Taguchi Grey Relational Analysis for Multi-Response Optimization of Bacillus Bacteria Flocculation Recovery from Fermented Broth by Chitosan to Enhance Biocontrol Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Inoculum Preparation and Cultivation Parameters
2.3. Antimicrobial Activity
2.3.1. Well-Diffusion Assay
2.3.2. Disk-Diffusion Assay
2.4. Flocculation Experiments
2.5. Taguchi Method and Grey Relational Analysis (GRA)
3. Results and Discussion
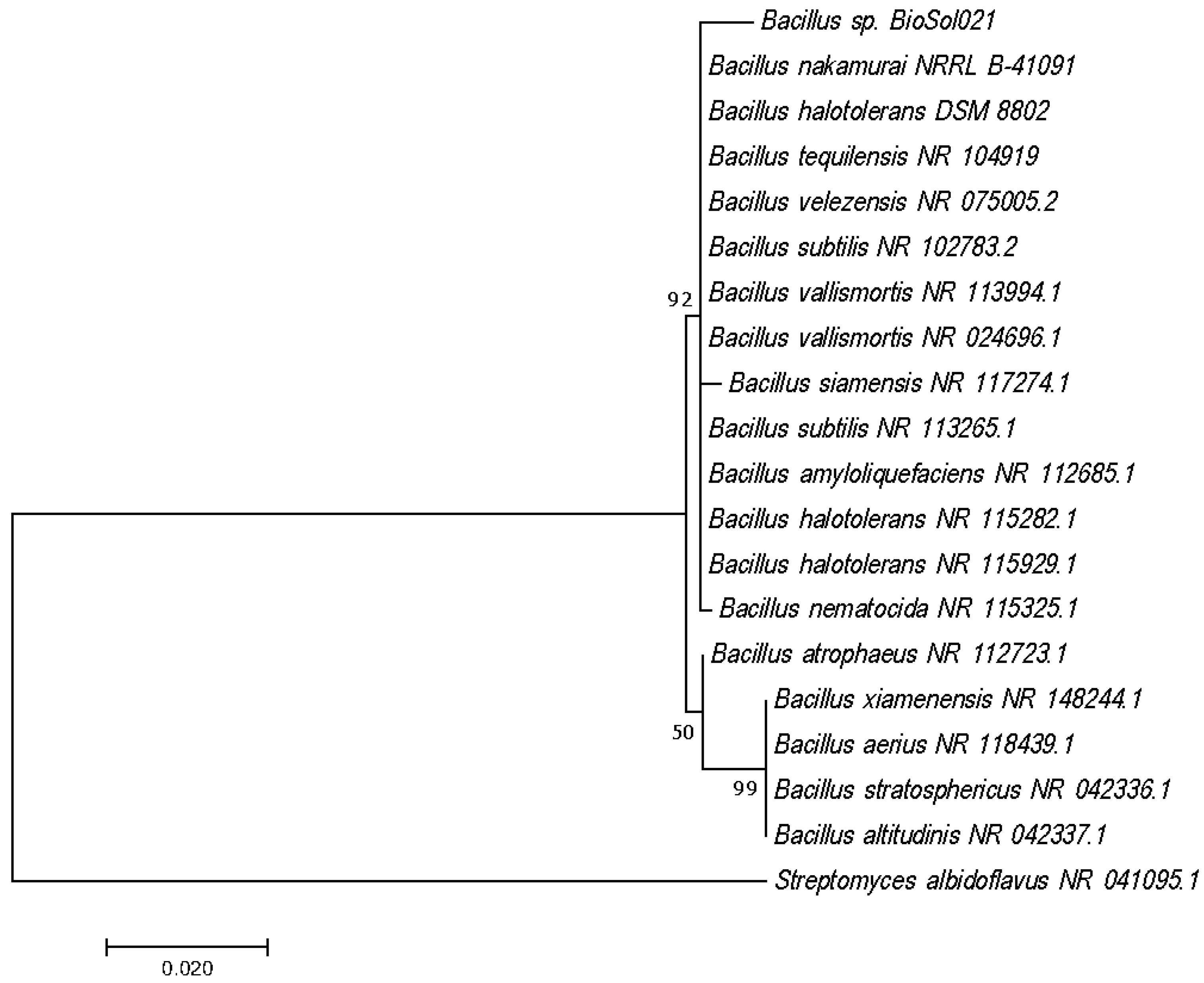
3.1. Identification of The Producing Microorganism
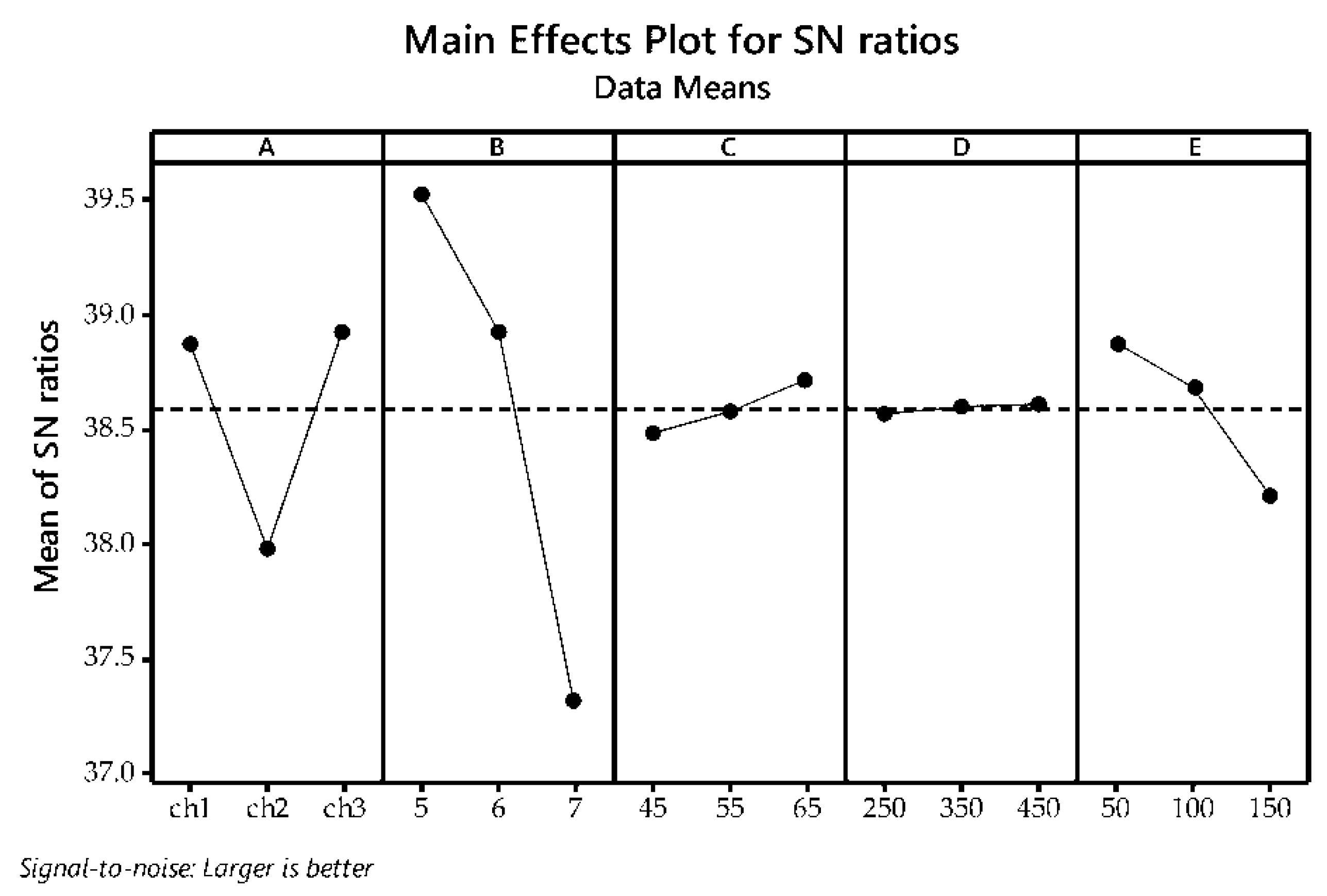
3.2. Taguchi Optimization of The Flocculation Process
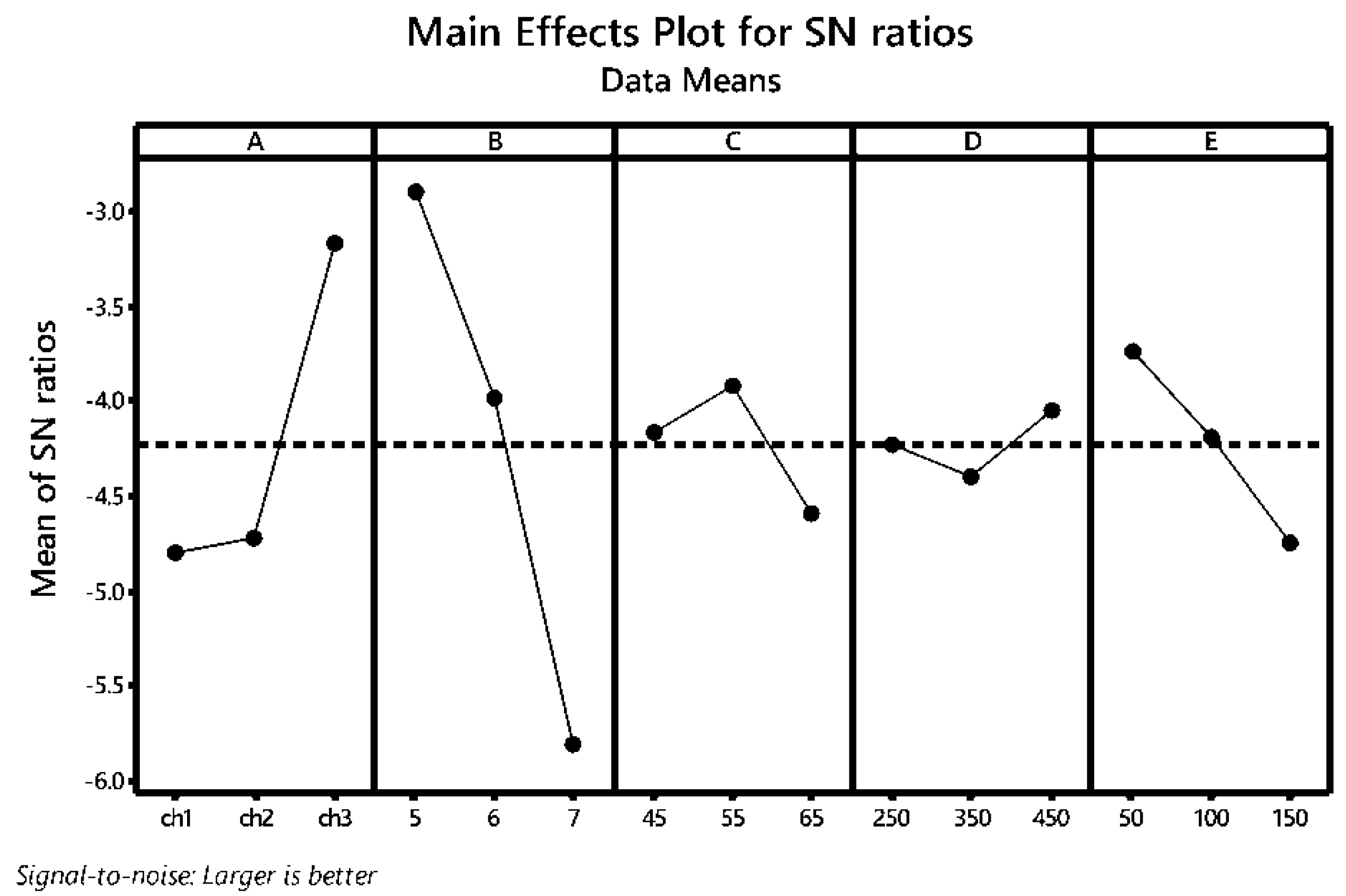
3.3. Taguchi Grey Relational Analysis (GRA) for Multi-Response Optimization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Singh, A. Biopesticides for integrated crop management: Environmental and regulatory aspects. J. Biofertil. Biopestici. 2014, 5, e121. [Google Scholar] [CrossRef]
- Pacifico, M.G.; Eckstein, B.; Bettiol, W. Screening of Bacillus for the development of bioprotectans for the control of Fusarium oxysporum f. sp. vasinfectum and meloidogye incognita. Biol. Control. 2021, 164, 104764. [Google Scholar] [CrossRef]
- Awad, H.M.; El-Enshasy, H.A.; Hanapi, S.Z.; Hamed, E.R.; Rosidi, B. A new chitinase-producer strain Streptomyces glauciniger WICC-A03: Isolation and identification as a biocontrol agent for plants phytopathogenic fungi. Nat. Prod. Res. 2014, 28, 2273–2277. [Google Scholar] [CrossRef]
- Hamed, E.R.; Awad, H.M.; Ghazi, E.A.; El-Gamal, N.G.; Shehata, H.S. Trichoderma sp. isolated from salinity soil using rice straw waste as biocontrol agent for cowpea plant pathogens. J. App. Pharm. Sci. 2015, 5 (Suppl. 2), 091–098. [Google Scholar] [CrossRef]
- Thiéry, I.; Hamon, S.; Delécluse, A.; Orduz, S. The introduction into Bacillus sphaericus of the Bacillus thuringiensis subsp. medellin cyt1Ab1 gene results in higher susceptibility of resistant mosquito larva populations to B. sphaericus. Appl. Environ. Microbiol. 1998, 64, 3910–3916. [Google Scholar] [CrossRef] [PubMed]
- Kunst, F.; Ogasawara, N.; Moszer, I.; Albertini, A.M.; Alloni, G.O.; Azevedo, V.; Bertero, M.G.; Bessières, P.; Bolotin, A.; Borchert, S.; et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 1997, 390, 249–256. [Google Scholar] [CrossRef]
- Nicholson, W.L. Roles of Bacillus endospores in the environment. Cell. Mol. Life Sci. 2002, 59, 410–416. [Google Scholar] [CrossRef]
- Gautam, S.; Chauhan, A.; Sharma, R.; Sehgal, R.; Shirkot, C.K. Potential of Bacillus amyloliquefaciens for biocontrol of bacterial canker of tomato incited by Clavibacter michiganensis ssp. michiganensis. Microb. Pathog. 2019, 130, 196–203. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, S.U.; Khan, W.U.; Saleh, T.A.; Khan, M.H.U.; Ullah, S.; Ali, A.; Ikram, M. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. Comptes Rendus Biol. 2019, 342, 124–135. [Google Scholar] [CrossRef]
- Piggot, P.J.; Hilbert, D.W. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 2004, 7, 579–586. [Google Scholar] [CrossRef]
- Sundin, G.W.; Castiblanco, L.F.; Yuan, X.; Zeng, Q.; Yang, C.H. Challenges in bacterial molecular plant pathology. Mol. Plant Pathol. 2016, 17, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Teixidó, N.; Usall, J.; Torres, R. Insight into a successful development of biocontrol agents: Production, formulation, packaging, and shelf life as key aspects. Horticulture 2022, 8, 305. [Google Scholar] [CrossRef]
- Mu, D.; Mu, X.; Xu, Z.; Du, Z.; Chen, G. Removing Bacillus subtilis from fermentation broth using alumina nanoparticles. Bioresour. Technol. 2015, 197, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Whittington, P.N. Fermentation broth clarification techniques. Appl. Biochem. Biotechnol. 1990, 23, 91–121. [Google Scholar] [CrossRef]
- Gregory, J. Monitoring particle aggregation processes. Adv. Colloid Interface Sci. 2009, 147–148, 109–123. [Google Scholar] [CrossRef]
- Aljuboori, A.H.R.; Idris, A.; Abdullah, N.; Mohamad, R. Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresour. Technol. 2013, 127, 489–493. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.L.; Qi, Q.S.; Wang, F.S.; Zhang, Y.Z. Isolation and characterization of temperature and alkaline stable bioflocculant from Agrobacterium sp. M-503. New Biotechnol. 2010, 27, 789–794. [Google Scholar] [CrossRef]
- Rudén, C. Acrylamide and cancer risk--expert risk assessments and the public debate. Food Chem. Toxicol. 2004, 42, 335–349. [Google Scholar] [CrossRef]
- Wu, H.; Yang, R.; Li, R.; Long, C.; Yang, H.; Li, A. Modeling and optimization of the flocculation processes for removal of cationic and anionic dyes from water by an amphoteric grafting chitosan-based flocculant using response surface methodology. Environ. Sci. Pollut. Res. Int. 2015, 22, 13038–13048. [Google Scholar] [CrossRef]
- Lananan, F.; Yunos, F.H.M.; Nasir, N.M.; Bakar, N.S.A.; Lam, S.S.; Jusoh, A. Optimization of biomass harvesting of microalgae, Chlorella sp. utilizing auto-flocculating microalgae, Ankistrodesmus sp. as bio-flocculant. Int. Biodeterior. Biodegrad. 2016, 113, 391–396. [Google Scholar] [CrossRef]
- Liu, J.; Tao, Y.; Zhang, Y.; Li, A.; Li, T.; Sang, M.; Zhang, C. Freshwater microalgae harvested via flocculation induced by pH decrease. Biotechnol. Biofuels 2013, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Purton, S.; Baganz, F. Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour. Technol. 2013, 129, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.J.; Su, W.H. Antibacterial activity of shrimp chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243. [Google Scholar] [CrossRef]
- Young, D.H.; Kauss, H. Release of calcium from suspension-cultured glycine max cells by chitosan, other polycations, and polyamines in relation to effects on membrane permeability. Plant Physiol. 1983, 73, 698–702. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Sebti, I.; Martial-Gros, A.; Carnet-Pantiez, A.; Grelier, S.; Coma, V. Chitosan polymer as bioactive coating and film against Aspergillus niger contamination. J. Food Sci. 2006, 70, 100–104. [Google Scholar] [CrossRef]
- Cuero, R.G.; Osuji, G.; Washington, A. N-carboxymethyl chitosan inhibition of aflatoxin production: Role of zinc. Biotechnol. Lett. 1991, 13, 441–444. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polím. Ciênc. Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro-and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Coma, V.; Deschamps, A.; Martial-Gros, A. Bioactive packaging materials from edible chitosan polymer-antimicrobial activity assessment on dairy-related contaminants. J. Food Sci. 2003, 68, 2788–2792. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Park, P.J.; Kim, S.K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar] [CrossRef]
- Jung, E.J.; Youn, D.K.; Lee, S.H.; No, H.K.; Ha, J.G.; Prinyawiwatkul, W. Antibacterial activity of chitosans with different degrees of deacetylation and viscosities. Food Sci. Technol. 2010, 45, 676–682. [Google Scholar] [CrossRef]
- Lim, S.H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- No, H.K.; Kim, S.H.; Lee, S.H.; Park, N.Y.; Prinyawiwatkul, W. Stability and antibacterial activity of chitosan solutions affected by storage temperature and time. Carbohydr. Polym. 2006, 65, 174–178. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Arul, J.; Grenier, J.; Asselin, A. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology 1992, 82, 398–402. [Google Scholar] [CrossRef]
- Sahai, A.S.; Manocha, M.S. Chitinases of fungi and plants: Their involvement in morphogenesis and host-parasite interaction. FEMS Microbiol. Rev. 1993, 11, 317–338. [Google Scholar] [CrossRef]
- Eweis, M.; Elkholy, S.S.; Elsabee, M.Z. Antifungal efficacy of chitosan and its thiourea derivatives upon the growth of some sugar-beet pathogens. Int. J. Biol. Macrom. 2006, 38, 1–8. [Google Scholar] [CrossRef]
- Stössel, O.; Leuba, J.L. Effect of chitosan, chitin and some aminosugars on growth of various soilborne phytopathogenic fungi. J. Phytopathol. 1984, 111, 82–90. [Google Scholar] [CrossRef]
- Bandara, S.; Du, H.; Carson, L.; Bradford, D.; Kommalapati, R. Agricultural and biomedical applications of chitosan-based nanomaterials. Nanomaterials 2020, 10, 1903. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Mujtaba, M.; Rahman, M.U.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The multifunctional role of chitosan in horticultural crops; A review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef]
- Orzali, L.; Corsi, B.; Forni, C.; Riccioni, L. Chitosan in agriculture: A new challenge for managing plant disease. In Biological Activities and Application of Marine Polysaccharides; Shalaby, E., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Zhang, M.; Zhang, F.; Li, C.; An, H.; Wan, T.; Zhang, P. Application of chitosan and its derivative polymers in clinical medicine and agriculture. Polymers 2022, 14, 958. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- Vlajkov, V.; Anđelić, S.; Pajčin, I.; Grahovac, M.; Budakov, D.; Jokić, A.; Grahovac, J. Medium for the production of Bacillus-based biocontrol agent effective against aflatoxigenic Aspergillus flavus: Dual approach for modelling and optimization. Microorganisms 2022, 10, 1165. [Google Scholar] [CrossRef]
- Vlajkov, V.; Grahovac, M.; Budakov, D.; Loc, M.; Pajčin, I.; Milić, D.; Novaković, T.; Grahovac, J. Distribution, Genetic Diversity and Biocontrol of Aflatoxigenic Aspergillus flavus in Serbian Maize Fields. Toxins 2021, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Carbú, M.; Moraga, J.; Cantoral, J.M.; Collado, I.G.; Garrido, C. Recent approaches on the genomic analysis of the phytopathogenic fungus Colletotrichum spp. Phytochem. Rev. 2020, 19, 589–601. [Google Scholar] [CrossRef]
- An, S.Q.; Potnis, N.; Dow, M.; Vorhölter, F.J.; He, Y.Q.; Becker, A.; Teper, D.; Li, Y.; Wang, N.; Bleris, L.; et al. Mechanistic insights into host adaptation, virulence and epidemiology of the phytopathogen Xanthomonas. FEMS Microbiol. Rev. 2020, 44, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Itako, A.T.; Tolentino, J.B., Jr.; da Silva, T.A.F., Jr.; Soman, J.M.; Maringoni, A.C. Chemical products induce resistance to Xanthomonas perforans in tomato. Braz. J. Microbiol. 2015, 46, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.R.; Lin, C.H.; Chang, C.P.; Ni, H.F.; Tsai, W.S.; Huang, C.J. Distribution of copper resistance gene variants of Xanthomonas citri subsp. citri and Xanthomonas euvesicatoria pv. perforans. Plant Protect. Sci. 2021, 57, 206–216. [Google Scholar] [CrossRef]
- Mann, A.; Nehra, K.; Rana, J.S.; Dahiya, T. Antibiotic resistance in agriculture: Perspectives on upcoming strategies to overcome upsurge in resistance. Curr. Res. Microb. Sci. 2021, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture, Forestry and Water Management of the Republic of Serbia-Plant Protection Directorate-List of Registered Plant Protection Agents Allowed for Application in Organic Agriculture. Available online: http://www.minpolj.gov.rs/download/Lista-sredstava-za-zastitu-bilja-koja-se-mogu-koristiti-u-organskoj-poizvodnji-na-dan-07.04.2022.-godine.pdf?script=lat (accessed on 15 May 2022).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.; Amatulli, M.T.; Buonaurio, R. PCR-based assay for the detection of Xanthomonas euvesicatoria causing pepper and tomato bacterial spot. Appl. Microbiol. 2009, 49, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Pajčin, I.; Vlajkov, V.; Frohme, M.; Grebinyk, S.; Grahovac, M.; Mojićević, M.; Grahovac, J. Pepper bacterial spot control by Bacillus velezensis: Bioprocess solution. Microorganisms 2020, 8, 1463. [Google Scholar] [CrossRef]
- Sreenivasaprasad, S.; Sharada, K.; Brown, A.E.; Mills, P.R. PCR–based detection of Colletotrichum acutatum on strawberry. Plant Pathol. 1996, 45, 650–655. [Google Scholar] [CrossRef]
- Grahovac, J.; Grahovac, M.; Dodić, J.; Bajić, B.; Balaž, J. Optimization of cultivation medium for enhanced production of antifungal metabolites by Streptomyces hygroscopicu. Crop Prot. 2014, 65, 143–152. [Google Scholar] [CrossRef]
- Schilling, A.G.; Möller, E.M.; Geiger, H.H. Polymerase chain reaction-based assays for species-specific detection of Fusarium culmorum, F. graminearum and F. avenaceum. Phytopathology 1996, 86, 515–522. [Google Scholar] [CrossRef]
- Turner, A.S.; Lees, A.K.; Rezanoor, H.N.; Nicholson, P. Refinement of PCR-detection of Fusarium avenaceum and evidence from DNA marker studies for phenetic relatedness to Fusarium tricinctum. Plant Pathol. 1998, 47, 278–288. [Google Scholar] [CrossRef]
- Tarlanović, J.; Petreš, M.; Grahovac, M.; Milić, B.; Magazin, N.; Hrustić, J.; Mihajlović, J. Effects of 1-MCP and dynamic controlled atmosphere on apple fruit rot caused by Fusarium avenaceum. Pestic. Phytomed. 2018, 33, 109–117. [Google Scholar] [CrossRef]
- Jiménez-Delgadillo, R.; Valdés-Rodríguez, S.E.; Olalde-Portugal, V.; Abraham-Juárez, R.; García-Hernández, J.L. Effect of pH and temperature on the growth and antagonistic activity of Bacillus subtilis on Rhizoctonia solani. Rev. Mex. Fitopatol. 2018, 36, 256–275. [Google Scholar] [CrossRef]
- Hazan, R.; Que, Y.A.; Maura, D.; Rahme, L.G. A method for high throughput determination of viable bacteria cell counts in 96-well plates. BMC Microbiol. 2012, 12, 259. [Google Scholar] [CrossRef]
- Malbaša, R.; Vitas, J.; Vukmanović, S. Analytical Chemistry: A Practicum with a Workbook; University of Technology Novi Sad: Novi Sad, Serbia, 2021. [Google Scholar]
- Cunniff, P. Association of Official Analytical Chemists. In Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1995; Section 33.2.11, Method 991.20. [Google Scholar]
- Kackar, R.N. Off-line quality control, parameter design, and the Taguchi method. J. Qual. Technol. 1985, 17, 176–188. [Google Scholar] [CrossRef]
- Phadke, M.S.; Dehnad, K. Optimization of product and process design for quality and cost. Qual. Reliab. Eng. Int. 1988, 4, 105–112. [Google Scholar] [CrossRef]
- Jozić, S.; Bajić, D.; Celent, L. Application of compressed cold air cooling: Achieving multiple performance characteristics in end milling process. J. Clean. Prod. 2015, 100, 325–332. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.P.; Vandvik, M.S.; Vårum, K.M.; Østgaard, K. Screening of Chitosans and Conditions for Bacterial Flocculation. Biomacromolecules 2001, 2, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Ramsden, D.K.; Symes, K.C. The flocculation of bacteria using cationic synthetic flocculants and chitosan. Biotechnol. Tech. 1990, 4, 55–60. [Google Scholar] [CrossRef]
- Weir, S.; Ramsden, D.K.; Hughes, J. The effect of complex growth media on microbial flocculation by the cationic polyelectrolyte chitosan. Biotechnol. Tech. 1993, 7, 111–116. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Roussy, J.; Van Vooren, M.; Guibal, E. Influence of chitosan characteristics on coagulation and flocculation of organic suspensions. J. Appl. Polym. Sci. 2005, 98, 2070–2079. [Google Scholar] [CrossRef]
- Kaseamchochoung, C.; Lertsutthiwong, P.; Phalakornkule, C. Influence of Chitosan Characteristics and Environmental Conditions on Flocculation of Anaerobic Sludge. Water Environ. Res. 2006, 78, 2210–2216. [Google Scholar] [CrossRef]
- Sheng, W.J.; Peng, X.; Lee, D.J.; Su, A. Coagulation of particles through rapid mixing. Dry. Technol. 2006, 24, 1271–1276. [Google Scholar] [CrossRef]
- Rossini, M.; Garcia Garrido, J.; Galluzzo, M. Optimization of the coagulation–flocculation treatment: Influence of rapid mix parameters. Water Res. 1999, 33, 1817–1826. [Google Scholar] [CrossRef]
- Rkhaila, A.; Chtouki, T.; Erguig, H.; El Haloui, N.; Ounine, K. Chemical proprieties of biopolymers (chitin/chitosan) and their synergic effects with endophytic Bacillus species: Unlimited applications in agriculture. Molecules 2021, 26, 1117. [Google Scholar] [CrossRef]
| Parameter | Notation | Levels | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Chitosan type | A | ch1 | ch 2 | ch 3 |
| pH value | B | 5 | 6 | 7 |
| Chitosan dosage [mg/mL] | C | 45 | 55 | 65 |
| Rapid mixing [rpm] | D | 250 | 350 | 450 |
| Slow mixing [rpm] | E | 50 | 100 | 150 |
| Parameter | Value | Parameter | Value | Parameter | Value |
|---|---|---|---|---|---|
| β-xylosidase | + | Myo-inositol | + | Pyruvate | + |
| L-Lysine arylamidase | - | Methyl-α-D- glucopyranoside acidification | + | α-glucosidase | + |
| L-Aspartate arylamidase | - | Ellman | + | D-tagatose | - |
| Leucine arylamidase | + | Methyl-D-xyloside | - | D-trehalose | + |
| Phenylalanine arylamidase | + | α-mannosidase | - | Inulin | - |
| L-Proline arylamidase | - | Maltotriose | - | D-glucose | + |
| β-galactosidase | - | Glycine arylamidase | + | D-ribose | + |
| L-Pyrrolidonyl arylamidase | + | D-mannitol | + | Putrescine assimilation | - |
| α-galactosidase | + | D-mannose | + | Growth in 6.5% NaCl | + |
| Alanine arylamidase | + | D-melezitose | - | Kanamycin resistance | - |
| Tyrosine arylamidase | + | N-acetyl-D-glucosamine | - | Oleandomycin resistance | - |
| β-N-acetyl- glucosaminidase | - | Palatinose | + | Esculin hydrolysis | + |
| Ala-Phe-Pro arylamidase | - | L-rhamnose | - | Tetrazolium red | + |
| Cyclodextrin | - | β-glucosidase | + | Polymyxin B resistance | + |
| D-galactose | - | β-mannosidase | - | ||
| Glycogen | - | Phosphoryl choline | - |
| Parameter | Value |
|---|---|
| Cellulose content (g/L) | 3.4 |
| Total nitrogen content (g/L) | 0.37 |
| Biomass content (CFU/mL) | 2∙108 |
| pH value | 7.51 |
| Run | Parameter Combination | EF (%) | S/N |
|---|---|---|---|
| 1 | A1B1C1D1E1 | 98.60 | 39.8776 |
| 2 | A1B2C2D2E2 | 97.63 | 39.7918 |
| 3 | A1B3C3D3E3 | 76.63 | 37.6883 |
| 4 | A2B1C1D2E2 | 89.23 | 39.0104 |
| 5 | A2B2C2D3E3 | 76.06 | 37.6230 |
| 6 | A2B3C3D1E1 | 73.76 | 37.3566 |
| 7 | A3B1C2D1E3 | 97.27 | 39.7598 |
| 8 | A3B2C3D2E1 | 97.60 | 39.7886 |
| 9 | A3B3C1D3E2 | 79.83 | 38.0431 |
| 10 | A1B1C3D3E2 | 95.55 | 39.6045 |
| 11 | A1B2C1D1E3 | 86.36 | 38.7263 |
| 12 | A1B3C2D2E1 | 75.27 | 37.5323 |
| 13 | A2B1C2D3E1 | 94.19 | 39.4797 |
| 14 | A2B2C3D1E2 | 83.35 | 38.4176 |
| 15 | A2B3C1D2E3 | 63.10 | 36.0008 |
| 16 | A3B1C3D2E3 | 93.90 | 39.4531 |
| 17 | A3B2C1D3E1 | 91.56 | 39.2346 |
| 18 | A3B3C2D1E2 | 72.97 | 37.2631 |
| Source | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| A | 2 | 3.3618 | 1.68088 | 7.72 | 0.017 |
| B | 2 | 15.7743 | 7.88714 | 36.24 | 0.000 |
| C | 2 | 0.1697 | 0.08483 | 0.39 | 0.691 |
| D | 2 | 0.0064 | 0.00318 | 0.01 | 0.986 |
| E | 2 | 1.4295 | 0.71477 | 3.28 | 0.099 |
| Error | 7 | 1.5234 | 0.21763 | ||
| Total | 17 | 22.2650 |
| Run | EF (%) | Antimicrobial Activity/Inhibition Zone Diameter (mm) | |||
|---|---|---|---|---|---|
| Xanthomonas sp. | Colletotrichum sp. | Fusarium sp. | Aspergillus sp. | ||
| Y1 | Y2 | Y3 | Y4 | Y5 | |
| 1 | 98.60 | 42.00 | 40.33 | 36.33 | 36.33 |
| 2 | 97.63 | 42.67 | 34.67 | 37.00 | 32.33 |
| 3 | 76.63 | 22.33 | 29.33 | 35.33 | 41.00 |
| 4 | 89.23 | 43.33 | 35.00 | 35.00 | 41.67 |
| 5 | 76.06 | 43.00 | 35.00 | 35.00 | 39.33 |
| 6 | 73.76 | 41.33 | 33.00 | 34.33 | 35.33 |
| 7 | 97.27 | 40.33 | 41.00 | 36.67 | 44.33 |
| 8 | 97.60 | 40.00 | 38.33 | 37.67 | 41.33 |
| 9 | 79.83 | 43.67 | 39.33 | 34.67 | 40.67 |
| 10 | 95.55 | 29.00 | 32.00 | 38.00 | 26.00 |
| 11 | 86.36 | 31.00 | 32.67 | 36.67 | 33.00 |
| 12 | 75.27 | 32.33 | 32.67 | 35.00 | 29.67 |
| 13 | 94.19 | 40.00 | 41.00 | 37.00 | 37.33 |
| 14 | 83.35 | 37.33 | 34.67 | 35.67 | 35.33 |
| 15 | 63.10 | 31.33 | 32.00 | 31.33 | 40.67 |
| 16 | 93.90 | 40.00 | 35.00 | 35.33 | 40.33 |
| 17 | 91.56 | 44.67 | 35.33 | 34.67 | 40.00 |
| 18 | 72.97 | 34.00 | 31.67 | 34.00 | 43.67 |
| Run | Normalized Values | Deviation Sequences | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | Y5 | Y1 | Y2 | Y3 | Y4 | Y5 | |
| 1 | 1.0000 | 0.8806 | 0.9429 | 0.7500 | 0.5636 | 0.0000 | 0.1194 | 0.0571 | 0.2500 | 0.4364 |
| 2 | 0.9727 | 0.9104 | 0.4571 | 0.8500 | 0.3455 | 0.0273 | 0.0896 | 0.5429 | 0.1500 | 0.6545 |
| 3 | 0.3812 | 0.0000 | 0.0000 | 0.6000 | 0.8182 | 0.6188 | 1.0000 | 1.0000 | 0.4000 | 0.1818 |
| 4 | 0.7361 | 0.9403 | 0.4857 | 0.5500 | 0.8545 | 0.2639 | 0.0597 | 0.5143 | 0.4500 | 0.1455 |
| 5 | 0.3650 | 0.9254 | 0.4857 | 0.5500 | 0.7273 | 0.6350 | 0.0746 | 0.5143 | 0.4500 | 0.2727 |
| 6 | 0.3003 | 0.8507 | 0.3143 | 0.4500 | 0.5091 | 0.6997 | 0.1493 | 0.6857 | 0.5500 | 0.4909 |
| 7 | 0.9626 | 0.8060 | 1.0000 | 0.8000 | 1.0000 | 0.0374 | 0.1940 | 0.0000 | 0.2000 | 0.0000 |
| 8 | 0.9717 | 0.7910 | 0.7714 | 0.9500 | 0.8364 | 0.0283 | 0.2090 | 0.2286 | 0.0500 | 0.1636 |
| 9 | 0.4712 | 0.9552 | 0.8571 | 0.5000 | 0.8000 | 0.5288 | 0.0448 | 0.1429 | 0.5000 | 0.2000 |
| 10 | 0.9141 | 0.2985 | 0.2286 | 1.0000 | 0.0000 | 0.0859 | 0.7015 | 0.7714 | 0.0000 | 1.0000 |
| 11 | 0.6552 | 0.3881 | 0.2857 | 0.8000 | 0.3818 | 0.3448 | 0.6119 | 0.7143 | 0.2000 | 0.6182 |
| 12 | 0.3428 | 0.4478 | 0.2857 | 0.5500 | 0.2000 | 0.6572 | 0.5522 | 0.7143 | 0.4500 | 0.8000 |
| 13 | 0.8756 | 0.7910 | 1.0000 | 0.8500 | 0.6182 | 0.1244 | 0.2090 | 0.0000 | 0.1500 | 0.3818 |
| 14 | 0.5703 | 0.6716 | 0.4571 | 0.6500 | 0.5091 | 0.4297 | 0.3284 | 0.5429 | 0.3500 | 0.4909 |
| 15 | 0.0000 | 0.4030 | 0.2286 | 0.0000 | 0.8000 | 1.0000 | 0.5970 | 0.7714 | 1.0000 | 0.2000 |
| 16 | 0.8675 | 0.7910 | 0.4857 | 0.6000 | 0.7818 | 0.1325 | 0.2090 | 0.5143 | 0.4000 | 0.2182 |
| 17 | 0.8018 | 1.0000 | 0.5143 | 0.5000 | 0.7636 | 0.1982 | 0.0000 | 0.4857 | 0.5000 | 0.2364 |
| 18 | 0.2781 | 0.5224 | 0.2000 | 0.4000 | 0.9636 | 0.7219 | 0.4776 | 0.8000 | 0.6000 | 0.0364 |
| Run | Grey Relational Coefficient | GRG | S/N | Rank | ||||
|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | Y5 | ||||
| 1 | 1.0000 | 0.8072 | 0.8974 | 0.6667 | 0.5340 | 0.7811 | −2.14629 | 3 |
| 2 | 0.9482 | 0.8481 | 0.4795 | 0.7692 | 0.4331 | 0.6956 | −3.15261 | 5 |
| 3 | 0.4469 | 0.3333 | 0.3333 | 0.5556 | 0.7333 | 0.4805 | −6.36628 | 16 |
| 4 | 0.6545 | 0.8933 | 0.4930 | 0.5263 | 0.7746 | 0.6684 | −3.49982 | 8 |
| 5 | 0.4405 | 0.8701 | 0.4930 | 0.5263 | 0.6471 | 0.5954 | −4.50383 | 11 |
| 6 | 0.4168 | 0.7701 | 0.4217 | 0.4762 | 0.5046 | 0.5179 | −5.71558 | 15 |
| 7 | 0.9304 | 0.7204 | 1.0000 | 0.7143 | 1.0000 | 0.8730 | −1.17951 | 1 |
| 8 | 0.9464 | 0.7053 | 0.6863 | 0.9091 | 0.7534 | 0.8001 | −1.93719 | 2 |
| 9 | 0.4860 | 0.9178 | 0.7778 | 0.5000 | 0.7143 | 0.6792 | −3.36039 | 7 |
| 10 | 0.8533 | 0.4161 | 0.3933 | 1.0000 | 0.3333 | 0.5992 | −4.44838 | 10 |
| 11 | 0.5919 | 0.4497 | 0.4118 | 0.7143 | 0.4472 | 0.5229 | −5.63086 | 14 |
| 12 | 0.4321 | 0.4752 | 0.4118 | 0.5263 | 0.3846 | 0.4460 | −7.01354 | 18 |
| 13 | 0.8008 | 0.7053 | 1.0000 | 0.7692 | 0.5670 | 0.7685 | −2.28754 | 4 |
| 14 | 0.5378 | 0.6036 | 0.4795 | 0.5882 | 0.5046 | 0.5427 | −5.30826 | 12 |
| 15 | 0.3333 | 0.4558 | 0.3933 | 0.3333 | 0.7143 | 0.4460 | −7.01333 | 17 |
| 16 | 0.7906 | 0.7053 | 0.4930 | 0.5556 | 0.6962 | 0.6481 | −3.76703 | 9 |
| 17 | 0.7161 | 1.0000 | 0.5072 | 0.5000 | 0.6790 | 0.6805 | −3.34368 | 6 |
| 18 | 0.4092 | 0.5115 | 0.3846 | 0.4545 | 0.9322 | 0.5384 | −5.37790 | 13 |
| Scheme. | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| A | 2 | 10.2073 | 5.1037 | 3.01 | 0.114 |
| B | 2 | 26.1180 | 13.0590 | 7.71 | 0.017 |
| C | 2 | 1.3837 | 0.6918 | 0.41 | 0.680 |
| D | 2 | 0.3583 | 0.1791 | 0.11 | 0.901 |
| E | 2 | 3.0274 | 1.5137 | 0.89 | 0.451 |
| Error | 7 | 11.8635 | 1.6948 | ||
| Total | 17 | 52.9581 |
| Output | Initial Controllable Parameters | Optimal Controllable Parameters | |
|---|---|---|---|
| Prediction | Experiment | ||
| Parameter Set | A3B1C2D1E3 | A3B1C2D3E1 | A3B1C2D3E1 |
| Y1 | 97.27 | – | 97.50 |
| Y2 | 40.33 | – | 43.00 |
| Y3 | 41.00 | – | 40.33 |
| Y4 | 36.67 | – | 37.00 |
| Y5 | 44.33 | 44.85 | |
| GRG | 0.8730 | 0.8673 | 0.8956 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrović, S.; Pajčin, I.; Lukić, N.; Vlajkov, V.; Grahovac, M.; Grahovac, J.; Jokić, A. Taguchi Grey Relational Analysis for Multi-Response Optimization of Bacillus Bacteria Flocculation Recovery from Fermented Broth by Chitosan to Enhance Biocontrol Efficiency. Polymers 2022, 14, 3282. https://doi.org/10.3390/polym14163282
Dmitrović S, Pajčin I, Lukić N, Vlajkov V, Grahovac M, Grahovac J, Jokić A. Taguchi Grey Relational Analysis for Multi-Response Optimization of Bacillus Bacteria Flocculation Recovery from Fermented Broth by Chitosan to Enhance Biocontrol Efficiency. Polymers. 2022; 14(16):3282. https://doi.org/10.3390/polym14163282
Chicago/Turabian StyleDmitrović, Selena, Ivana Pajčin, Nataša Lukić, Vanja Vlajkov, Mila Grahovac, Jovana Grahovac, and Aleksandar Jokić. 2022. "Taguchi Grey Relational Analysis for Multi-Response Optimization of Bacillus Bacteria Flocculation Recovery from Fermented Broth by Chitosan to Enhance Biocontrol Efficiency" Polymers 14, no. 16: 3282. https://doi.org/10.3390/polym14163282
APA StyleDmitrović, S., Pajčin, I., Lukić, N., Vlajkov, V., Grahovac, M., Grahovac, J., & Jokić, A. (2022). Taguchi Grey Relational Analysis for Multi-Response Optimization of Bacillus Bacteria Flocculation Recovery from Fermented Broth by Chitosan to Enhance Biocontrol Efficiency. Polymers, 14(16), 3282. https://doi.org/10.3390/polym14163282









