. Environ. Processes 2017, 4, 463–476. [Google Scholar] [CrossRef]
- King, D.J.; Noss, R.R. Toxicity of Polyacrylamide and Acrylamide Monomer. Rev. Environ. Health 1989, 8, 3–16. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant Extract-Loaded Bacterial Cellulose Composite Membrane for Potential Biomedical Applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Lourenço, A.F.; Gamelas, J.A.F.; Nunes, T.; Amaral, J.; Mutjé, P.; Ferreira, P.J. Influence of TEMPO-Oxidised Cellulose Nanofibrils on the Properties of Filler-Containing Papers. Cellulose 2017, 24, 349–362. [Google Scholar] [CrossRef]
- Lourenço, A.F.; Gamelas, J.A.F.; Sarmento, P.; Ferreira, P.J.T. A Comprehensive Study on Nanocelluloses in Papermaking: The Influence of Common Additives on Filler Retention and Paper Strength. Cellulose 2020, 27, 5297–5309. [Google Scholar] [CrossRef]
- Heinze, T.; Seoud, O.A.E.; Koschella, A. Cellulose Derivatives: Synthesis, Structure, and Properties; Springer Series on Polymer and Composite Materials; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-73168-1. [Google Scholar]
- Aguado, R.; Lourenço, A.F.; Ferreira, P.J.; Moral, A.; Tijero, A. Cationic Cellulosic Derivatives as Flocculants in Papermaking. Cellulose 2017, 24, 3015–3027. [Google Scholar] [CrossRef]
- Aguado, R.; Moral, A.; Tijero, A. Cationic Fibers from Crop Residues: Making Waste More Appealing for Papermaking. J. Clean. Prod. 2018, 174, 1503–1512. [Google Scholar] [CrossRef]
- Diab, M.; Curtil, D.; El-shinnawy, N.; Hassan, M.L.; Zeid, I.F.; Mauret, E. Biobased Polymers and Cationic Microfibrillated Cellulose as Retention and Drainage Aids in Papermaking: Comparison between Softwood and Bagasse Pulps. Ind. Crops Prod. 2015, 72, 34–45. [Google Scholar] [CrossRef]
- Korhonen, M.H.J.; Laine, J. Flocculation and Retention of Fillers with Nanocelluloses. Nord. Pulp Pap. Res. J. 2014, 29, 119–128. [Google Scholar] [CrossRef]
- Li, G.; Fu, Y.; Shao, Z.; Zhang, F. Preparing Cationic Cellulose Derivative in NaOH / Urea Aqueous Solution and Its Performance as Filler Modifier. BioResources 2015, 10, 7782–7794. [Google Scholar] [CrossRef]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic Surface Functionalization of Cellulose Nanocrystals. Soft Matter 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Kono, H.; Kusumoto, R. Preparation, Structural Characterization, and Flocculation Ability of Amphoteric Cellulose. React. Funct. Polym. 2014, 82, 111–119. [Google Scholar] [CrossRef]
- Moral, A.; Aguado, R.; Ballesteros, M.; Tijero, A. Cationization of Alpha-Cellulose to Develop New Sustainable Products. Int. J. Polym. Sci. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Pedrosa, J.F.S.; Rasteiro, M.G.; Neto, C.P.; Ferreira, P.J.T. Effect of Cationization Pretreatment on the Properties of Cationic Eucalyptus Micro/Nanofibrillated Cellulose. Int. J. Biol. Macromol. 2022, 201, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.; Hyvakko, U.; Liimatainen, H.; Niinimaki, J.; Hormi, O. Periodate Oxidation of Cellulose at Elevated Temperatures Using Metal Salts as Cellulose Activators. Carbohydr. Polym. 2011, 83, 1293–1297. [Google Scholar] [CrossRef]
- Sirviö, J.; Honka, A.; Liimatainen, H.; Niinimäki, J.; Hormi, O. Synthesis of Highly Cationic Water-Soluble Cellulose Derivative and Its Potential as Novel Biopolymeric Flocculation Agent. Carbohydr. Polym. 2011, 86, 266–270. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Gamelas, J.A.F.; Rasteiro, M.G. Environmentally Friendly Cellulose-Based Polyelectrolytes in Wastewater Treatment. Water Sci. Technol. 2017, 76, 1490–1499. [Google Scholar] [CrossRef]
- Hubbe, M.A. Flocculation and Redispersion of Cellulosic Fiber Suspensions: A Review of Effects of Hydrodynamic Shear and Polyelectrolytes. BioResources 2007, 2, 296–331. [Google Scholar]
- Merayo, N.; Balea, A.; de la Fuente, E.; Blanco, Á.; Negro, C. Interactions between Cellulose Nanofibers and Retention Systems in Flocculation of Recycled Fibers. Cellulose 2017, 24, 677–692. [Google Scholar] [CrossRef]
- Shin, J.-H.; Han, S.H.; Sohn, C.; Ow, S.K.; Mah, S. Highly Branched Cationic Polyelectrolytes: Filler Flocculation. Tappi J. 1997, 80, 179–185. [Google Scholar]
- Saritha, V.; Srinivas, N.; Srikanth Vuppala, N.V. Analysis and Optimization of Coagulation and Flocculation Process. Appl. Water Sci. 2017, 7, 451–460. [Google Scholar] [CrossRef]
- Rasteiro, M.G.; Garcia, F.A.P.; Del Mar Perez, M. Applying LDS to Monitor Flocculation in Papermaking. Part. Sci. Technol. 2007, 25, 303–308. [Google Scholar] [CrossRef]
- Rasteiro, M.G.; Garcia, F.A.P.; Ferreira, P.; Blanco, A.; Negro, C.; Antunes, E. The Use of LDS as a Tool to Evaluate Flocculation Mechanisms. Chem. Eng. Processing: Process Intensif. 2008, 47, 1323–1332. [Google Scholar] [CrossRef]
- Antunes, E.; Garcia, F.A.P.; Blanco, A.; Negro, C.; Rasteiro, M.G. Evaluation of the Flocculation and Reflocculation Performance of a System with Calcium Carbonate, Cationic Acrylamide Co-Polymers, and Bentonite Microparticles. Ind. Eng. Chem. Res. 2015, 54, 198–206. [Google Scholar] [CrossRef]
- Blanco, A.; Fuente, E.; Negro, C.; Tijero, J. Flocculation Monitoring: Focused Beam Reflectance Measurement as a Measurement Tool. Can. J. Chem. Eng. 2002, 80, 1–7. [Google Scholar] [CrossRef]
- Rasteiro, M.G.; Garcia, F.A.P.; Ferreira, P.; Blanco, A.; Negro, C.; Antunes, E. Evaluation of Flocs Resistance and Reflocculation Capacity Using the LDS Technique. Powder Technol. 2008, 183, 231–238. [Google Scholar] [CrossRef]
- Lourenço, A.F.; Godinho, D.; Gamelas, J.A.F.; Sarmento, P.; Ferreira, P.J.T. Carboxymethylated Cellulose Nanofibrils in Papermaking: Influence on Filler Retention and Paper Properties. Cellulose 2019, 5, 3489–3502. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Gamelas, J.A.F.; Cayre, O.J.; Rasteiro, M.G. Flocculation of Silica Nanoparticles by Natural, Wood-Based Polyelectrolytes. Sep. Purif. Technol. 2020, 231, 115888. [Google Scholar] [CrossRef]
- Grenda, K.; Gamelas, J.A.F.; Arnold, J.; Cayre, O.J.; Rasteiro, M.G. Evaluation of Anionic and Cationic Pulp-Based Flocculants with Diverse Lignin Contents for Application in Effluent Treatment From the Textile Industry: Flocculation Monitoring. Front. Chem. 2020, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Du, C.; Liu, H.; Chen, Y.; Yu, H.; Zhou, Z. Preparation and Characterization of Aldehyde-Functionalized Cellulosic Fibers through Periodate Oxidization of Bamboo Pulp. BioResources 2016, 11, 8386–8395. [Google Scholar] [CrossRef]
- Grenda, K.; Gamelas, J.A.F.; Arnold, J.; Cayre, O.J.; Rasteiro, M.G. Cationization of Eucalyptus Wood Waste Pulps with Diverse Lignin Contents for Potential Application in Colored Wastewater Treatment. RSC Adv. 2019, 9, 34814–34826. [Google Scholar] [CrossRef]
- Campano, C.; Lopez-Exposito, P.; Blanco, A.; Negro, C.; van de Ven, T.G.M. Hairy Cationic Nanocrystalline Cellulose as a Novel Flocculant of Clay. J. Colloid Interface Sci. 2019, 545, 153–161. [Google Scholar] [CrossRef]
- Bushell, G. Forward Light Scattering to Characterise Structure of Flocs Composed of Large Particles. Chem. Eng. J. 2005, 111, 145–149. [Google Scholar] [CrossRef]
- Liao, J.Y.H.; Selomulya, C.; Bushell, G.; Bickert, G.; Amal, R. On Different Approaches to Estimate the Mass Fractal Dimension of Coal Aggregates. Part. Part. Syst. Charact. 2005, 22, 299–309. [Google Scholar] [CrossRef]
- Biggs, S.; Habgood, M.; Jameson, G.J.; Yan, Y. Aggregate Structures Formed via a Bridging Flocculation Mechanism. Chem. Eng. J. 2000, 80, 13–22. [Google Scholar] [CrossRef]
- Jarvis, P.; Jefferson, B.; Parsons, S.A. Breakage, Regrowth, and Fractal Nature of Natural Organic Matter Flocs. Environ. Sci. Technol. 2005, 39, 2307–2314. [Google Scholar] [CrossRef]
- Im, W.; Lae Lee, H.; Youn, H.J.; Seo, D. Structure Characterization of Ground Calcium Carbonate Flocs by Fractal Analysis and Their Effects on Handsheet Properties. TAPPI J. 2013, 12, 17–23. [Google Scholar] [CrossRef]
- Kekäläinen, K.; Liimatainen, H.; Illikainen, M.; Maloney, T.C.; Niinimäki, J. The Role of Hornification in the Disintegration Behaviour of TEMPO-Oxidized Bleached Hardwood Fibres in a High-Shear Homogenizer. Cellulose 2014, 21, 1163–1174. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Gan, W.; Zhou, J.; Zhang, L. Flocculation Properties and Antimicrobial Activities of Quaternized Celluloses Synthesized in NaOH / Urea Aqueous Solution. Ind. Eng. Chem. Res. 2010, 49, 1242–1246. [Google Scholar] [CrossRef]
- Odabas, N.; Amer, H.; Bacher, M.; Henniges, U.; Potthast, A.; Rosenau, T. Properties of Cellulosic Material after Cationization in Different Solvents. ACS Sustain. Chem. Eng. 2016, 4, 2295–2301. [Google Scholar] [CrossRef]
- Huang, X.; Dognani, G.; Hadi, P.; Yang, M.; Job, A.E.; Hsiao, B.S. Cationic Dialdehyde Nanocellulose from Sugarcane Bagasse for Efficient Chromium(VI) Removal. ACS Sustain. Chem. Eng. 2020, 8, 4734–4744. [Google Scholar] [CrossRef]
- Otoni, C.G.; Figueiredo, J.S.L.; Capeletti, L.B.; Cardoso, M.B.; Bernardes, J.S.; Loh, W. Tailoring the Antimicrobial Response of Cationic Nanocellulose-Based Foams through Cryo-Templating. ACS Appl. Bio Mater. 2019, 2, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Y.; Wang, Q.; Xu, J.; Fan, X.; Wang, P.; Yuan, J. Biological–Chemical Modification of Cellulose Nanocrystal to Prepare Highly Compatible Chitosan-Based Nanocomposites. Cellulose 2019, 26, 5267–5279. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, P.; Li, Y.; He, X.; Dai, Y.; Tan, Z. Preparation and Antibacterial Activity of a Cellulose-Based Schiff Base Derived from Dialdehyde Cellulose and L-Lysine. Ind. Crops Prod. 2020, 145, 112126. [Google Scholar] [CrossRef]
- Calvini, P.; Gorassini, A.; Luciano, G.; Franceschi, E. FTIR and WAXS Analysis of Periodate Oxycellulose: Evidence for a Cluster Mechanism of Oxidation. Vib. Spectrosc. 2006, 40, 177–183. [Google Scholar] [CrossRef]
- Antunes, E.; Garcia, F.A.P.; Ferreira, P.; Blanco, A.; Negro, C.; Rasteiro, M.G. Effect of Water Cationic Content on Flocculation, Flocs Resistance and Reflocculation Capacity of PCC Induced by Polyelectrolytes. Ind. Eng. Chem. Res. 2008, 47, 6006–6013. [Google Scholar] [CrossRef]



| CHPTAC Method | ||||||||||||
| Sample | Cellulose Activation | Cellulose Cationization | ||||||||||
| C 1 (wt %) | IPA (% v/v) | NaOH/AGU 2 (mol/mol) | T (°C) | t (h) | CHPTAC/AGU 3 (mol/mol) | T (°C) | t (h) | |||||
| CH0.08 | 2 | - | 12 | 5 | 1 | 3 | 65 | 8 | ||||
| CH0.16 | 5 | 50 | 3.4 | 70 | 1 | 2 | 70 | 2 | ||||
| CH0.13_F | 3 | - | 9 | 20 | 0.3 | 6 | 65 | 8 | ||||
| GT method | ||||||||||||
| Sample | Cellulose Oxidation | Cellulose Cationization | ||||||||||
| C 1 (wt %) | IPA (% v/v) | T (°C) | t (h) | SP/AGU 4 (mol/mol) | Dsa 5 | C 1 (wt %) | AA (% v/v) | IPA (% v/v) | GT/ald.6 (mol/mol) | T (°C) | t (h) | |
| GT0.32 | 2.5 | 10 | 70 | 4 | 0.50 | 0.50 | 5 | - 7 | - | 1 | 70 | 1.5 |
| GT1.06_P | 1.50 | 1.59 | 10 | 60 | ||||||||
| GT0.02_F | 0.10 | 0.11 | - | |||||||||
| GT0.04_F | 0.20 | 0.19 | - | |||||||||
| GT0.16_F | 0.55 | 0.48 | - | 0.7 | ||||||||
| GT0.36_FP | 0.65 | 0.85 | - | 0.6 | ||||||||
| DS | CD (mmol/g) | ZP (mV) 1 | YF (%) | SF (%) | avgMw (Da) 2,3/PDI 4 | |
|---|---|---|---|---|---|---|
| BEKP | - | 0.14 ± 0.01 5 | −23 ± 5 | - | - | - |
| CH0.08 | 0.08 ± 0.02 | 0.46 ± 0.04 | +29 ± 3 | - | - | - |
| CH0.16 | 0.16 ± 0.00 | 0.92 ± 0.04 | +18 ± 4 | - | - | - |
| CH0.13_F 6 | 0.13 ± 0.01 | 0.78 ± 0.01 | +26 ± 3 | 29.0 ± 0.4 | 0.0 ± 0.0 | - |
| GT0.32 | 0.32 ± 0.01 | 1.62 ± 0.09 | +21 ± 4 | - | - | - |
| GT1.06_P | 1.06 ± 0.00 | 3.44 ± 0.00 | +35 ± 2 | - | 100.0 ± 0.0 | 2150 ± 12/1.01 ± 0.00 972 ± 8.5/1.02 ± 0.00 |
| GT0.02_F 6 | 0.02 ± 0.01 | 0.23 ± 0.04 | +24 ± 2 | 6.4 ± 0.6 | 6.1 ± 1.0 | - |
| GT0.04_F 6 | 0.04 ± 0.01 | 0.32 ± 0.03 | +29 ± 5 | 11.5 ± 1.0 | 6.5 ± 1.6 | - |
| GT0.16_F 6 | 0.16 ± 0.01 | 0.90 ± 0.02 | +27 ± 2 | 98.4 ± 0.5 | 39.9 ± 1.4 | 1081 ± 3.5/1.11 ± 0.00 526 ± 0.5/1.02 ± 0.00 |
| GT0.36_FP 6 | 0.36 ± 0.01 | 1.68 ± 0.04 | +30 ± 3 | 100.0 ± 0.0 | 99.6 ± 0.4 | 1025 ± 43/1.06 ± 0.02 543 ± 4.5/1.01 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, J.F.S.; Alves, L.; Neto, C.P.; Rasteiro, M.G.; Ferreira, P.J.T. Assessment of the Performance of Cationic Cellulose Derivatives as Calcium Carbonate Flocculant for Papermaking. Polymers 2022, 14, 3309. https://doi.org/10.3390/polym14163309
Pedrosa JFS, Alves L, Neto CP, Rasteiro MG, Ferreira PJT. Assessment of the Performance of Cationic Cellulose Derivatives as Calcium Carbonate Flocculant for Papermaking. Polymers. 2022; 14(16):3309. https://doi.org/10.3390/polym14163309
Chicago/Turabian StylePedrosa, Jorge F. S., Luís Alves, Carlos P. Neto, Maria G. Rasteiro, and Paulo J. T. Ferreira. 2022. "Assessment of the Performance of Cationic Cellulose Derivatives as Calcium Carbonate Flocculant for Papermaking" Polymers 14, no. 16: 3309. https://doi.org/10.3390/polym14163309
APA StylePedrosa, J. F. S., Alves, L., Neto, C. P., Rasteiro, M. G., & Ferreira, P. J. T. (2022). Assessment of the Performance of Cationic Cellulose Derivatives as Calcium Carbonate Flocculant for Papermaking. Polymers, 14(16), 3309. https://doi.org/10.3390/polym14163309








