Abstract
In recent decades, the enhancement of the properties of electrolytes and electrodes resulted in the development of efficient electrochemical energy storage devices. We herein reported the impact of the different polymer electrolytes in terms of physicochemical, thermal, electrical, and mechanical properties of lithium-ion batteries (LIBs). Since LIBs use many groups of electrolytes, such as liquid electrolytes, quasi-solid electrolytes, and solid electrolytes, the efficiency of the full device relies on the type of electrolyte used. A good electrolyte is the one that, when used in Li-ion batteries, exhibits high Li+ diffusion between electrodes, the lowest resistance during cycling at the interfaces, a high capacity of retention, a very good cycle-life, high thermal stability, high specific capacitance, and high energy density. The impact of various polymer electrolytes and their components has been reported in this work, which helps to understand their effect on battery performance. Although, single-electrolyte material cannot be sufficient to fulfill the requirements of a good LIB. This review is aimed to lead toward an appropriate choice of polymer electrolyte for LIBs.
Keywords:
electrolyte; ionic liquid; polymer; hybrid; composite; lithium-ion conductivity; lithium-ion 1. Introduction
In recent decades, battery technologies have made considerable progress, mainly in LIBs. LIB demand has been increasing since 2017 from USD 29.86 billion and is expected to reach USD 139.36 billion by 2026 [1]. LIBs show very high energy density and have been widely applied in vehicles, power grids, and electrical appliances [2]. The energy density of the LIBs can commonly be improved by high-voltage active cathode materials, anode materials, and electrodes. One of the main problems regarding high-voltage cathodes and LIBs is the decomposition of the electrolyte at more than 4.2 V Li/Li+ [3,4,5,6,7,8,9]. Hence, to develop Li-ion conductivity, the stability of the material succeeding the electrolyte can also be considered a crucial electrochemical parameter in the implementation of next-generation devices that hold higher cell potential. The fast development of electric vehicles and power stations in terms of safety and energy density engenders new challenges for Li-ion batteries (LIBs) [10,11,12,13]. The progress observed in LIB technology reposes eventually on basic comprehension of the electronic, chemical and structural modifications of the battery elements, which influence the charge-discharge cycle. A LIB is made with a cathode, electrolyte, separator, and anode. The enhancement of each LIB component contributes significantly to its performance. The implementation of these batteries is highly dependent on novel electrolyte materials with outstanding transport properties, low interfacial resistance, and good mechanical strength. In lithium ion batteries, lithium ions move from the negative electrode (typically graphite) through an electrolyte to the positive electrode during discharge and back when charging. During the oxidation reaction that occurs in the process of discharging/cycling, the SEI layer fissures continuously with the mass variation of active material [14,15]. The interface stability of the active material increases the battery retention capacity during cycling and a long-time for energy storage [16,17]. The change in the content and mechanical properties of the surface of active materials has drawn particular attention [18]. For electroactive materials to be considered latent candidates for LIBs, the immediate requirement is to obtain the reversible capacity, excellent ionic conductivity, good life span, and excellent diffusion rate of lithium into active materials. The graphite (C6) permits the intercalation of only a single Li with the six carbon atoms that result in the stoichiometry of lithiated graphite (LiC6), providing the equivalent reversible specific capacity of 372 mA h g−1. Therefore, it is urgent to replace graphite anodes with materials having higher capacity, energy, and power density. The modification of electrolytes is a well-accepted way to improve the performance of LIBs. A small amount of electrolyte additives incorporated is often introduced to develop the SEI layer at the interface between the active material and electrolyte [19,20,21,22]. Electrolyte additive materials are often used in a small amount to create the SEI layer at the active material/electrolyte interface [23,24,25,26]. Solid-state polymer electrolytes (SPEs), a specific class of polymer, are considered promising candidates to replace current organic liquid electrolytes due to their mechanical properties and electrochemical stability against lithium metal [27]. However, current SPEs exhibit low ionic conductivity at room temperature and high contact resistance. To overcome this, there is a particular interest in gel polymer electrolytes. Gel polymer electrolytes, which contain liquid plasticizers, have received considerable attention in the field of energy storage because of their specific characteristic, high ionic conductivity, and enhanced interfacial charge transfer [28,29]. Changing the electrolyte properties is a good approach to enhancing LIB storage capacity. Figure 1 shows an illustrative schema of a LIB during the charge and discharge.
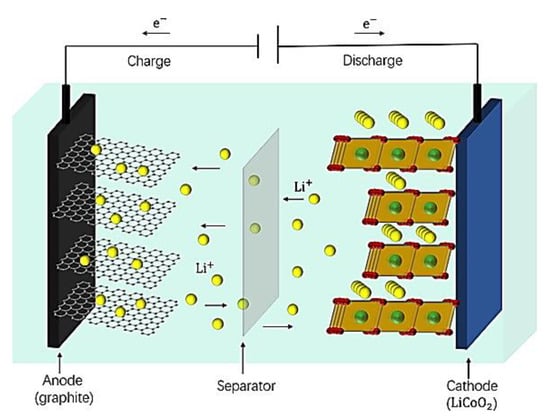
Figure 1.
Schematic illustration of LIBs [30].
The transfer of lithium ions inside the battery is accompanied by the flow of charge in the external circuit, so the efficiency of lithium-ion transfer in the electrolyte affects the capacity of the battery.
The chemistry of secondary batteries in the aprotic electrolyte is very close to the chemistry of primary ones. There are a few recent studies that demonstrate that utilizing a liquid electrolyte in conjunction with a porous polymer structure can provide good transport properties and cycling performance in different configurations of lithium battery [31,32,33,34,35]. In the 1 M of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) with 1,3-dioxolane, Dimethoxyethane (DOL-DME) and 0.5 M LiPS (lithium polysulfide) electrolyte, the transference number was reported as 0.98, which may be the highest transference number ever reported for a gel polymer electrolyte containing free salt, along with 1.14 × 10−3 S cm−1 ionic conductivity [27]. Wang and his team used a similar crosslinked polymer as a coating on a polyethylene separator, in the presence of 1 M Lithium hexafluor phosphate (LiPF6) with ethylene carbonate-ethyl methyl carbonate-diethyl carbonate (EC-EMC-DEC) [34]. The transference number was 0.72 along with 10−4 S cm−1 ionic conductivity at room temperature. The impact of polyethylene dimethacrylate (PEGDMA) by the addition of water in the prepolymer solution was studied. As a result, it was found that conventional Li-ion battery electrolyte contained in the porous PEGDMA network leads to augmenting the lithium transference number to 0.79 while maintaining the ionic conductivity in the order of 10−3 S cm−1 [27].
LIBs also suffer from many drawbacks, such as the poor rechargeability of fluoromethane sulfonyl and susceptibility to misuse leading to fire or even explosion. An uncommon phenomenon of the swelling has been reported in LIBs after a complete discharge [36]. The amount of gas (hydrogen fluoride) produced upon lower voltage battery anodes is more considerable than that generated on the same electrode but with higher voltages [18]. In LIBs, the hydrogen fluoride gas generated is caused by the shortening of electrolytes on the anode after oxidation and decomposition of the SEI layer after the entire removal of lithium ions [18]. Herein, we study the impact of electrolytes on the performance of the full cell. This review focuses on recent research on various types of electrolytes for lithium battery application, mainly on investigating the effect of the additives and solvents on the electrolyte properties. In this work, IL-based polymer electrolytes, solid polymer electrolytes, gel polymer electrolytes, porous gel polymer electrolytes, aqueous polymer electrolytes, non-aqueous electrolytes, ionic conductor-based composite polymer electrolytes, and solid polymer electrolytes have been reported. This work orients in developing safer electrolytes with high voltage stability for high-energy-density LIB applications.
2. Base Electrolyte Salt
2.1. Electrolytes
The choice of a good electrolyte is one of the most important ways to resist environmental redox reactions at the electrodes and should perfectly work within the cell without decomposition. In addition, electrolytes should be inert, well-balanced, and able to operate at a certain temperature.
Carbonate-containing liquid electrolytes are mainly used to dissolve lithium salts and ease the ions’ migration within the electrolyte due to their comparatively low viscosity, but their flammability has been the main drawback that results in the use of room-temperature ionic liquids (RTILs) [37]. Ionic liquids (ILs) are non-flammable and able to operate at higher potential windows relative to carbonates and demonstrate good thermal stability with low vapor pressure. In addition, to operate, carbonates might have been inserted at a defined voltage to create an adequate SEI [37]. Fernicola and his co-workers developed and characterized a new polymer electrolyte by dissolving Li-TFSI salt in ionic liquid (IL) called: N-butyl-N-ethylpiperidinium N,N-bis(trifluoro-methane)sulfonamide (PP24TFSI) and the mixture was incorporated into the polymer matrix [38]. They have also studied the dynamics of atoms 1H, 19F, and 7Li, and their migration effect in neat ionic liquid (PP24TFSI), the mixture of salts (Li-TFSI: PP24TFSI), and Li-TFSI-doped PP24TFSI polymer electrolyte were investigated by the Nuclear Magnetic Resonance (NMR) method. The results indicated a significant modification of the dynamical systems due to the shifting of the minimum ignition temperature (T1) to higher temperatures. Equivalent behaviors were noticed for 19F and 7Li through the typical sequences [37]. Li-ion diffusivity in the mixed salt (Li-TFSI: PP24TFSI) showed a value inferior to that of PP24 and TFSI ions, because of the strong coordination that exists between Li+ ion and TFSI-anions via a coulombic interaction [37]. Nevertheless, the Li-ion transference number in the matrix was much higher, owing to the decrease in the coefficient ratios of TFSI/Li self-diffusion from 2 to about 1 moving from the salt combination to the polymer membrane. This augmentation of the transference number is greatly likable for battery applications.
The details of the methodology and defies encountered with promising electrolytes have been communicated in the literature [23,24,25,26,38]. Examples of materials reported are solid-state polymer electrolytes (SPEs) [39], with characteristically high molecular weight, gel electrolytes, or additive-based composite materials [40,41,42,43,44]. NMR spectra demonstrated a correlation between the dynamics of the polymer segment and the mobility of free charges. In this case, the results showed that the ions’ migration takes place principally in the amorphous region of the polymer at T > Tg (glass transition temperature). In addition, a rise in the diffusion coefficients of Li+ along the polymer chain direction was observed, which influenced the ordering of lithium cations [41]. A Li-ion transfer study was carried out in mechanically aligned polymer properties [42], or Li-ion transport has also been studied in mechanically balanced polymers [42] or polymers with balanced mechanical properties [43]. This statement is directly opposed to the ion transfer observed in polyethylene oxide (PEO), which emphasized that the migration of lithium ions occurred along the aligned axis of helical canals of the matter [44].
2.2. Electrolytes for LIBs
In LIBs, electrolytes are conventionally made with integrated binary carbonate solvents and contained lithium salts and exhibit low viscosity and high permittivity. Lithium salts include LiPF6, LiTFSI, lithium tetrafluoroborate (LiBF4), etc. [36]. However, these electrolytes demonstrate a low wet sensibility causing the formation of hydrogen fluoride gas [45] and the continual development of an SEI layer [46]. Organic solvents with a reduction potential of 1.0 V (vs. +/Li) are typically used in LIBs [47]. The SEI layer is formed when Li-ions, anions, and solvents interacted with each other on the surface of electrodes. However, the layer expansion affects the dendritic growth of lithium, which leads to weakening the coulombic efficiency and limits the battery life span [48]. Additionally, electrolytes made with traditional organic solvents are highly flammable, such as acetone, acetonitrile, acetic acid, benzene, acetic acid, etc., and volatile, owing to the non-stability of these organic solvents for a large temperature range [47], which can lead to the device’s degradation and conflagration. For this reason, the use of common non-organic solvents such as carbon tetrachloride, sulfur dioxide, sulfuryl chloride fluoride, dinitrogen tetroxide, antimony trichloride, and bromine trifluoride, with the potential possibilities, has led to the manufacture of non-flammable electrolytes with electrochemical and thermal stabilities.
3. Types of Electrolytes
3.1. Ionic Liquid-Based Polymer Electrolyte for LIBs
Ionic liquids (ILs) as compared to organic solvents are non-flammable with low vapor pressure, which demonstrate good characteristics to be a suitable candidate for electrolytes because of their wide potential range and their wide potential window, and excellent thermal stability. In general, ILs are classed into three main groups depending on the chemical composition of cations as shown in Figure 2 [49].
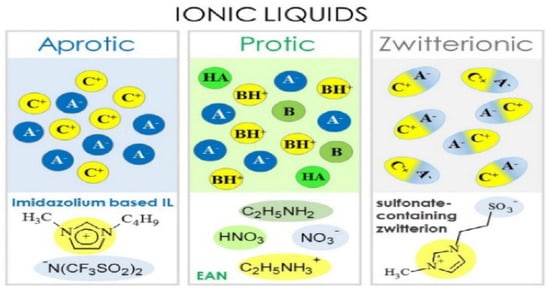
Figure 2.
Classes of ionic liquids (aprotic, protic, and zwitterionic) (A−: anion, B: base, C+: cation, BH+: Brønsted base, HA−: Brønsted acid) [49].
Among these classes of ILs, aprotic ILs appear to be an appropriate electrolyte material for LIBs, since they consist of ions only. However, the choice of a specific combination of anion–cation defines the IL properties, such as conductivity, viscosity, solubility, and melting point. Mostly imidazolium, pyrrolidinium, pyridinium, tetraalkylammonium, and tetraalkylphosphonium have been studied. Anions, unlike cations, were widely studied. Some examples are given in Figure 3.
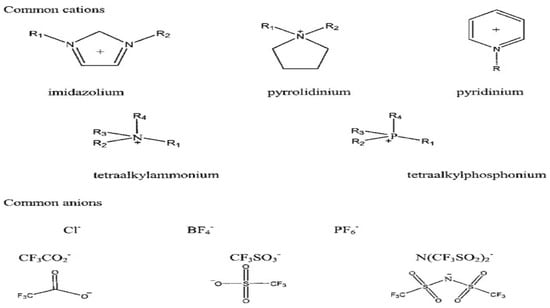
Figure 3.
Structures of common cations and anions of ionic liquids [50].
The mentioned anions are inorganic anions such as halides, e.g., chloride (Cl−), bromide (Br−), iodide (I−), Fluorine (F−), polyatomic inorganic (BF4−, PF6−), or organic anions such as trifluoroacetic anhydride (CF3CO2−), triflate (CF3SO3−), bis(trifluoromethanesulfonimide (N(CF3SO2)2), methanesulfonate (CH3SO3−), and acetate (CH3COO−) [51,52,53,54,55].
Electrolytes used for LIBs are typically made with 1 M LiPF6 in binary carbonate solvents or 1 M LiTFSI in ternary ether solvents but suffer from stability and safety issues due to their flammability and volatility properties. To overcome this problem, Zaghib has increased the thermal stability of electrolytes using the ionic liquid 1-Ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([EMI][TFSI]) as an additive for LIBs application [56]. Chen and his team revealed that using LiTFSI and the N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide [PP13][TFSI] new electrolyte, crucially, enhances the rate capacity and performance at low temperatures and is more secure than using traditional electrolytes [57]. Additionally, the enhancement of the cycle stability and high-performance LIB was achieved using low-melting-point-inorganic alkali salts. Zhibin and his co-workers used dual-salt-mixed potassium bis(fluorosulfonyl)imide ([KFSI]) and lithium bis(fluorosulfonyl)imide ([LiFSI]), which showed good ionic conductivity (10−3~10−2 S cm−1) in a temperature range of 40−150 °C [58]. The change in IL properties via the addition of promising materials and the development of new ionic systems were reported. Three ionic liquids ([EMI][TFSI], 1-propyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([PMI][TFSI]) and 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([BMI][TFSI]) were chosen as electrolytes and their features were studied [59]. As a result, IL ([EMI] [TFSI]), as compared to others, demonstrated the best thermal stability and electrochemical performance. Yang and his co-workers used the following five low viscosity quaternary ammonium-based ILs for LIB application: N-ethyl-N,N,N-tri-(2-methoxyethyl)ammonium bis(trifluoromethanesulfonyl)imide([N2(2o1)3][TFSI]), N-propyl-N,N,N-tri-(2-methoxyethyl)ammonium bis(trifluoromethanesulfonyl)imide ([(N3(2o1)3)][TFSI]), N-butyl-N,N,N-tri-(2-methoxyethyl)ammonium bis(trifluoromethanesulfonyl)imide ([(N4(2o1)3)][TFSI]), N-ethyl-N,N-di-(2-methoxyethyl)-N-2-ethoxyethylammonium bis(trifluoromethanesulfonyl)imide ([(N2(2o1)2(2o2)][TFSI]) and N-ethyl-N,N,N-tri-(2-methoxyethyl)ammonium bis(trifluoromethanesulfonyl)imide ([N2(2o1)3][TFSI]). Among them, ionic liquid ([N2(2o1)2(2o2)][TFSI]) and ([N2(2o1)3][TFSI]) electrolytes exhibited better properties at the current rate of 0.1 C [60]. Recently, Hirano and his team prepared a hybrid electrode using organosilicon functionalized ammonium ILs and oligo (ethylene oxide) substitute mixed with a commercial carbonate electrolyte. As a result, 30 vol% LFP-doped electrode/Li half-cell exhibited good cycle stability and reversible capacity, and efficiently drove back the decomposition of electrolyte due to the good stability of the SEI layer, thus ameliorating lithium storage performance [61]. Paillard et al. investigated the performance of the LiNi0.5Co0.2Mn0.3O2 (NCM 523)/graphite full cell. A recent study reported that 1-Butyl-1-methylpyrrolidinium ([Pyr14]), 1-Methyl-1-propylpyrrolidinium ([Pyr13]), and N-methyl-N-pentylpyrrolidinium ([Pyr15]) have high ionic conductivity and superior electrochemical performance, which could be used for LIB application [62,63,64]. The results revealed that 1-Butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide ([Pyr14][TFSI]), 1-Butyl-1-Methylpyrrolidinium Dicyanamide ([Pyr14][DCA]) and N-butyl- N-methyl pyrrolidinium trifluoromethanesulfonyl-N-cyanoamide ([Pyr14][TFSAM]) showed better electrochemical performances than electrolytes based on organic carbonate solvent in LiNi0.5Co0.2Mn0.3O2 (NCM 523)/graphite full cells [65]. An emerging way to achieve high oxidation stability (>5.5 V vs. Li+/Li) for a graphite and Li metal anode was performed by Qian et al. 4 M LiTFSI salt was added to IL:1-Methyl-1-propyl-3-fluoropyrrolidinium and bis(fluorosulfonyl)-imide ([PfMpyr][FSI]), resulting in a higher potential window [66]. Howlett et al. [67] manufactured a high-energy-density LIB using LiFSI salt and N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide anion ([C3mpyr][FSI]) that eliminated dendritic growth despite the rapid rate of charging. In terms of stability, although IL-doped electrolytes show lower ionic conductivity than traditional carbonate electrolytes (8–12 S cm−1), the entire performance of IL electrolyte-based LIB remains almost constant and shows rather higher stability. However, the full cell operated at high current densities, which enhanced the coulombic efficiency of 0.96 at 20 mA cm−2 with a 0.2 V polarization [47]. The morphology structure revealed the growth of uniform sediment without dendrites with low electrode resistance, as shown in Figure 4.
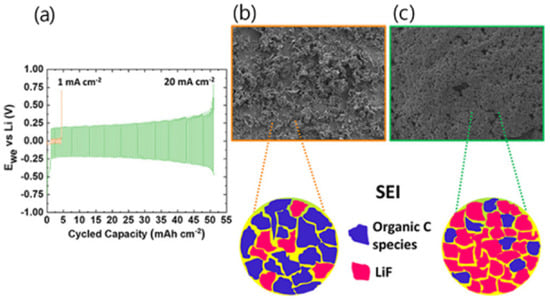
Figure 4.
(a) Form of symmetric voltage for various current densities; (b) SEM picture of accumulated lithium at 1 mA cm−2; (c) micrograph of accumulated lithium at 20 mA cm−2 [68].
3.2. Solid Polymer Electrolytes
Solid lithium batteries can be developed using two types of electrolytes: (a) Inorganic ionic conductors or (b) solid polymer electrolytes. It has been reported that solid lithium battery In–Lix/Li1−xCoO2 was made using Li3PO4-Li2S-SiS2 glass-ceramic electrolyte. As a result, the charging and discharging behavior remain constant over 80 cycles, with excellent cyclic behavior, and the cell can operate under high-pressure circumstances [69]. Figure 5 shows the entire performance of a 10 Wh LiCoO2-based solid LIB made with a solid polymer electrolyte and put on test at 60 °C. The cell shows a good cycling life at 70% DOD and no real change was observed in the discharge voltage after 355 cycles without capacity loss [70].
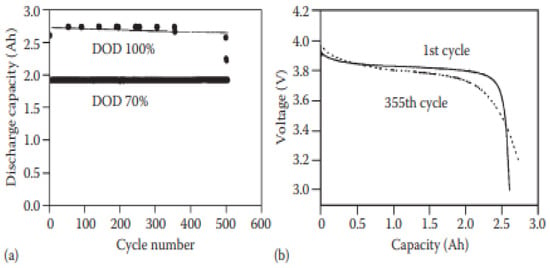
Figure 5.
Cycling feature of a 10 Wh solid LIB: (a) at various drops of discharge and (b) discharge curves in different cycles. The LIB consists of LiCoO2 as the positive electrode and Li metal as the negative electrode; ethylene oxide and propylene oxide (EO–PO) copolymer are used as electrolyte [70].
In 1991, Armand reported a Li-ion-conducting electrolyte made with novel LiTFSI salt as an ion lithium-ion conductive electrolyte for LIBs and later on a new type of single-ion solid polymer [71]. Jungdon Suk et al. [72] developed highly conductive electrolytes with an ionic conductivity of 7.6 × 10−4 S cm−1 for solid-state LIBs. The polymer electrolyte film was made with lithium-salt via the in situ radical polymerization of a precursor solution containing Li-salt and polyethylene glycol dimethyl ether as a plasticizer, and a combination of pentaerythritol tetrakis (3-mercapto propionate) and a synthesized hexakis(allyloxy)cyclotriphosphazene (thiol-ene PAL) employed as cross-linker. They assembled the battery using lithium iron phosphate (LiFePO4) as the positive electrode, solid polymer electrolytes (SPE), and lithium foil as the anode. The cell exhibits good performance, the capacity of initial discharge was 147 mAh g−1 at 0.1 C and 132 mAh g−1 at 0.5 C, and 97% of the capacity of retention was found to be 0.97 at the 100th cycle [72]. The SPE-based LIB demonstrated a stability potential window of 5.66V and good mechanical stability. Figure 6 shows the entire performance of a LiFePO4-based solid LIB made with a solid polymer electrolyte.
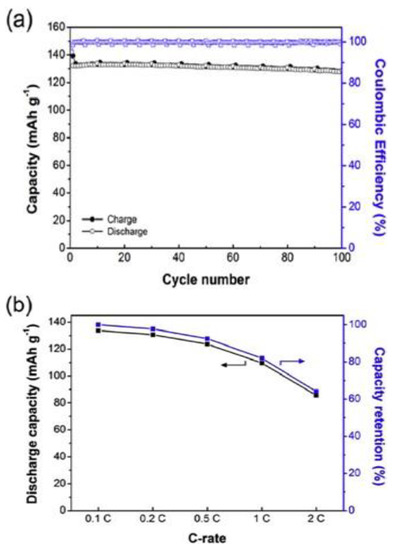
Figure 6.
(a) Cycling performance of the solid electrolyte-based battery evaluated with a stable charge-discharge of 0.5 C at 30 °C and (b) capacity retention values of various discharge capacities [72].
3.3. Gel Polymer Electrolytes
3.3.1. Gel Polymer Electrolytes for LIBs
A gel polymer electrolyte (GPE) is formed when an organic electrolyte is trapped in the porous structure. A gel-based battery is fabricated by transforming the H2SO4 aqueous solution into a gel. Gel-LIBs show different benefits such as high flexibility, lower interface resistance, better behavior at low temperature, no leakage, better safety, lower self-discharge, good working performance, higher reliability, longer cycling life, and a higher energy density. It should be noted that these characteristics mentioned above were not the same at the initial level but increased progressively until they achieved this stage of performance. As an example, Ultralife Co. Ltd. [73] assembled gel lithium batteries, which exhibit good performance as shown in Figure 7. Gel polymer electrolyte was prepared by addition of poly (vinylidene fluoride-hexafluoropropylene (PVDF-HFP) copolymer and 1 M LiPF6 solution in ethylene carbonate/dimethyl carbonate (EC: DEC) (v/v = 2:1). The cell performance indicates a good cycling life and better durability to overcharge than ordinary lithium-ion batteries [74]. In general, gel-LIBs operate better than batteries based on liquid electrolytes.
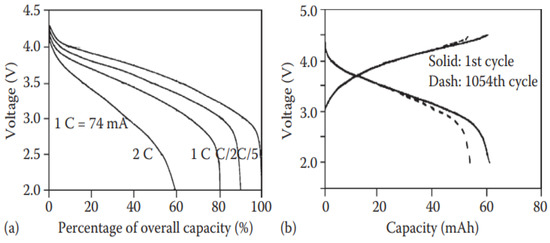
Figure 7.
(a) Discharge curves for various rates and (b) charge/discharge curves in different cycles for the gel lithium-ion batteries (74 mAh) fabricated by Ultralife Co. Ltd [73].
3.3.2. Porous Polymer Gel Electrolytes
Boz and his team [27] developed new porous polymer gel electrolytes for LiB application. The electrolyte of O-PEGDMA-VS-0 was prepared by trapping the conventional electrolyte (1 M LiPF6 in EC-DEC) in a microporous polymer network. The prepared porous polymer gel electrolytes show a conductivity of 10−3 S cm−1 and the cation transference number rises to 0.79 compared to non-porous. Condensed polymer electrolyte developed with an equal tendency shows lower conductivity and a transference number of 7.6 × 10−4 S cm−1 and 0.65, respectively [74]. The incorporation of the porous electrolyte within Li-metal/LiFePO4-based cells showed an increase in its rate capability, the capacity of retention, and the efficiency of the cell caused by the enhanced ion transfer properties of the porous polymer electrolyte compared to a commercial separator [74].
Zhang et al. developed a PVDF-HFP-based gel polymer membrane with multi-sized honeycomb-like porous architectures for lithium batteries beneficial to ameliorated safety [75]. The manufactured porous membrane demonstrates high porosity (78%) that drives to a high electrolyte uptake of 86.2%, exhibits an ionic conductivity of 1.03 × 10−3 S cm−1 at room temperature, and is better than using a commercial membrane [75]. The capacity of the full cell was 63.1 mAh g−1 at 5 C higher than that obtained with thinner, and conventional separators.
3.4. Aqueous Polymer Electrolytes
Wang and his co-workers manufactured solid-state aqueous polymer electrolytes by healing water-in-salt electrolytes (WiSE) into the network of poly (methyl methacrylate) (PMMA). The prepared solid-state aqueous polymer electrolyte (SAPE) showed higher ionic conductivity compared with traditional solid polymer electrolytes [76]. The use of WiSE contributes significantly to Li-ion transport compared to that of SPE using ionic liquids as reported recently. The aqueous SAPE can resist cutting and function in an open cell condition due to its strong bonding, which maintains the water and salt in the framework of the polymer [63]. They placed a water-free thin PEO-LiTFSI-KOH SPE interface between the anode and electrolyte to further increase the current efficiency. After a test, the LiMn2O4/Li4Ti5O12 full cell based on SAPE@SPE electrolyte offers a practical capacity ratio of 1.14 and 12m with unparalleled high initial coulombic efficiency of 90.50%, and the average value was found to be 99.97% at 0.5 C [76]. It was found that the energy of the entire cell can be ameliorated by extending the area capacity of the active material up to 1.5 mAh cm−1, and the potential window of aqueous electrolytes showed an extension from 3.0 V of 21 m WiSE to ~3.86 V of 12 m UV-cured SAPE [76]. However, solid-state aqueous polymer electrolytes offer a good opportunity to overcome the safety problems of LIBs and enable them to attain high voltage and high energy modules of the battery [76]. Zhao and co-workers [77] studied a new cell—LiFePO4/C (cathode) |9 M LiNO3 aqueous solution|LiV3O8 (anode)—to achieve a higher rate capability and better cyclic stability. It showed a capacity of 88.7 mAh g−1 after 100 cycles at a rate of 10 C and a discharge capacity of 60 mAh g−1 after 500 cycles at 50 C. Lithium sulfate (Li2SO4) as an aqueous electrolyte was used in the cell: LiTi2 (PO4)3 (cathode)|0.5 M Li2SO4 aqueous solution|LiFePO4 (anode). It showed a capacity of retention of over 90% up to 1000 cycles. This aqueous electrolyte was used to neaten the PH values of the electrolyte by oxygen elimination [65]. A complete LIB of 2.3 V based on water-in-salt electrolytes (aqueous solutions) was tested to have a coulombic efficiency of nearly 100% with several cycles > 1000, which could perfectly challenge LIBs made with non-aqueous solutions in terms of energy and power density (Figure 8).
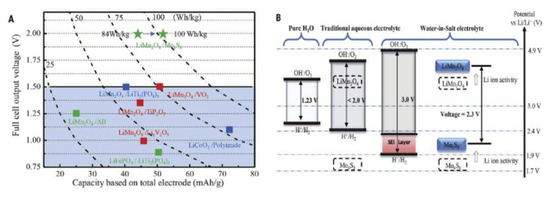
Figure 8.
(A) Performance of each aqueous LIB based on different redox couples. Cycling stability: red color, 1000 cycles. (B) Representation of enlarged potential window for WiSEs together with the modulated redox couples of LiMn2O4 cathode and Mo6S8 anode due to high content of salt [66].
3.5. Non-Aqueous Electrolytes
Since 1990, commercial LIBs have been dominated by the use of a non-aqueous solution that consists of salt (LiPF6) dissolved in organic carbonates, especially the mixture of EC with dimethyl carbonate (DMC), propylene carbonate (PC), ethyl methyl carbonate (EMC), and/or DEC [78,79]. However, new organic solvents and Li salts are needed for LIB performance enhancement. In general, conventional organic carbonate solvents exhibit a very low potential stability, and this drives the development of electrolytes with a wide potential window [80]. This instability was observed by Lucht et al. [81] when they studied the interfacial properties between an electrolyte prepared with 1M LiPF6 dissolved in EC:DMC:DEC at equal proportions (v/v/v = 1:1:1) and LiNi0.5Mn1.5O4-based electrodes. The common electrolyte appears to be unstable when the charge exceeds 4.5V after testing at different potentials [4–5.3 V vs. Li].
For this reason, organic fluoro-compounds have been employed as high-voltage organic solvents since the fluorinated molecules exhibit higher electrochemical stability potential windows because of the intense electron-removing effect of fluorine atoms [80]. Zhang et al. [82] studied the electrochemical stability of various fluorinate-based electrolytes at high-voltage conditions, and the E5 electrolytes made with the composition (1.2 M LiPF6 in F-AEC/F-ethyl methyl carbonate (EMC)/F-EPE (v/v/v = 2:6:2)) showed a higher potential window than that of the Gen2 electrolyte based on EC/EMC. In addition, the absence of EMC/F-EMC and EC/F-AEC has ameliorated the maximal potential as shown in Figure 9.
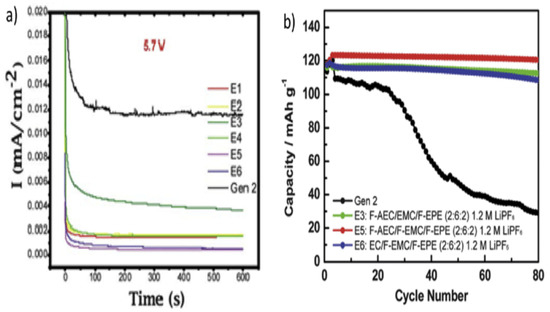
Figure 9.
(a) Electrochemical potential window of Gen2 and fluorinated electrolytes E1–E6 at 5.7 V utilizing a traditional cell with three electrodes. (b) Cycling capacity retention of lithium titanate (Li4Ti5O12) (LTO)/LNMO cells with baseline electrolyte (Gen2) and fluorinated electrolytes E3, E5, and E6 at 55 °C. Gen2: 1.2 M LiPF6 in EC/EMC (3:7); E1: EC/EMC/F-EPE (2:6:2) 1.2 M LiPF6; E2: EC/EMC/F-EPE (2:5:3) 1.2 M LiPF6; E3: F-AEC/EMC/F-EPE (2:6:2) 1.2 M LiPF6; E4: F-AEC/EC/EMC/F-EPE (1:1:6:2) 1.2 M LiPF6; E5: F-AEC/F-EMC/F-EPE (2:6:2) 1.2 M LiPF6; E3: EC/F-EMC/F-EPE (2:6:2) 1.2 M LiPF6 [83].
Solvents have a crucial impact on the fabrication of a good electrolyte. The physical and chemical properties of some commonly used solvents are shown in Table 1.

Table 1.
Properties of some conventional organic solvents [83].
Chen and his co-workers [82] developed a new non-aqueous fluorinated lithium borate cluster salt (Li2B12F12xHx) electrolyte with lithium difluoro(oxalate)borate (LiDFOB) as the additive, which showed higher thermal stability and better cycle life than LiPF6-electrolyte. The novel Li2B12F9H3 electrolyte showed a capacity of retention of 70% with 1200 cycles under 55 °C. Li et al. [83] used a mixture of LiPF6 (1 M) dissolved in DMC, EMC, and EC (1:1:1) as electrolytes with a lithium manganite (Li2MnO3)-doped Li2Ni0.3Co0.3 Mn0.3O2 cathode material. The Li2MnO3-based electrolyte ameliorated the cycling life and demonstrated a high coefficient of Li+ diffusion and good rate capacity.
3.6. Ionic Conductor-Based Composite Polymer Electrolytes
3.6.1. Mechanism of Li-ion Transport
Inorganic solid electrolytes have received attention due to their good properties, such as non-flammability and high ionic conductivity up to 10−2 S cm−1 at room temperature (RT) [84,85], but showing a poor interaction with electrodes which has limited their use in commercial LIBs [86]. To overcome this inconvenience, many types of research have been conducted to enhance the interfacial properties of inorganic solid electrolytes by doping inorganic solid electrolytes used as fillers to solid polymer electrolytes (SPEs) to combine both the properties of inorganic polymer electrolytes (good conductivity, strength) and SPEs (flexibility and good interfacial properties) [87]. Polymer is not only expected to dissolve Li-salt but also be enabled to connect with Li+ ions. In this case, the polar groups (— O —, — S —, etc.) present in the polymer are essential for Li-salt dissolution. For this reason, researchers have been directed to PEO polymer and its offshoots. In PEO, the oxygen atom (—O—) present on the polymer chain, and lithium coordinate with each other by coulombic interactions. Under the electric field, Li+ cations move from one coordination point to another along the polymer segment. The mechanism of lithium-ion transfer in PEO-doped polymer electrolytes is shown in Figure 10 [79].
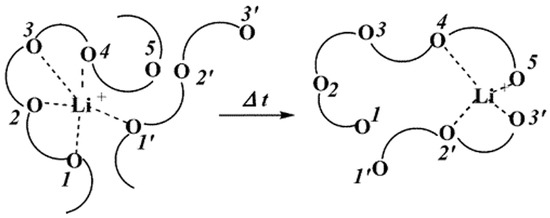
Figure 10.
Structural representation of Li+ transport in PEO-doped polymer electrolyte (PE) [74].
In the PEO-based composite system, Li+ ion transport is influenced by the large size of the polymer segment and the bound effect of the crystalline regions. However, lithium-ion conductivity relies on the ion’s density and the polymer chain mobility. The polymer aptitude to dissolve lithium salt defines the number of ions that can move within the polymer matrix, and thus polymer with high dielectric constant and low lattice energy lithium salt favors the dissociation [88]. Under normal conditions, the ionic conductivity () can be calculated using the following expression [89]:
Here, is expressed in S m−1, is the Faraday constant in C mol−1, is the carrier density in m−3, is the number of charges in C, and is the mobility in m2 (V⋅s)−1. It appears clearly that the increase in density of free charges and the speed of migrated ions can improve the ionic conductivity in polymer electrolytes.
The motion of ions in SPEs can be explained by three theories: Arrhenius theory, Vogel–Tammann–Fulcher (VTF), William–Landel–Ferry (WLF), and combining all these theories [90].
Arrhenius established the relationship between temperature and ion migration caused by polymer chain displacement as [91]:
Here, represents the pre-exponential factor in S m−1, is the activation energy in J, K is the Boltzmann constant in J/K and T is the thermodynamic temperature in K.
The conductivity is generally affected by the mobility of the polymer segment or/and the relaxation of the polymer chain and jump motion of ions along the polymer segment, where = f () is a nonlinear curve [92]. VTF describes the relation between of polymer electrolyte and T [91].
Here, is an action factor (with energy dimension) in J/K, and is the reference temperature in K, normally found in the range [10–50 °K] below the experimental glass transition temperature (. At ambient temperature, low could have a significant impact on the conductivity enhancement if only the impact of the polymer segment on σ was taken into account.
William–Landel–Ferry (WLF) established a relationship between conductivity, temperature, and frequency. The WLF theory takes into account the relaxation process of molecular chain motion in an amorphous system. The WLF equation is:
Here, σ() is the ionic conductivity in S m−1 atand and are the WLF free volume parameters.
is a crucial parameter in the conductivity improvement in the polymer electrolyte. The conductivity is very low if and increases undeniably if. However, a reduction in leads to an increase in the ionic conductivity.
The mechanism of ion transport of PEO-doped polymer electrolytes has been well-explained by the three above-mentioned theories. The amorphous regions of the polymer contribute efficiently to the diffusion of ions. This theory is also applicable to other polymer-based solid electrolytes.
Figure 11 shows various composites that were doped into polymers, including fast-ion conductive ceramics [92,93,94,95], inert ceramic fillers [92,96], lithium salts [97], ionic liquid [98], etc.
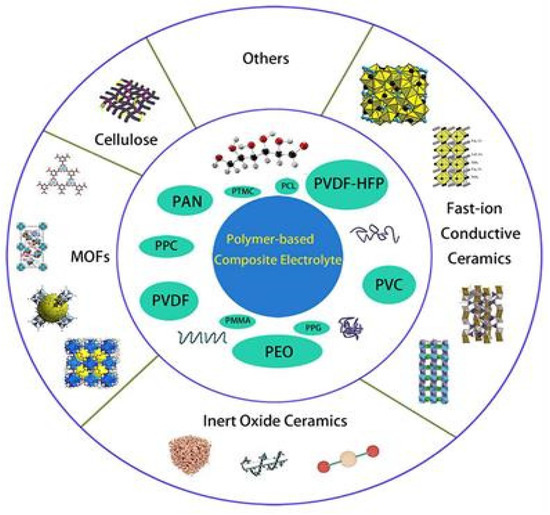
Figure 11.
Classification of certain composite electrolytes doped with polymers [99].
3.6.2. Electrolyte/Electrode Interface
A solid lithium-ion battery is typically made of LiFePO4 or LiCoO2 (cathode) and lithium metal (anode). Each electrode/electrolyte contact should have good properties that help the battery to be working well. Cathode/electrolyte contact desires a highly flexible and solid polymer electrolyte to reduce the interface resistance while the electrolyte/anode contact desires a solid electrolyte to resist the lithium dendrite growth [100,101,102]. High flexible solid polymer electrolyte allows for lowering the resistance at the interface, but poor mechanical stability could not confront the perforation caused by metallic lithium dendrites. To overcome this problem, an inorganic ceramic electrolyte with good mechanical properties is employed to sustain the metallic lithium dendrites but still suffers from poor interface contact with the electrodes (large interface resistance) [103]. Additionally, the non-stability at the interface creates side reactions and the SEI layer is formed on the electrode surface, which may conduct to reduced battery cycle life [103]. To consider the beneficial properties of both polymer and inorganic ceramic electrolyte in terms of good interface contact and excellent mechanical properties, respectively, polymer composite inorganic ceramic electrolyte has been developed [99].
3.6.3. Categories of Polymer-Based Composite Electrolytes for Lithium Batteries
Inert-Oxide-Ceramic-Based Solid Polymer Electrolytes
To improve the mechanical features, reduce the degree of crystallinity in polymer and improve the ionic conductivity of SPE, inert oxide ceramics have been used in polymer electrolytes. Weston and his colleague [104] ameliorated ionic conductivity and mechanical properties of solid polymer composite electrolytes using PEO-doped with Al2O3 as inert oxide ceramic particles. Tambelli and his co-workers [105] revealed that the incorporation of Al2O3 into PEO can also significantly diminish the crystallinity and of PEO. This indicates that the reduction in crystallinity in the polymer leads to improving its ionic conductivity. This decrease in crystallinity can then allow a high number of free polymer segments and speed up the segmental motion that facilitates the lithium diffusion. Liang and his team [106] reported the preparation of PEO-PMMA-LiTFSI–Al2O3 composite electrolyte using the solution casting technique. The addition of Al2O3 as filler improved the ionic conductivity from 6.71 × 10−7 to 9.39 × 10−7 S cm−1. Lee et al. developed SPE using PEO-EC-PC and SiO2 as inert ceramic fillers. The conductivity of the SPE was found to be 2 × 10−4 S cm−1 at RT with a composition of 2.5wt% SiO2 [107]. Lin et al. [108] developed a new PEO-silica aerogel composite polymer electrolyte (CPE), and SPE shows a high Young’s modulus of 0.43 GPa and high ionic conductivity of 6 × 10−4 S/cm. The investigation shows that the dispersion of SiO2 powder into polymer has enhanced the mechanical properties and ionic conductivity. Pal and his group [109] prepared PMMA-LiClO4-TiO2-based SPEs via the solution casting method, the study showed that incorporation of composite nanosized TiO2 into SPE exhibits ionic conductivity of 3 × 10−4 S cm−1 at RT, and this can ameliorate the thermal stability as well. LiCoO2-based SPE/graphite demonstrated a specific capacity of 30 mAh g−1 in twenty cycles at ambient temperature. In recent research work, Tao and his team [110] fabricated PEO-LiTFSI-based SPE using Mg2B2O5 nanowires. The prepared composite polymer electrolyte showed excellent mechanical properties and remarkable potential windows. CPE shows good ionic conductivity due to the facility of ions to migrate upon the surface of Mg2B2O5 nanowires, and also due to the high-speed mobility of Mg2B2O5 and the coordination of Mg2B2O5 with TFSI− (Figure 12).
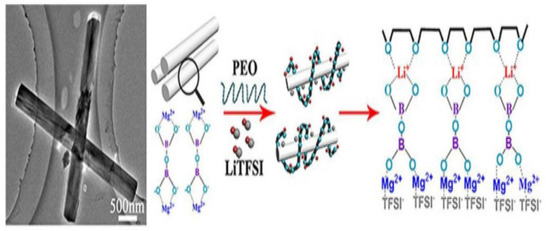
Figure 12.
Schematic representation of Li-ion transfer in Mg2B2O5 improved based on solid CPEs [110].
MOF-Based Composite Electrolytes
Metal-organic frameworks (MOFs) are new porous materials containing bridging organic ligands and metal ions [111,112,113,114]. MOFs are widely employed in different domains, including molecular separation, gas adsorption, and drug delivery [115,116,117], because of their porosity, high surface area, and polymetallic sites [118]. Yuna and his co-workers [118] developed a new composite polymer electrolyte using Zn-based MOF-5, LiTFSI, and PEO polymer. The addition of MOFs to the polymer host (PEO) enhanced the mechanical and electrochemical properties of SPEs. The enhancement of ionic conductivity up to 3.16 × 10−5 S cm−1 at 25 °C may be caused by: (a) the restraint of the PEO crystallization due to the interaction between the polymer chain with the Lewis acidic sites on the MOF-5 and the lithium salt, which eases the formation of Li+ ion conductive channels, and (b) the adsorption of solvent through the MOF pores, which can speed up the migration of lithium ions. Gerbaldi et al. [119] used Al-based MOF as new filler material in the PEO matrix. Composite polymer electrolytes (CPEs) showed an ionic conductivity of two orders of magnitude higher than non-mixed MOFs. The composite film was utilized in LIB (Li/LiFePO4) as the electrolyte, and the composite-based cell demonstrated a high specific capacitance and remarkable electrochemical performance.
Cellulose-Based Solid Polymer Electrolytes
Cellulose is used in polymer electrolytes because it possesses good mechanical properties with a large specific surface area, it is completely safe and cheap, and it uses environmentally friendly materials [120,121,122]. The incorporation of cellulose into the polymer matrix will not only improve the mechanical properties of polymer electrolytes but also acts physically as a block to stop the formation of Li dendrites. The polar groups in cellulose can also facilitate salt dissociation in the polymer matrix [123]. During the interaction, the cellulose/polymer separation forms a channel that eases the migration of ions. Nair and his co-workers [124] prepared cellulose-based polymer electrolytes to enhance the electrical and mechanical properties of solid electrolytes. The cellulose-based electrolytes exhibit ionic conductivity of 2.0 × 10−4 S cm−1 at RT and good flexibility, which can be appropriate for solid flexible LIBs. Mixing an ionic liquid with cellulose is also an innovative solution to eliminate the leakage problem in IL-composite electrolytes. Asghar et al. [125] designed quasi-solid polymer electrolytes using network cellulose (NC) in PEG-LiCloO4. The solid electrolyte doped with 12.8 wt% NC indicates ionic conductivity of 10−4 S cm−1 at RT with a decomposition voltage of up to 4.7 V.
Garnet-Type Composite Polymer Electrolytes
Wu et al. [126] reported that a garnet-type-based lithium solid–polymer electrolyte shows a large electrochemical potential window and high ionic conductivity. However, the implementation of garnet-type ceramics into the entire battery always exhibits low conductivity at the contact between electrolyte and electrode, due to increased interfacial resistance and low ionic conductivity leading to lowered battery performance [127]. However, the combination of polymer and garnet in composite electrolytes contributes to the overall electrochemical performance of the cell. A nanoscale garnet ceramic filler with a great specific surface area enhances the ionic transition rate [128], which increases the ionic conductivity. A composite electrolyte was prepared using PEO and Li7La3 Zr2O12 (LLZO) particles doped at 52 wt.%, and the ionic conductivity of CPE was 4.42 × 10−4 S cm−1 at 55 °C [129]. Hybrid electrolytes were developed with Li6.75La3Zr1.75Ta0.25O12 (LLZTO) ceramic filler in PVDF [130]. It was reported that a 10 wt.% LLZTO-doped hybrid electrolyte demonstrated the highest conductivity of 5 × 10−4 S cm−1, which appears to be seven times greater than that with no LLZTO. This resulted in the reaction between Li+ and LLZO fillers via acid-base interaction. Lithium salt dissociation will contribute to the carriers’ concentration, which increases ionic conductivity. Furthermore, the garnet ceramic filler acts also as a plasticizer in lowering the degree of polymer crystallinity, which improves the segmental motion of the polymer and eases the ions migration and so enhances the ionic conductivity. The morphological aspect of ceramic fillers such as particles, distribution of nanowire, and 3D framework in polymer composite electrolyte may impact the ionic conductivity. Amongst them, aligned nanowires when combined with polymers can offer an uninterrupted Li+ migration track. Figure 13 shows the morphological impact on the ion migration in solid polymer electrolytes.
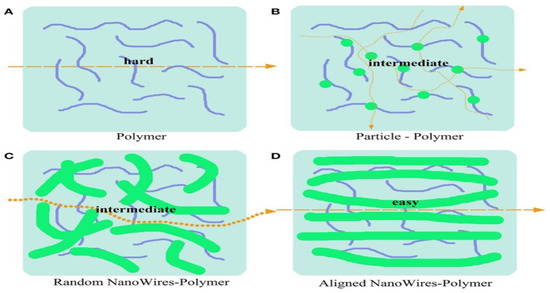
Figure 13.
Schematic diagram of ion transport mechanism in SPEs incorporated with different varieties of ceramic fillers. (A) Ion transfer pathway in the pure polymer. (B–D) Ion transfer pathway in nanoparticle-polymer-based CPEs (B), random nanowires (C), and aligned nanowires (D) [99].
It was found that a CPE doped with aligned inorganic nanowires demonstrates better conduction than non-aligned ceramic-filler-based solid polymer. This result was also confirmed by Cui and his co-workers [116,131]. They found that polymer electrolytes based on well-aligned inorganic nanowires (LLZO) reveal an ionic conductivity of 6.05 × 10−5 S cm−1 at RT, which is superior by one order of magnitude to that of nanoparticles or randomly aligned nanowire-based CPEs. The improved ionic conductivity results in the free motion of Li+ throughout the matrix with no crossing junctions on the nanowire surface.
Sulfide-Type Polymer Electrolytes
Sulfide-type PEs demonstrate high ionic conductivities of two orders of magnitude at RT [132]. Zao et al. developed a solid electrolyte membrane via the combination of thio-LISICON Li10GeP2S12 with PEO [133]. The electrolyte membrane exhibits 10−5 S cm−1 of ionic conductivity, which is greater than common PEO-based PE with an electrochemical stability window of 5.7V. Glass-doped glass-ceramic Li2S-P2S5 and ceramic thio-LISICON Li4-xGe1-xPx S4 (0 < x < 1) are the most suitable materials to enhance the performance of solid polymer electrolytes in lithium metal stability and offer additional choices in the selection of positive electrode materials.
Perovskite-Type Composite Polymer Electrolytes
Perovskite-type solid electrolytes Li3xLa2/3−xTiO3 (LLTO) are well known for their interesting properties, such as their stability at high voltages. They exhibit a cubic crystal system with a space group of P4/mmm and C-mmm [134]. Recently, Zhu et al. investigated a PEO:LiClO4 polymer electrolyte with Li0.33La0.557TiO3 nanowires. The ionic conductivity of Li+ was found to be 2.4 × 10−4 S cm−1 at 25 °C [135]. The addition of LLTO nanowire to PAN achieved higher ionic conductivity than pristine PAN film. The composite electrolyte contains a 3D long-distance Li+ conduction network (3D), which reduces the detrimental impact of agglomerated inorganic ceramic nanoparticles in polymers [99]. This artificial 3D infiltration network with a large specific surface area provides a continuous Li+ transfer channel.
Fast-Ion Conductive Ceramic-Based SPEs
Fast-ion conductive ceramics show a high ionic conductivity of up to 10−2 S cm−1 at RT, but their poor contact at the interface limits their employment as solid electrolytes. The combination of polymer and fast-ion conductor ceramics can result in promising electrolytes for LIBs. Fast ion conductors are thermally stable and commonly composed of garnet-type, NASICON-type, and LISICON-type ceramics.
4. Summary
In this review, different types of electrolytes and their electrical and mechanical properties have been reported and studied to evaluate their effect on LIB performance. It was noticed that the electrolyte component and solvent in polymer electrolytes have a great influence on the ionic conductivity, Li+ migration, interfacial contact between electrolyte and electrode, mechanical properties, and the performance of the entire battery. The morphology of incorporated additive materials (nanoparticles, nanowires, nanofillers, salt, etc.) may well contribute to the amelioration of the ion transport pathway, which raises the lithium-ion conductivity. A basic understanding of the chemical reaction routes and the electrolyte structure would facilitate innovation in the battery. The structural, electrochemical, and mechanical properties of new promising materials should be investigated in advance for application in advanced lithium-ion batteries. The electrochemical behavior is inextricably related to the structure. IL-based solid polymer electrolytes appear as a promising material for long-term lithium-ion batteries despite showing low ionic conductivity but exhibiting more advantages than conventional carbonate electrolytes such as good safety, stability, good electrochemical performance, good mechanical stability, and enhanced energy density. Since solid electrolytes exhibit low ionic conductivity, ILs used in SPEs increased their conductivity. In a battery, porous materials appear to offer good properties in terms of lithium ionic conductivity, with no leakage and low interface resistance, and gel-based LIBs demonstrate a good working performance, long cycling life, and high energy density. However, commercial LIBs have been widely developed, and the use of liquid electrolytes in the battery showed some drawbacks, such as low electrochemical stability and poor safety, which limits their use in many fields. As an alternative, the development of solid CPEs has been found as a promising solution to replace liquid electrolytes because they are highly flexible, non-flammable, thermally stable, and highly safe. In this work, a basic comprehension of the mechanism of ion transport and interfaces for polymer-based composite electrolytes has also been explained. A summary of the recent investigations on CPEs, including polymer/ionic liquid, polymer/inert ceramics, polymer/MOFs, polymer/cellulose, polymer/garnet type, polymer/sulfide type, polymer/perovskite-type composite electrolytes, and polymer/fast ion conductive ceramics, has been reviewed. However, polymers combined with fast ion conductors compared to other SPEs have been largely commercialized because of their excellent conductivity and good interface contact.
For future development of promising efficient and low-cost solid electrolytes for battery application, the database of materials genome should be exploited in the analysis, characterization, and design of composite materials. Advanced methods of characterization should be also used to make a deep understanding of the material mechanisms, such as cryo-electron microscopy. This technique reveals the entire structure of atoms present in the battery materials and interfaces. The cryogenic TEM method appears to be one suitable candidate for a good interface analysis of solid electrolytes. Since the advanced research on the manufacturing of novel electrolytes for LIBs combines all the advantages, it still has to be studied in depth. Good polymer electrolytes need to be highly conductive, safe, highly mechanically and thermally stable, and easy for film formation.
Author Contributions
Conceptualization, A.M.T. and N.B.; methodology, A.M.T. and S.A.A. and H.A.A.-A.; software, A.M.T. and A.L.; validation, N.B. and P.K.S.; writing—review and editing, N.B. and M.N.F.N. and G.N. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding was enabled and organized by Projekt DEAL through financial support from the Institute of Materials Science, Technical University Darmstadt, 64289 Darmstadt Federal Republic of Germany. This research was funded by The Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, the project number (0005-1442-S).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors are thankful for the support and cooperation from their host institutions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, M.; Zheng, Z.; Wang, Q.; Zhang, Y.; Yubin, Z.; Xiaotu, M.; Chao, S.; Dapeng, X.; Jin, L.; Yangtao, L.; et al. High Performance Cathode Recovery from Different Electric Vehicle Recycling Streams. Sci. Rep. 2009, 9, 1654. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Meng, Y.S.; Bréger, J.; Grey, C.P.; Ceder, G. Electrodes with High Power and High Capacity for Rechargeable Lithium Batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A. Materials challenges and opportunities of lithium ion batteries. J. Phys. Chem. Lett. 2011, 2, 176–184. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Von Cresce, A.; Xu, K. Electrolyte Additive in Support of 5 V Li Ion Chemistry. J. Electrochem. Soc. 2011, 158, A337. [Google Scholar] [CrossRef]
- Dedryvère, R.; Foix, D.; Franger, S.; Patoux, S.; Daniel, L.; Gonbeau, D. Electrode/electrolyte interface reactivity in high-voltage spinel LiMn1.6Ni0.4O4/Li4Ti5O12 lithium-ion battery. J. Phys. Chem. C 2010, 114, 10999–11008. [Google Scholar] [CrossRef]
- Brédas, J.L.; Norton, J.E.; Cornil, J.; Coropceanu, V. Molecular understanding of organic solar cells: The challenges. Acc. Chem. Res. 2009, 42, 1691–1699. [Google Scholar] [CrossRef]
- Capiglia, C.; Saito, Y.; Kageyama, H.; Mustarelli, P.; Iwamoto, T.; Tabuchi, T.; Tukamoto, H. 7Li and 19F diffusion coefficients and thermal properties of non-aqueous electrolyte solutions for rechargeable lithium batteries. J. Power Sources 1999, 81, 859–862. [Google Scholar] [CrossRef]
- Capiglia, C.; Saito, Y.; Kataoka, H.; Kodama, T.; Quartarone, E.; Mustarelli, P. Structure and transport properties of polymer gel electrolytes based on PVdF-HFP and LiN(C2F5SO2)2. Solid State Ion. 2000, 131, 291–299. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, J.P.; Xu, C.X. Facile preparation of Mn3O4 octahedra and their long-term cycle life as an anode material for Li-ion batteries. J. Mater. Chem. A 2014, 2, 87–93. [Google Scholar] [CrossRef]
- Liu, Q.; Hou, J.G.; Xu, C.; Chen, Z.Z.; Qin, R.; Liu, H. TiO2 particles wrapped onto macroporous germanium skeleton as high performance anode for lithium-ion batteries. Chem. Eng. J. 2020, 381, 122649. [Google Scholar] [CrossRef]
- Michan, A.L.; Parimalam, B.S.; Leskes, M.; Kerber, R.N.; Yoon, T.; Grey, C.P. TiO2 particles wrapped onto macroporous germanium skeleton as high performance anode for lithium-ion batteries. Chem. Mater. 2016, 28, 8149–8159. [Google Scholar] [CrossRef]
- Abe, K.; Yoshitake, H.; Kitakura, T.; Hattori, T.; Wang, H.Y.; Yoshio, M. Additives-containing functional electrolytes for suppressing electrolyte decomposition in lithium-ion batteries. Electrochim. Acta 2004, 49, 4613–4622. [Google Scholar] [CrossRef]
- Schmitz, R.W.; Murmann, P.; Schmitz, R.; Müller, R.; Krämer, L.; Kasnatschew, J.; Isken, P.; Niehof, P.; Nowak, S.; Röschenthaler, G.-V.; et al. Investigations on novel electrolytes, solvents and SEI additives for use in lithium-ion batteries: Systematic electrochemical characterization and detailed analysis by spectroscopic methods. Prog. Solid State Chem. 2014, 42, 65–84. [Google Scholar] [CrossRef]
- Li, Y.; Wan, S.; Veith, G.M.; Unocic, R.R.; Paranthaman, M.P.; Dai, S.; Sun, X.G. A novel electrolyte salt additive for lithium-ion batteries with voltages greater than 4.7 V. Adv. Energy Mater. 2016, 7, 1601397. [Google Scholar] [CrossRef]
- Meng, F.; Zhu, S.; Gao, J.; Zhang, F.; Li, D. Effect of electrolyte additives on the performance of lithium-ion batteries. Ionics 2021, 27, 3821–3827. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, Z.; Gao, J.; Li, J.; Xu, J.; Wang, Z.; Yang, Y. Vinyl ethylene sulfite as a new additive in propylene carbonate-based electrolyte for lithium ion batteries. Energy Environ. Sci. 2009, 2, 1102–1108. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Wang, Z.; Zhang, H. Electrochemical analysis graphite/electrolyte interface in lithium-ion batteries: P-toluenesulfonyl isocyanate as electrolyte additive. Nano Energy 2017, 34, 131–140. [Google Scholar] [CrossRef]
- Ushirogata, K.; Sodeyama, K.; Okuno, Y.; Tateyama, Y. Additive effect on reductive decomposition and binding of carbonate-based solvent toward solid electrolyte interphase formation in lithium-ion battery. J. Am. Chem. Soc. 2017, 135, 11967–11974. [Google Scholar] [CrossRef] [PubMed]
- Leggesse, E.; Jiang, J.-C. Theoretical study of the reductive decomposition of ethylene sulfite: A film-forming electrolyte additive in lithiumion batteries. J. Phys. Chem. A 2012, 116, 11025–11033. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Armand, M. Polymer electrolytes. Annu. Rev. Mater. Sci. 1986, 16, 245–261. [Google Scholar] [CrossRef]
- Scrosati, B. Challenge of portable power. Nature 1995, 373, 557–558. [Google Scholar] [CrossRef]
- Gray, F.M. Solid Polymer Electrolyte—Fundamentals and Technological Applications; VCH: New York, NY, USA, 1991; p. 245. [Google Scholar]
- Boz, B.; Ford, H.O.; Salvadori, A.; Schaefer, J.L. Porous Polymer Gel Electrolytes Influence Lithium Transference Number and Cycling in Lithium-Ion Batteries. Electron. Mater. 2021, 2, 154–173. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in li-Ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Baik, J.-H.; Kim, S.; Hong, D.G.; Lee, J.-C. Gel Polymer electrolytes based on polymerizable lithium salt and poly (ethylene glycol) for lithium battery applications. ACS Appl. Mater. Interfaces 2019, 11, 29718–29724. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent progress of advanced anode materials of lithium-ion batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Lin, M.; Fu, C.; Li, L.; Mayilvahanan, K.S.; Watkins, T.; Perdue, B.R.; Zavadil, K.R.; Helms, B.A. Nanoporous polymer films with a high cation transference number stabilize lithium metal anodes in light-weight batteries for electrified transportation. Nano Lett. 2019, 19, 1387–1394. [Google Scholar]
- Ma, L.; Nath, P.; Tu, Z.; Tikekar, M.; Archer, L.A. Highly conductive, sulfonated, UV-Cross-Linked separators for Li–S batteries. Chem. Mater. 2016, 28, 5147–5154. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Wang, J.; Ye, F.; Wang, S.; Xu, Y.; Liu, J.; Xu, G.; Zhang, Y.; Zhang, Y.; et al. Asymmetric gel polymer electrolyte with high lithium ion conductivity for dendrite-free lithium metal batteries. J. Mater. Chem. A 2020, 8, 8033–8040. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, L.; Shi, L.; Wang, Z.; Zhu, J.; Zhao, Y.; Yuan, S. Gel polymer electrolyte with high li transference number enhancing the cycling stability of lithium anodes. ACS Appl. Mater. Interfaces 2019, 11, 5168–5175. [Google Scholar] [CrossRef]
- Baran, M.J.; Carrington, M.E.; Sahu, S.; Baskin, A.; Song, J.; Baird, M.A.; Teat, S.J.; Meckler, S.M.; Fu, C.; Prendergast, D.; et al. Diversity-oriented synthesis of polymers of intrinsic microporosity with explicit solid solvation cages for lithium ions. Nature 2021, 592, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Flamme, B.; Garcia, G.; Weil, M.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidal, V.; Chagnes, A. Guidelines to design organic electrolytes for lithium-ion batteries: Environmental impact, physicochemical and electrochemical properties. Green Chem. 2017, 19, 1828–1849. [Google Scholar] [CrossRef]
- Greenbaum, S.G.; Sideris, P.J. NMR Studies of Materials for Electrochemical Energy Storage. Encycl. Sustain. Sci. Technol. 2013, 6067–6097. [Google Scholar]
- Fernicola, A.; Weise, F.; Greenbaum, S.; Kagimoto, J.; Scrosati, B.; Soleto, A. Lithium Ion Batteries, Electrochemical Reactions in. J. Electrochem. Soc. 2009, 156, A514–A520. [Google Scholar] [CrossRef][Green Version]
- Yong, A.; Xue, H.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond. Nano MICRO Small 2021, 18, 2103617. [Google Scholar]
- Kalita, M.; Bukat, M.; Ciosek, M.; Siekierski, M.; Chung, S.H.; Rodriguez, T.; Greenbaum, S.G.; Kovarsky, R.; Golodnitsky, D.; Peled, E.; et al. Effect of calixpyrrole in PEO–LiBF4 polymer electrolytes. Electrochim. Acta 2005, 50, 3942–3948. [Google Scholar] [CrossRef]
- Periasamy, P.; Tatsumi, K.; Shikano, M.; Fujieda, T.; Saito, Y.; Sakai, T.; Mizuhata, M.; Kajinami, A.; Deki, S. An electrochemical investigation on polyvinylidene fluoride-based gel polymer electrolytes. J. Power Sources 2000, 88, 269–273. [Google Scholar] [CrossRef]
- Pawlowska, A.; Zukowska, G.; Kalita, M.; Solgala, A.; Parzuchowski, P.; Siekierski, M. The effect of receptor-polymer matrix compatibility on properties of PEO-based polymer electrolytes containing a supramolecular additive Part 1. Studies on phenomenon of compatibility. J. Power Sources 2007, 173, 755–764. [Google Scholar] [CrossRef]
- Bloise, A.; Donoso, J.; Magon, C.; Rosario, A.; Pereira, E. NMR and conductivity study of PEO-based composite polymer electrolytes. Electrochim. Acta 2003, 48, 2239–2246. [Google Scholar] [CrossRef]
- Masuda, Y.; Seki, M.; Nakayama, M.; Wakihara, M.; Mita, H. Study on ionic conductivity of polymer electrolyte plasticized with PEG-aluminate ester for rechargeable lithium ion battery. Solid State Ion. 2006, 117, 843–846. [Google Scholar] [CrossRef]
- Lux, S.F.; Lucas, I.T.; Pollak, E.; Passerini, S.; Winter, M.; Kostecki, R. HF Formation in LiPF6-Based Organic Carbonate Electrolytes. Electrochem. Commun. 2012, 14, 47–50. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion. YNPJ Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Eunhwan, K.; Juyeon, H.; Seokgyu, R.; Youngkyu, C.; Jeeyoung, Y. Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices. Materials 2001, 14, 4000. [Google Scholar]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Paluch, M. Recent progress on dielectric properties of protic ionic liquids. J. Phys. Condens. Matter 2015, 27, 073202. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly (ionic liquids): Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef]
- Kasprzak, D.; Stepniak, I.; Galínski, M. Acetate- and lactate-based ionic liquids: Synthesis, characterisation and electrochemical properties. J. Mol. Liq. 2018, 264, 233–241. [Google Scholar] [CrossRef]
- Naushad, M.; ALOthman, Z.A.; Khan, A.B.; Ali., M. Effect of ionic liquid on activity, stability, and structure of enzymes: A review. Int. J. Biol. Macromol. 2012, 51, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Shiflett, M.B.; Drew, D.W.; Cantini, R.A.; Yokozeki, A. Carbon Dioxide Capture Using Ionic Liquid 1-Butyl-3-methylimidazolium Acetate. Energy Fuels 2010, 24, 5781–5789. [Google Scholar] [CrossRef]
- Guerfi, A.; Dontigny, M.; Charest, P.; Petitclerc, M.; Lagacé, M.; Vijh, A.; Zaghib, K. Improved electrolytes for Li-ion batteries: Mixtures of ionic liquid and organic electrolyte with enhanced safety and electrochemical performance. J. Power Sources 2010, 195, 845–852. [Google Scholar] [CrossRef]
- Xiang, H.F.; Yin, B.; Wang, H.; Lin, H.W.; Ge, X.W.; Xie, S.; Chen, C.H. Improving electrochemical properties of room temperature ionic liquid (RTIL) based electrolyte for Li-ion batteries. Electrochim. Acta 2010, 55, 5204–5209. [Google Scholar] [CrossRef]
- Mai, Y.J.; Luo, H.; Zhao, X.Y.; Wang, J.L.; Davis, J.; Lyons, L.J.; Zhang, L.Z. Organosilicon functionalized quaternary ammonium ionic liquids as electrolytes for lithium-ion batteries. Ionics 2014, 20, 1207–1215. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Correia, D.M.; Gonçalves, R.; de Zea, V.; Bermudez, V.; Silva, M.M.; Lanceros-Mendez, S.; Costa, C.M. Enhanced ionic conductivity in poly (vinylidene fluoride) electrospun separator membranes blended with different ionic liquids for lithium ion batteries. Colloid J. Interface Sci. 2021, 582, 376–386. [Google Scholar] [CrossRef]
- Fang, S.; Jin, Y.; Yang, L.; Hirano, S.I.; Tachibana, K.; Katayama, S. Functionalized ionic liquids based on quaternary ammonium cations with three or four ether groups as new electrolytes for lithium battery. Electrochim. Acta 2011, 56, 4663–4671. [Google Scholar] [CrossRef]
- Beltrop, K.; Qi, X.; Hering, T.; Röser, S.; Winter, M.; Placke, T. Enabling bis (fluorosulfonyl) imide-based ionic liquid electrolytes for application in dualion batteries. J. Power Sources 2018, 373, 193–202. [Google Scholar] [CrossRef]
- Liu, Q.; Hsu, C.W.; Dzwiniel, T.L.; Pupek, K.Z.; Zhang, Z.; Liu, Q.; Hsu, C.-W.; Dzwiniel, T.; Pupek, K.Z.; Zhang, Z. A fluorine-substituted pyrrolidinium-based ionic liquid for high-voltage Li-ion battery. Chem. Commun. 2020, 56, 7317–7320. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Feng, W.; Nie, J.; Li, H.; Huang, X.; Zhou, Z. Molten salt of lithium bis(fluorosulfonyl)imide (LiFSI)-potassium bis(fluorosulfonyl)imide (KFSI) as electrolyte for the natural graphite/LiFePO4 lithium-ion cell. Electrochim. Acta 2014, 135, 217–223. [Google Scholar] [CrossRef]
- Kärnä, M.; Lahtinen, M.; Valkonen, J. Preparation and characterization of new low melting ammonium-based ionic liquids with ether functionality. J. Mol. Struct. 2009, 922, 64–76. [Google Scholar] [CrossRef]
- Hoffknecht, J.P.; Drews, M.; He, X.; Paillard, E. Investigation of the N-butyl-N-methyl pyrrolidinium trifluoromethanesulfonyl-N-cyanoamide (PYR14TFSAM) ionic liquid as electrolyte for Li-ion battery. Electrochim. Acta 2017, 250, 25–34. [Google Scholar] [CrossRef]
- Li, Q.; Ardebili, H. Flexible thin-film battery based on solid-like ionic liquid-polymer electrolyte. J. Power Sources 2016, 303, 17–21. [Google Scholar] [CrossRef]
- Kim, E.; Han, J.; Ryu, S.; Choi, Y.; Yoo, J. Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices. Materials 2021, 14, 4000. [Google Scholar] [CrossRef]
- Periyapperuma, K.; Arca, E.; Harvey, S.; Pathirana, T.; Ban, C.; Burrell, A.; Pozo-Gonzalo, C.; Howlett, P.C. High Current Cycling in a Superconcentrated Ionic Liquid Electrolyte to Promote Uniform Li Morphology and a Uniform LiF-Rich Solid Electrolyte Interphase. ACS Appl. Mater. Interfaces 2020, 12, 42236–42247. [Google Scholar] [CrossRef]
- Takada, K.; Inada, T.; Kajiyama, A.; Sasaki, H.; Kondo, S.; Watanabe, M.; Murayama, M.; Kanno, R. Solid–state lithium battery with graphite anode. Solid State Ion. 1995, 158, 269–274. [Google Scholar] [CrossRef]
- Aihara, Y.; Kuratomi, J.; Bando, T.; Iguchi, T.; Yoshida, H.; Kuwana, K. Investigation on solvent-free solid polymer electrolytes for advanced lithium batteries and their performance. J. Power Sources 2003, 114, 96–104. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Suk, J.; Lee, Y.H.; Kim, D.Y.; Cho, S.Y.; Kim, J.M.; Kang, Y. Semi-interpenetrating solid polymer electrolyte based on thiol-ene cross-linker for all-solid-state lithium batteries. J. Power Sources 2016, 334, 154–161. [Google Scholar] [CrossRef]
- Cao, C.; Yuan, X.; Wu, Y.; van Ree, T. Electrochemical Performance of Lithium-Ion Batteries. In Electrochemical Energy Storage and Conversion; CRC Press: Boca Raton, FL, USA, 2015; pp. 507–524. [Google Scholar]
- Wu, Y.P.; Yuan, X.Y.; Dong, C.; Duan, Y.J. Lithium Ion Batteries: Practice and Applications; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Zhang, J.; Sun, B.; Huang, X.; Chen, S.; Wang, G. Honeycomb-like porous gel polymer electrolyte membrane for lithium-ion batteries with enhanced safety. Sci. Rep. 2014, 4, 6007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, C.; Wang, P.F.; Li, Q.; Chen, L.; Han, F.; Jin, T.; Liu, S.; Choudhary, H.; Raghavan, S.R.; et al. “Water-in-salt” polymer electrolyte for Liion batteries. Energy Environ. Sci. 2020, 13, 2878–2887. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, B.; Huang, G.; Zhang, H.; Song, X. Excellent rate capabilities of (LiFePO4/C)//LiV3O8 in an optimized aqueous solution electrolyte. J. Power Sources 2013, 232, 181–186. [Google Scholar] [CrossRef]
- Luo, J.Y.; Cui, W.J.; He, P.; Xia, Y.-Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2010, 2, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Croy, J.R.; Abouimrane, A.; Hang, Z. Next-generation lithium-ion batteries: The promise of near-term advancements. MRS Bull. 2014, 39, 407–415. [Google Scholar] [CrossRef]
- Li, Y.; Ravdel, B.; Lucht, B.L. Electrolyte reactions with the surface of high voltage LiNi0.5Mn1.5O4 cathodes for lithiumion batteries. Electrochem. Solid State Lett. 2010, 13, A95–A97. [Google Scholar]
- Zhang, Z.; Hu, L.; Wu, H.; Weng, W.; Koh, M.; Redfern, P.C.; Curtiss, L.A.; Amine, K. Fluorinated electrolytes for 5 V lithium-ion battery chemistry. Energy Environ. Sci. 2013, 6, 1806–1810. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2014, 7, 627–631. [Google Scholar] [CrossRef]
- Xia, S.; Wu, X.; Zhang, Z.; Cui, Y.; Liu, W. Practical Challenges and Future Perspectives of All-Solid-State Lithium-Metal Batteries. Chem 2019, 5, 753–785. [Google Scholar] [CrossRef]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic solid-state electrolytes for lithium batteries: Mechanisms and properties governing ion conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Sun, J.; Hsu, P.-C.; Li, Y.; Lee, H.-W.; Cui, Y. Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Lett. 2015, 15, 2740–2745. [Google Scholar] [CrossRef]
- Young, W.S.; Kuan, W.F.; Epps, T.H., III. Block Copolymer Electrolytes for Rechargeable Lithium Batteries. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1–16. [Google Scholar] [CrossRef]
- Wei-Min, W. Study on all solid-state composite polymer electrolyte. Adv. Mat. Res. 2012, 571, 13–16. [Google Scholar]
- Ratner, M.A.; Johansson, P.; Shriver, D.F. Electrolytes: Ionic transport mechanisms and relaxation coupling. Mrs Bullet. 2000, 25, 31–37. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Liu, K.; Ding, F.; Liu, X.J. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Pandey, G.P. Solid polymer electrolytes: Materials designing and all-solid-state battery applications: An overview. J. Phys. D Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Aliahmad, N.; Shrestha, S.; Varahramyan, K.; Agarwal, M. Poly (vinylidene fluoride-hexafluoropropylene) polymer electrolyte for paper-based and flexible battery applications. AIP Adv. 2016, 6, 065206. [Google Scholar] [CrossRef]
- Keller, M.; Appetecchi, G.B.; Kim, G.-T.; Sharova, V.; Schneider, M.; Schuhmacher, J.; Roters, A.; Passerini, S. Electrochemical performance of a solvent-free hybrid ceramic-polymer electrolyte based on Li7La3Zr2O12 in P(EO)15LiTFSI. J. Power Sources 2017, 353, 287–297. [Google Scholar] [CrossRef]
- Ling, S.-G.; Peng, J.-Y.; Yang, Q.; Qiu, J.-L.; Lu, J.-Z.; Li, H. Enhanced ionic conductivity in LAGP/LATP composite electrolyte. Chin. Phy. B 2018, 27, 038201. [Google Scholar] [CrossRef]
- Lin, D.; Liu, W.; Liu, Y.; Lee, H.R.; Hsu, P.-C.; Liu, K. High Ionic Conductivity of Composite Solid Polymer Electrolyte via In Situ Synthesis of Monodispersed SiO2 Nanospheres in Poly (ethylene oxide). Nano Lett. 2016, 16, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Do, J.S.; Chang, C.P.; Lee, T.J. Electrochemical properties of lithium salt-poly (ethylene oxide)-ethylene carbonate polymer electrolyte and discharge characteristics of LiMnO2. Solid State Ion. 1986, 89, 291–298. [Google Scholar] [CrossRef]
- Subianto, S.; Mistry, M.K.; Choudhury, N.R.; Dutta, N.K.; Knout, R. Composite polymer electrolyte containing ionic liquid and functionalized polyhedral oligomeric silsesquioxanes for anhydrous PEM applications. ACS Appl. Mater. Interfaces 2009, 1, 1173–1182. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef]
- Camacho-Forero, L.E.; Balbuena, P.B. Exploring interfacial stability of solid-state electrolytes at the lithium-metal anode surface. J. Power Sources 2018, 396, 782–790. [Google Scholar] [CrossRef]
- Wang, L.-P.; Zhang, X.-D.; Wang, T.-S.; Yin, Y.-X.; Shi, J.-L.; Wang, C.-R. Ameliorating the interfacial problems of cathode and solid-state electrolytes by interface modification of functional polymers. Adv. Energy Mater. 2018, 8, 1801528. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Cheng, X.-B.; Zhang, Q. Advances in interfaces between Li metal anode and electrolyte. Adv. Mater. Interfaces 2018, 5, 1701097. [Google Scholar] [CrossRef]
- Xu, R.C.; Xia, X.H.; Zhang, S.Z.; Xie, D.; Wang, X.L.; Tu, J.P. Interfacial challenges and progress for inorganic all-solid-state lithium batteries. Electrochimi. Acta 2018, 284, 177–187. [Google Scholar] [CrossRef]
- Weston, J.E.; Steele, B.C.H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly (ethylene oxide) polymer electrolytes. Solid State Ion. 1982, 7, 75–79. [Google Scholar] [CrossRef]
- Tambelli, C.C.; Bloise, A.C.; Rosario, A.; Pereira, E.C.; Magon, C.J.; Donoso, J.P. Characterisation of PEO–Al2O3 composite polymer electrolytes. Electrochim. Acta 2002, 47, 1677–1682. [Google Scholar] [CrossRef]
- Liang, B.; Tang, S.; Jiang, Q.; Chen, C.; Chen, X.; Li, S. Preparation and characterization of PEO-PMMA polymer composite electrolytes doped with nano-Al2O3. Electrochim. Acta 2015, 169, 334–341. [Google Scholar] [CrossRef]
- Nan, C.W.; Fan, L.Z.; Lin, Y.H.; Cai, Q. Polymer composite electrolytes containing ionically active mesoporous particles. Phys. Rev. Lett. 2003, 91, 266104. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Yuen, P.Y.; Liu, Y.; Liu, W.; Liu, N.; Dauskardt, R.H. A silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus. Adv. Mater. 2018, 30, 1802661. [Google Scholar] [CrossRef]
- Pal, P.; Ghosh, A. Influence of TiO2 nano-particles on charge carrier transport and cell performance of PMMA-LiClO4 based nano-composite electrolytes. Electrochim. Acta 2018, 260, 157–167. [Google Scholar] [CrossRef]
- Sheng, O.; Jin, C.; Luo, J.; Yuan, H.; Huang, H.; Gan, Y. Mg2B2O5 Nanowire Enabled Multifunctional Solid-State Electrolytes with High Ionic Conductivity, Excellent Mechanical Properties, and Flame-Retardant Performance. Nano Lett. 2018, 18, 3104–3112. [Google Scholar] [CrossRef]
- Xie, X.-C.; Huang, K.-J.; Wu, X. Metal–organic framework derived hollow materials for electrochemical energy storage. J. Mater. Chem. A 2018, 6, 6754–6771. [Google Scholar] [CrossRef]
- Stavila, V.A.; Talin, A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.M.; Nahm, K.S. Review on composite polymer electrolytes for lithium batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef]
- Indra, A.; Song, T.; Paik, U. Metal organic framework derived materials: Progress and prospects for the energy conversion and storage. Adv. Mater. 2018, 30, 1705146. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastre, J. Liquid phase adsorption by microporous coordination polymers: Removal of organosulfur compounds. J. Mater. Chem. 2008, 16, 626–636. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.R.; Li, J.R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.C. Potential applications of metal-organic frameworks. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworksChem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Han, P.; Lai, Y.; Zhang, Z.; Liu, J. Enhanced electrochemical performance of poly (ethylene oxide) based composite polymer electrolyte by incorporation of nano-sized metal-organic framework. J. Power Sources 2013, 240, 653–658. [Google Scholar] [CrossRef]
- Gerbaldi, C.; Nair, J.R.; Kulandainathan, M.A.; Kumar, R.S.; Ferrara, C.; Mustarelli, P.; Stephan, A.M. Innovative high performing metal organic framework (MOF)-laden nanocomposite polymer electrolytes for all-solid-state lithium batteries. J. Mater. Chem. A 2014, 2, 9948–9954. [Google Scholar] [CrossRef]
- Gonzalez, F.; Tiemblo, P.; Garcia, N.; Garcia-Calvo, O.; Fedeli, E.; Kvasha, A. High performance polymer/ionic liquid thermoplastic solid electrolyte prepared by solvent-free processing for solid state lithium metal batteries. Membranes 2018, 8, 55. [Google Scholar] [CrossRef]
- Baxter, J.; Bian, Z.; Chen, G.; Danielson, D.; Dresselhaus, M.S.; Fedorov, A.G. Nanoscale design to enable the revolution in renewable energy. Energy Environ. Sci. 2009, 2, 559–588. [Google Scholar] [CrossRef]
- Sheng, J.; Tong, S.; He, Z.; Yang, R. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Shi, Q.X.; Xia, Q.; Xiang, X.; Ye, Y.S.; Hai Yan, P.; Xue, Z.G. Self-Assembled Polymeric Ionic Liquid-Functionalized Cellulose Nano-crystals: Constructing 3D Ion-conducting Channels within Ionic Liquid-based Composite polymer eletrolytes. Chem. Eur. J. 2017, 23, 11881–11890. [Google Scholar] [CrossRef]
- Nair, J.R.; Gerbaldi, C.; Chiappone, A.; Zeno, E.; Bongiovanni, R.; Bodoardo, S. UV-cured polymer electrolyte membranes for Li-cells: Improved mechanical properties by a novel cellulose reinforcement. Electrochem. Commun. 2009, 11, 1796–1798. [Google Scholar] [CrossRef]
- Asghar, A.; Samad, Y.A.; Lalia, B.S.; Hashaikeh, R. PEG based quasi-solid polymer electrolyte: Mechanically supported by networked cellulose. J. Memb. Sci. 2012, 421, 85–90. [Google Scholar] [CrossRef]
- Wu, J.-F.; Pang, W.K.; Peterson, V.K.; Wei, L.; Guo, X. Garnet-Type Fast Li-Ion Conductors with High Ionic Conductivities for All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2017, 9, 12461–12468. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.-P.; Fan, L.-Z.; Nan, C.-W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Kumar, B.; Scanlon, L.G. Composite electrolytes for lithium rechargeable batteries. J. Electroceramics 2000, 5, 127–139. [Google Scholar] [CrossRef]
- Thokchom, J.S.; Gupta, N.; Kumar, B. Composite electrolytes for lithium rechargeable batteries. J. Electrochem. Soc. 2008, 155, A915–A920. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zhang, S.; Huang, X.; Xu, B.; Lin, Y.; Xu, B.; Li, L.; Nan, C.W.; Shen, Y. Synergistic Coupling between Li6.75La3Zr1.75Ta0.25O12 and Poly (vinylidene fluoride) Induces High Ionic Conductivity, Mechanical Strength, and Thermal Stability of Solid Composite Electrolytes. J. Am. Chem. Soc. 2017, 139, 13779–13785. [Google Scholar] [CrossRef]
- Liu, W.S.; Lee, W.; Lin, D.; Shi, F.; Wang, S.; Sendek, A.D.; Cui, Y. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat. Energy 2017, 2, 17035. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Han, F.; Yao, X.; Wang, C. Lithium/sulfide all-solid-state batteries using sulfide electrolytes. Adv. Mater. 2021, 33, 2000751. [Google Scholar] [CrossRef]
- Stramare, S.; Thangadurai, V.; Weppner, W. Lithium lanthanum titanates: A review. Chem. Mater. 2003, 15, 3974–3990. [Google Scholar] [CrossRef]
- Zhu, P.; Yan, C.; Dirican, M.; Zhu, J.; Zang, J.; Selvan, R.K.; Chung, C.-C.; Jia, H.; Li, Y.; Kiyak, Y.; et al. Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries. J. Mater. Chem. A 2018, 6, 4279–4285. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).