Synthesis of Carbosilane and Carbosilane-Siloxane Dendrons Based on Limonene
Abstract
:1. Introduction
2. Material and Methods
2.1. Dichloro(2-(4-methylcyclohex-3-en-1-yl)propyl)methylsilane (Lim-G0Cl2)
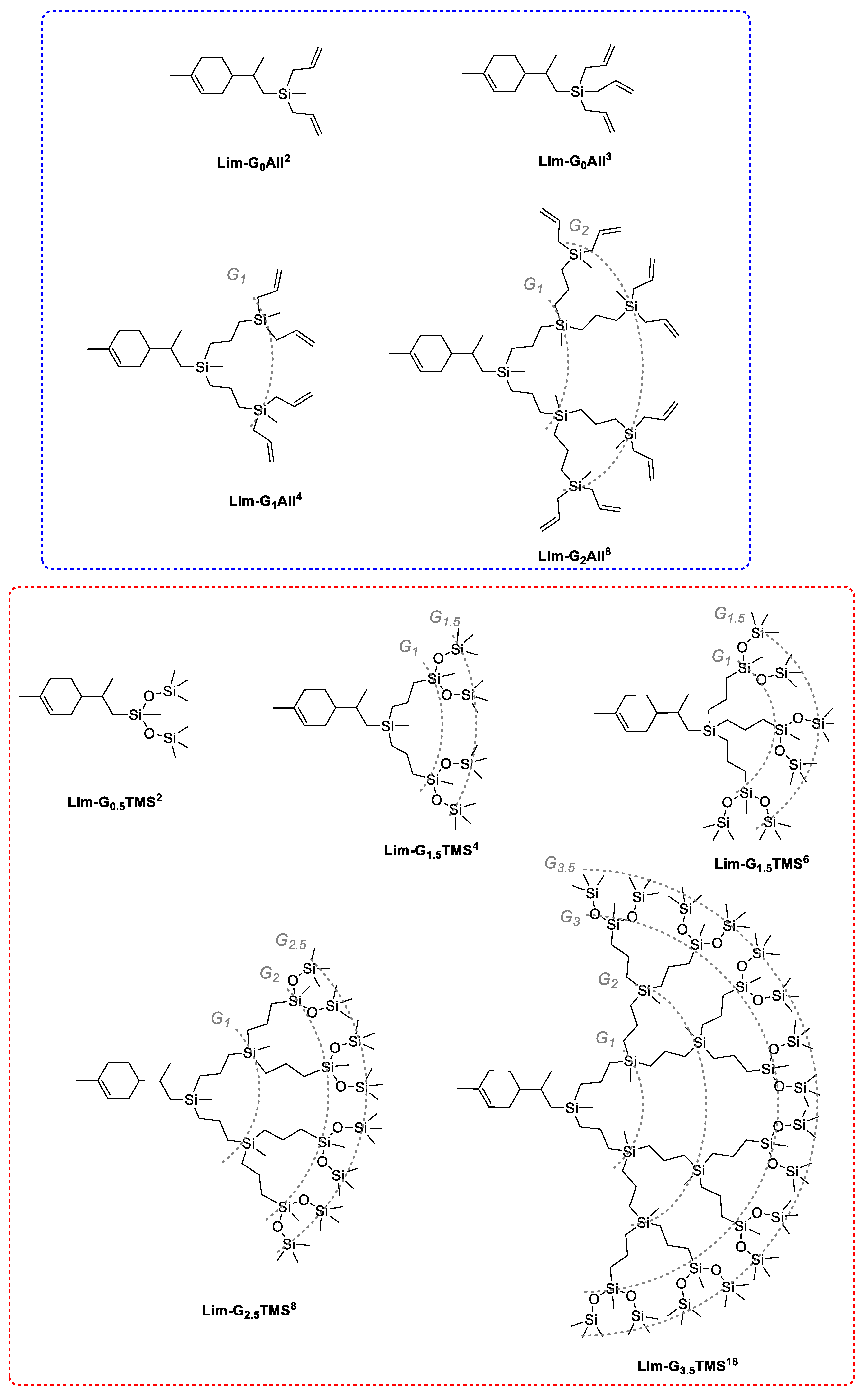
2.2. Diallyl(2-(4-methylcyclohex-3-en-1-yl)propyl)methylsilane (Lim-G0All2)
2.3. Trichloro(2-(4-methylcyclohex-3-en-1-yl)propyl)silane (Lim-G0Cl3)
2.4. Triallyl(2-(4-methylcyclohex-3-en-1-yl)propyl)silane (Lim-G0All3)
2.5. Dendrimer of the First Generation (Lim-G1Cl4)
2.6. Dendrimer of the First Generation (Lim-G1All4)
2.7. Dendrimer of the Second Generation (Lim-G2Cl8)
2.8. Dendrimer of the Second Generation (Lim-G2All8)
2.9. 1,1,3,5,5,5-Heptamethyltrisiloxane (HMS)
2.10. 1,1,3,5,5,5-Heptamethyl-3-(2-(4-methylcyclohex-3-en-1-yl)propyl)trisiloxane (Lim-G0,5TMS2)
2.11. Bis(heptamethylsilylpropyl)methylsilyllimonene (Lim-G1,5TMS4)
2.12. Tris(heptamethylsilylpropyl)silyllimonene (Lim-G1,5TMS6)
2.13. Dendrimer of the 2,5 Generation (Lim-G2,5TMS8)
2.14. Dendrimer of the 3,5 Generation (Lim-G3,5TMS18)
2.15. Dibuthyl(2-(4-methylcyclohex-3-en-1-yl)propyl)methylsilane (Lim-G0Bu2)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sikwal, D.R.; Kalhapure, R.S.; Govender, T. An emerging class of amphiphilic dendrimers for pharmaceutical and biomedical applications: Janus amphiphilic dendrimers. Eur. J. Pharm. Sci. 2017, 97, 113–134. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Villaraza, A.J.L.; Bumb, A.; Brechbiel, M.W. Macromolecules, Dendrimers, and Nanomaterials in Magnetic Resonance Imaging: The Interplay between Size, Function, and Pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. [Google Scholar] [CrossRef]
- Dutta, T.; Jain, N.K.; McMillan, N.A.J.; Parekh, H.S. RETRACTED: Dendrimer nanocarriers as versatile vectors in gene delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 25–34. [Google Scholar] [CrossRef]
- Yokoyama, S.; Otomo, A.; Nakahama, T.; Okuno, Y.; Mashiko, S. Dendrimers for Optoelectronic Applications. In Topics in Current Chemistry; Schalley, C.A., Vögtle, F., Dendrimers, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 228. [Google Scholar]
- Newkome, G.R.; Moorefield, C.N.; Vogtle, F. Dendrimers and Dendrons: Concepts, Syntheses, Applications; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Malkoch, M.; Gallego, S.G. (Eds.) Dendrimer Chemistry: Synthetic Approaches towards Complex Architectures; Royal Society of Chemistry: London, UK, 2020. [Google Scholar]
- Cuadrado, I.; Morán, M.; Losada, J.; Casado, C.M.; Pascual, C.; Alonso, B.; Lobete, F. Advances in Dendritic Macromolecules; Newkome, G.R., Ed.; Jai: Greenwich, UK, 1999; Volume 3, p. 151. [Google Scholar]
- Made, A.W.; Leeuwen, P.W.N.M. Silane dendrimers. J. Chem. Soc. Chem. Commun. 1992, 19, 1400–1401. [Google Scholar] [CrossRef]
- Zhou, L.L.; Hadjichristidis, N.; Toporowski, P.M.; Roovers, J. Synthesis and Properties of Regular Star Polybutadienes with 32 Arms. J. Rubber Chem. Technol. 1992, 65, 303–314. [Google Scholar] [CrossRef]
- Van der Made, A.W.; Van Leeuwen, P.N.M.W.; de Wilde, J.C.; Brandes, R.A.C. Dendrimeric silanes. Adv. Mater. 1993, 5, 466–468. [Google Scholar] [CrossRef]
- Rabiee, N.; Ahmadvand, S.; Ahmadi, S.; Fatahi, Y.; Dinarvand, R.; Bagherzadeh, M.; Rabiee, M.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Carbosilane dendrimers: Drug and gene delivery applications. J. Drug Deliv. Sci. Technol. 2020, 59, 101879. [Google Scholar] [CrossRef]
- Rasines, B.; Hernández-Ros, J.M.; de las Cuevas, N.; Copa-Patiño, J.L.; Soliveri, J.; Muñoz-Fernández, M.A.; Gómez, R.; de la Mata, F.J. Water-stable ammonium-terminated carbosilanedendrimers as efficient antibacterial agents. Dalton Trans. 2009, 40, 8704–8713. [Google Scholar] [CrossRef]
- Yamada, A.; Hatano, K.; Matsuoka, K.; Koyama, T.; Esumi, Y.; Koshino, H.; Hino, K.; Nishikawa, K.; Natori, Y.; Terunuma, D. Syntheses and Vero toxin-binding activities of carbosilane dendrimers periphery-functionalized with galabiose. Tetrahedron 2006, 62, 5074–5083. [Google Scholar] [CrossRef]
- Nishikawa, K.; Matsuoka, K.; Watanabe, M.; Igai, K.; Hino, K.; Hatano, K.; Yamada, A.; Abe, N.; Terunuma, D.; Kuzuhara, H.; et al. Identification of the Optimal Structure Required for a Shiga Toxin Neutralizer with Oriented Carbohydrates to Function in the Circulation. J. Infect. Dis. 2005, 191, 2097–2105. [Google Scholar] [CrossRef]
- Andres, R.; Jesus, E.; Fierro, J.L.G.; Terreros, P. Bifunctional carbosilane dendrons for the immobilization of zirconocene catalysts on silica. New J. Chem. 2011, 35, 2203–2211. [Google Scholar] [CrossRef]
- Gutierrez-Ulloa, C.E.; Buyanova, M.Y.; Apartsin, E.K.; Venyaminova, A.G.; de la Mata, F.J.; Valiente, M.; Gómez, R. Amphiphilic carbosilane dendrons as a novel synthetic platform toward micelle formation. Org. Biomol. Chem. 2017, 15, 7352. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Hernández-Ros, J.M.; Sánchez-García, L.; Maly, M.; Verdú-Expósito, C.; Soliveri, J.; de la Mata, F.J.; Copa-Patiño, J.L.; Pérez-Serrano, J.; Sánchez-Nieves, J.; et al. Ammonium and guanidine carbosilane dendrimers and dendrons as microbicides. Eur. Polym. J. 2018, 101, 159–168. [Google Scholar] [CrossRef]
- Barrios-Gumiel, A.; Sanchez-Nieves, J.; Pérez-Serrano, J.; Gómez, R.; de la Mata, F.J. PEGylated AgNP covered with cationic carbosilane dendrons to enhance antibacterial and inhibition of biofilm properties. Int. J. Pharm. 2019, 569, 118591. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, C.; Ceña-Diez, R.; Sepúlveda-Crespo, D.; de la Mata, F.J.; Gómez, R.; Leal, M.; Muñoz-Fernández, M.A.; Jiménez, J.L. Carbosilane dendrons with fatty acids at the core as a new potential microbicide against HSV-2/HIV-1 co-infection. Nanoscale 2017, 9, 17263–17273. [Google Scholar] [CrossRef]
- Percec, V.; Wilson, D.A.; Leowanawat, P.; Wilson, C.J.; Hughes, A.D.; Kaucher, M.S.; Hammer, D.A.; Levine, D.H.; Kim, A.J.; Bates, F.S.; et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science 2010, 328, 1009. [Google Scholar] [CrossRef]
- Ma, J.C.; Dougherty, D.A. The Cation−π Interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. 1998, 27, 89. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The nature of π-π interactions. J. Am. Chem. Soc. 1990, 112, 5525. [Google Scholar] [CrossRef]
- Kim, E.; Paliwal, S.; Wilcox, C.S. Measurements of Molecular Electrostatic Field Effects in Edge-to-Face Aromatic Interactions and CH-π Interactions with Implications for Protein Folding and Molecular Recognition. J. Am. Chem. Soc. 1998, 120, 11192–11193. [Google Scholar] [CrossRef]
- Kurbatov, A.O.; Balabaev, N.K.; Mazo, M.A.; Kramarenko, E.Y. Effects of generation number, spacer length and temperature on the structure and intramolecular dynamics of siloxane dendrimer melts: Molecular dynamics simulations. Soft Matter 2020, 16, 3792–3805. [Google Scholar] [CrossRef]
- Kurbatov, A.O.; Balabaev, N.K.; Mazo, M.A.; Kramarenko, E.Y. A Comparative Study of Intramolecular Mobility of Single Siloxane and Carbosilane Dendrimers via Molecular Dynamics Simulations. Polymers 2018, 10, 838. [Google Scholar] [CrossRef] [PubMed]
- Peterca, M.; Percec, V.; Imam, M.R.; Leowanawat, P.; Morimitsu, K.; Heiney, P.A. Molecular Structure of Helical Supramolecular Dendrimers. J. Am. Chem. Soc. 2008, 130, 14840–14852. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Cho, W.-D.; Ungar, G.; Yeardley, D.J.P. Synthesis and Structural Analysis of Two Constitutional Isomeric Libraries of AB2-Based Monodendrons and Supramolecular Dendrimers. J. Am. Chem. Soc. 2001, 123, 1302–1315. [Google Scholar] [CrossRef]
- Ding, S.-G.; Yu, L.; Wang, L.-H.; Wang, L.-D.; Yu, Z.-Q.; You, Y.-Z. Self-Assembling Janus Dendritic Polymer for Gene Delivery with Low Cytotoxicity and High Gene Transfection Efficiency. J. Mater. Chem. B 2016, 4, 6462–6467. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Jin, H.; Jiang, B.; Zhu, X.; Zhou, Y.; Lu, Z.; Yan, D. A Supramolecular Janus Hyperbranched Polymer and Its Photoresponsive Self-Assembly of Vesicles with Narrow Size Distribution. J. Am. Chem. Soc. 2013, 135, 4765–4770. [Google Scholar] [CrossRef]
- Trant, J.F.; Jain, N.; Mazzuca, D.M.; McIntosh, J.T.; Fan, B.; Haeryfar, S.M.M.; Lecommandoux, S.; Gillies, E.R. Synthesis, Self-Assembly, and Immunological Activity of α-Galactose-Functionalized Dendron−lipid Amphiphiles. Nanoscale 2016, 8, 17694–17704. [Google Scholar] [CrossRef]
- Percec, V.; Leowanawat, P.; Sun, H.-J.; Kulikov, O.; Nusbaum, C.D.; Tran, T.M.; Bertin, A.; Wilson, D.A.; Peterca, M.; Zhang, S.; et al. Modular Synthesis of Amphiphilic Janus Glycodendrimers and Their Self-Assembly into Glycodendrimersomes and Other Complex Architectures with Bioactivity to Biomedically Relevant Lectins. J. Am. Chem. Soc. 2013, 135, 9055–9077. [Google Scholar] [CrossRef]
- Hinman, S.S.; Ruiz, C.J.; Cao, Y.; Ma, M.C.; Tang, J.; Laurini, E.; Posocco, P.; Giorgio, S.; Pricl, S.; Peng, L.; et al. Mix and Match: Coassembly of Amphiphilic Dendrimers and Phospholipids Creates Robust, Modular, and Controllable Interfaces. ACS Appl. Mater. Int. 2017, 9, 1029–1035. [Google Scholar] [CrossRef]
- Gottis, S.; Rodriguez, L.-I.; Laurent, R.; Angurell, I.; Seco, M.; Rossell, O.; Majoral, J.-P.; Caminade, A.-M. Janus carbosilane/phosphorhydrazone dendrimers synthesized by the ‘click’ Staudinger reaction. Tetrahedron Lett. 2013, 54, 6864–6867. [Google Scholar] [CrossRef]
- Riegert, D.A.; Pla-Quintana, A.; Fuchs, S.; Laurent, R.; Turrin, C.-O.; Duhayon, C.; Majoral, J.-P.; Chaumonnot, A.; Caminade, A.-M. Diversified Strategies for the Synthesis of Bifunctional Dendrimeric Structures. Eur. J. Org. Chem. 2013, 2013, 5414–5422. [Google Scholar] [CrossRef]
- Muzafarov, A.M.; Rebrov, E.A. Current trends in the chemistry of dendrimers. Polym. Sci. Ser. C 2000, 42, 55–77. [Google Scholar]
- Muzafarov, A.M.; Gorbatsevich, O.B.; Rebrov, E.A.; Ignat’eva, G.M.; Chenskaya, T.B.; Myakushev, V.D.; Bulkin, A.F.; Papkov, V.S. Organosilicon dendrimers: Volume-growing polyallylcarbosilanes. Polym. Sci. Ser. C 1993, 35, 1575–1580. [Google Scholar]
- Lorenz, K.; Hölter, D.; Stühn, B.; Müllwupt, R.; Frey, H. A mesogen-functionalized carbosilane dendrimer: A dendritic liquid crystalline polymer. Adv. Mater. 1996, 8, 414–416. [Google Scholar] [CrossRef]
- Lorenz, K.; Mülhaupt, R.; Frey, H.; Rapp, U.; Mayer-Posner, F.J. Carbosilane-Based Dendritic Polyols. Macromolecules 1995, 28, 6657–6661. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Cara, P.D.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288. [Google Scholar] [CrossRef] [PubMed]
- Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and Fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Tanaka, R.; Iyoda, J.; Shiihara, I. Addition reaction of dichloromethylsilane to (+)-limonene and (+)-β-pinene in the presence of hexachloroplatinic acid catalyst. J. Chem. Soc. Jpn. 1968, 6, 71. [Google Scholar]
- Voronkov, M.V.; Yarosh, O.G.; Shchukina, L.V.; Albanov, A.I.; Rudakov, G.A. Organosilicon derivatives of p-menth-1-ene. J. Gen. Chem. 1996, 12, 66. [Google Scholar]
- Qian, Y.; Dong, F.; Guo, L.; Guo, J.; Shaghaleh, H.; Wang, Y. Preparation and properties of room temperature vulcanized silicone rubber using triethoxy(2-(4-methylcyclohex-3-en-1-yl)propyl)silane as a novel crosslinking agent. Polym. Degrad. Stab. 2020, 173, 109068. [Google Scholar] [CrossRef]
- Marciniec, B. (Ed.) Comprehensive Handbook on Hydrosilylation; Pergamon Press: Oxford, UK, 1992. [Google Scholar]
- Brzakalski, D.; Sztorch, B.; Frydrych, M.; Pakuła, D.; Dydek, K.; Kozera, R.; Boczkowska, A.; Marciniec, B.; Przekop, R.E. Limonene Derivative of Spherosilicate as a Polylactide Modifier for Applications in 3D Printing Technology. Molecules 2020, 25, 5882. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, F.V.; Cherkaev, G.V.; Buzin, M.I.; Muzafarov, A.M. Facile methods of siloxanes derivatives modification by azodyes based on eugenol. J. Organomet. Chem. 2018, 871, 135–139. [Google Scholar] [CrossRef]
- Drozdov, F.V.; Cherkaev, G.V.; Muzafarov, A.M. Synthesis of new functional siloxane derivatives of limonene. Part I: Combination of hydrosilylation and hydrothiolation reactions. J. Organomet. Chem. 2019, 880, 293–299. [Google Scholar] [CrossRef]
- Milenin, S.A.; Selezneva, E.V.; Tikhonov, P.A.; Vasil’ev, V.G.; Buzin, A.I.; Balabaev, N.K.; Muzafarov, A.M. Hybrid polycarbosilane-siloxane dendrimers: Synthesis and properties. Polymers 2021, 13, 606. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkov, A.I.; Drozdov, F.V.; Cherkaev, G.V.; Muzafarov, A.M. Synthesis of Carbosilane and Carbosilane-Siloxane Dendrons Based on Limonene. Polymers 2022, 14, 3279. https://doi.org/10.3390/polym14163279
Ryzhkov AI, Drozdov FV, Cherkaev GV, Muzafarov AM. Synthesis of Carbosilane and Carbosilane-Siloxane Dendrons Based on Limonene. Polymers. 2022; 14(16):3279. https://doi.org/10.3390/polym14163279
Chicago/Turabian StyleRyzhkov, Aleksei I., Fedor V. Drozdov, Georgij V. Cherkaev, and Aziz M. Muzafarov. 2022. "Synthesis of Carbosilane and Carbosilane-Siloxane Dendrons Based on Limonene" Polymers 14, no. 16: 3279. https://doi.org/10.3390/polym14163279
APA StyleRyzhkov, A. I., Drozdov, F. V., Cherkaev, G. V., & Muzafarov, A. M. (2022). Synthesis of Carbosilane and Carbosilane-Siloxane Dendrons Based on Limonene. Polymers, 14(16), 3279. https://doi.org/10.3390/polym14163279






