Facile Synthesis and Characterization of CeO2-Nanoparticle-Loaded Carboxymethyl Cellulose as Efficient Protective Films for Mild Steel: A Comparative Study of Experiential and Computational Findings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CeO2-Nanoparticle-Loaded Carboxymethyl Cellulose (CeO2-CMC)
2.2. Preparation of CeO2-CMC Coating Films
2.3. Characterization Techniques
2.4. Corrosion Protection Measurements
2.5. Computational Details
3. Results and Discussion
3.1. Material Characterizations
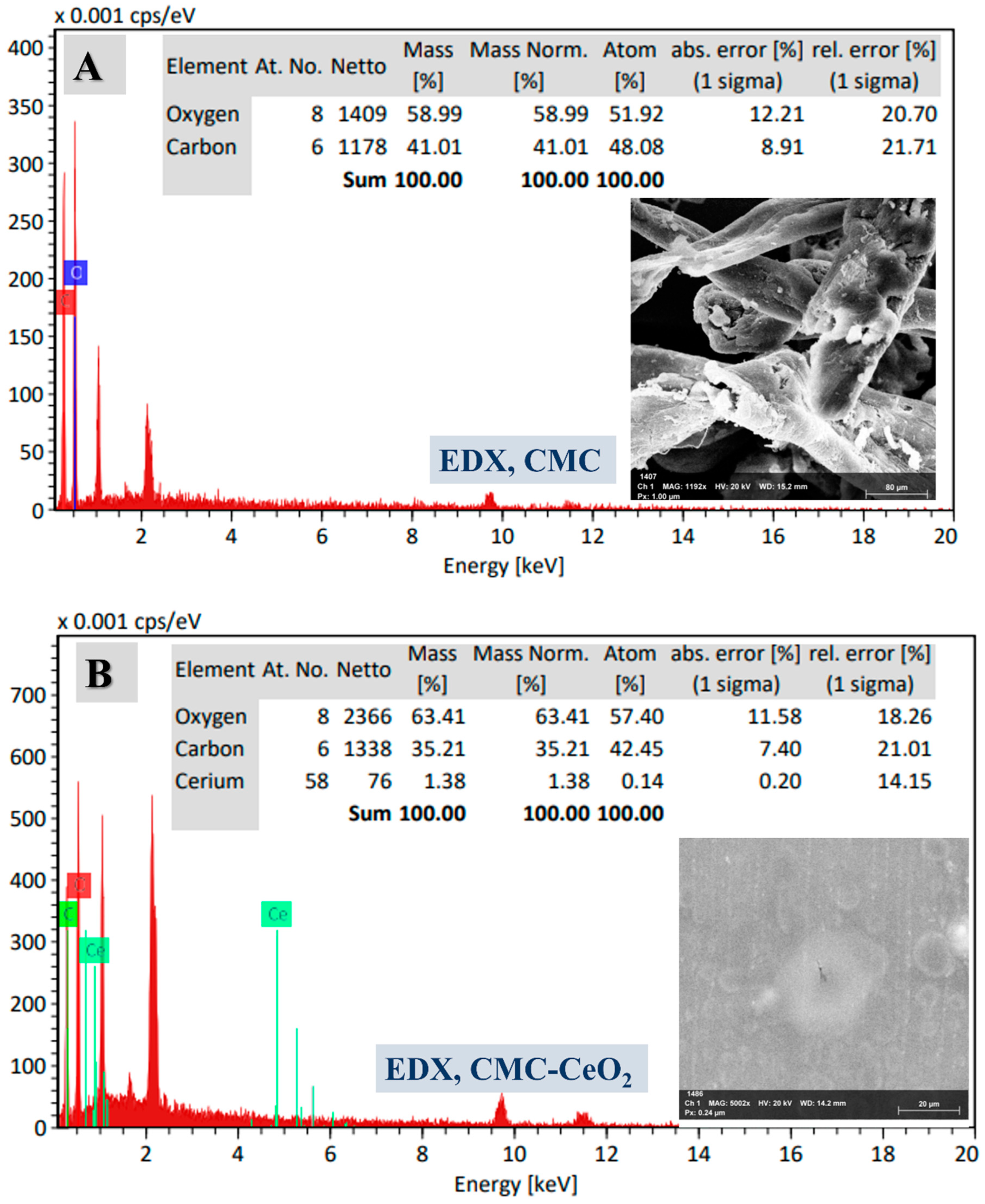
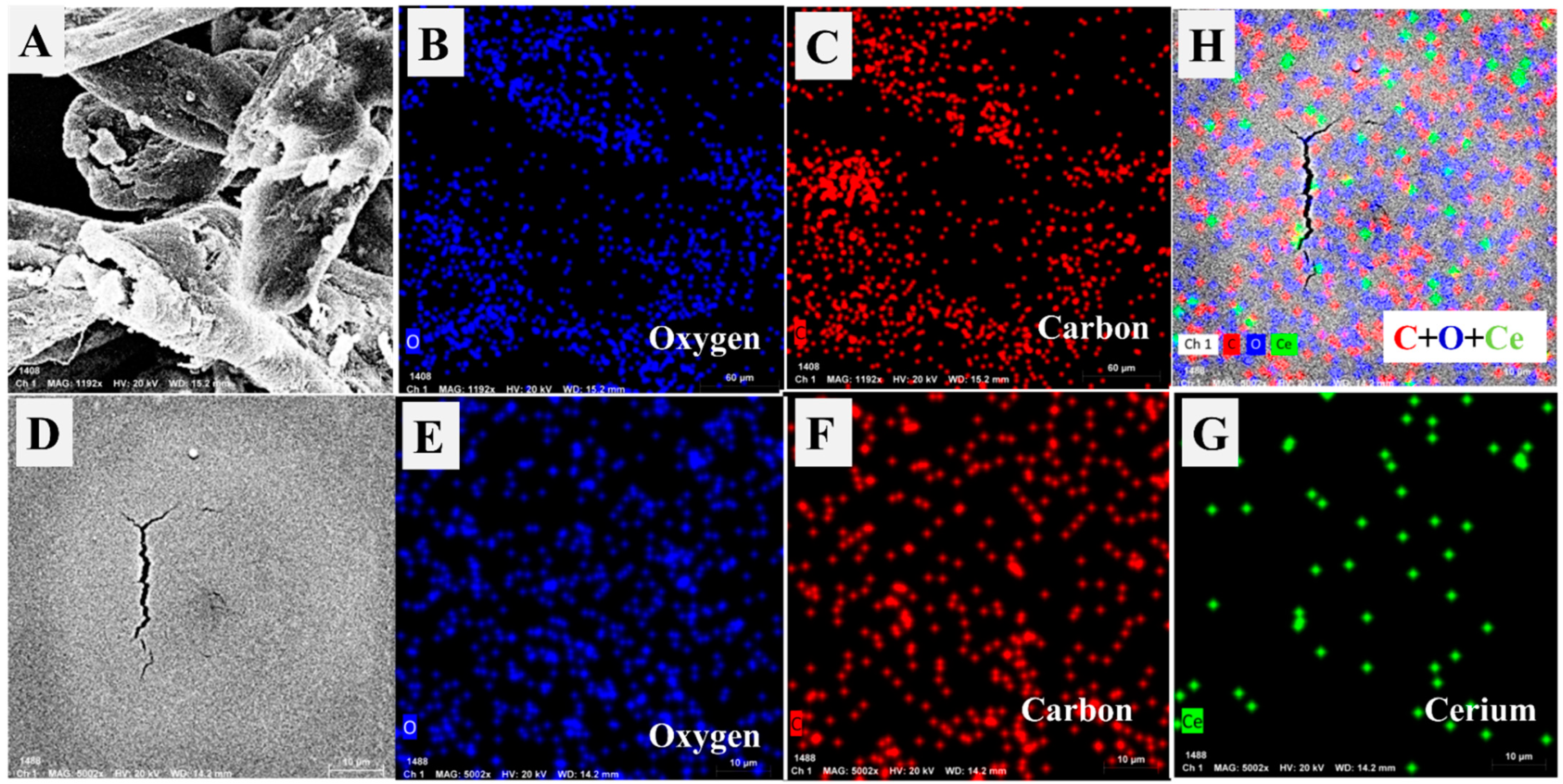
3.1.1. SEM and TEM Analyses
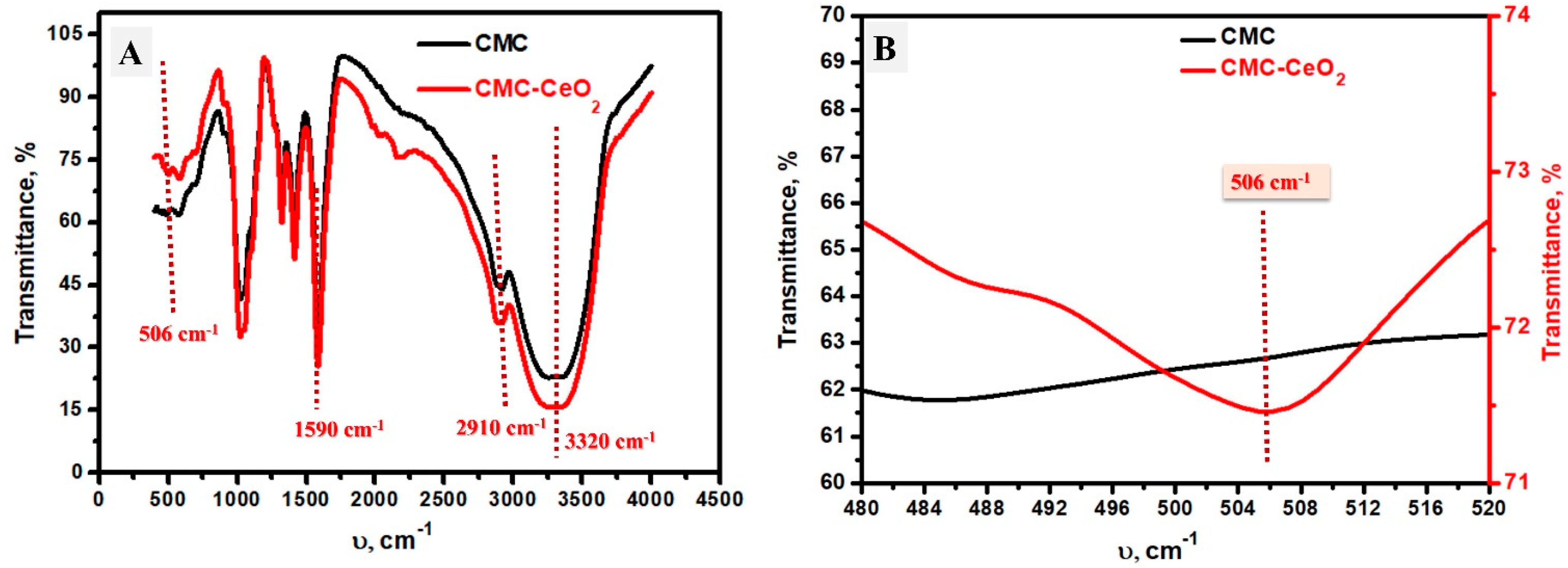
3.1.2. FT-IR Analysis
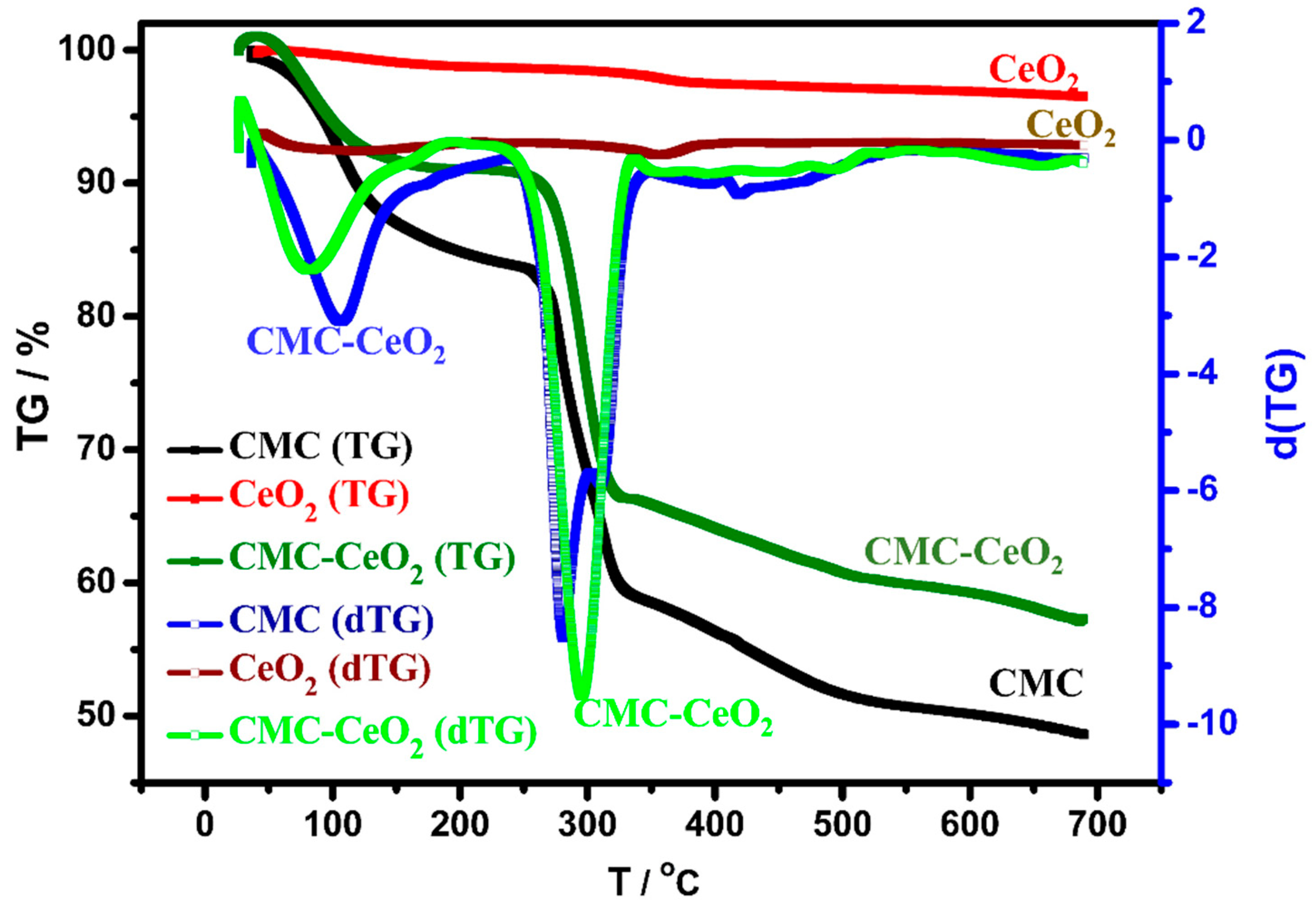
3.1.3. Thermal Properties
3.2. OCP vs. Time and PDP Studies
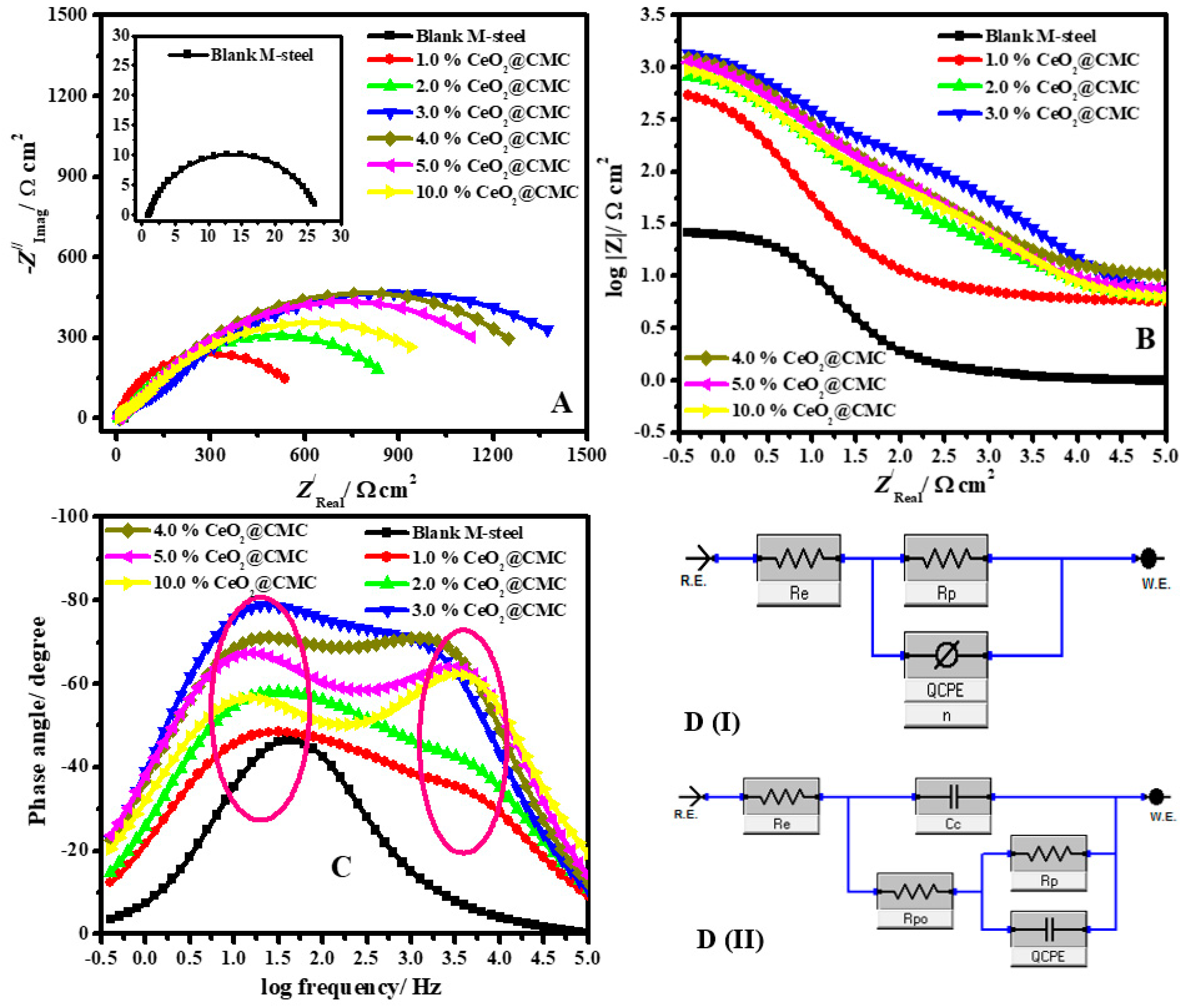
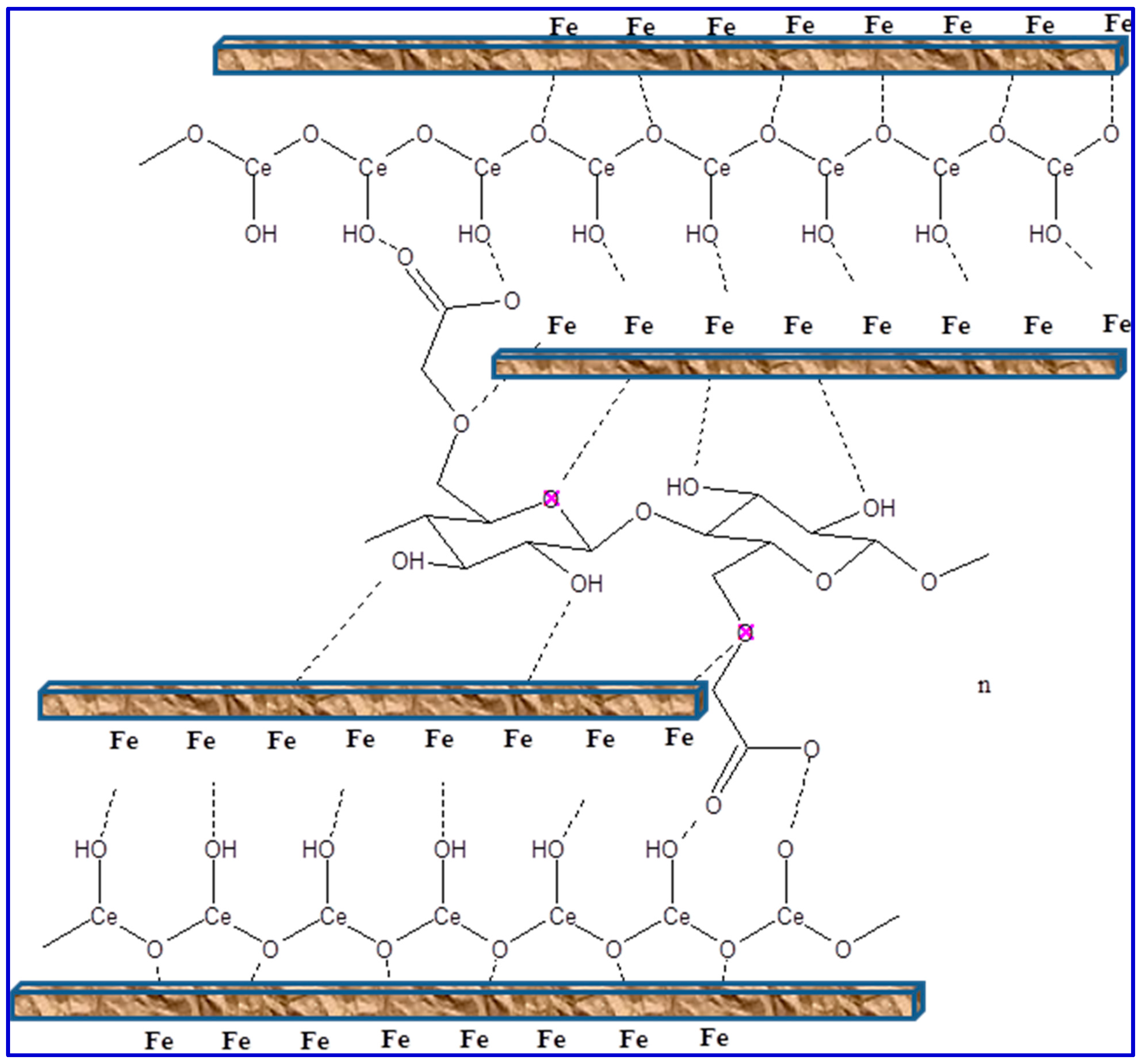
3.3. EIS studies
3.4. Computational Calculations (DFT)
3.5. MC Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, K.; Goyat, M.S.; Vishwakarma, P. Synthesis of Polymer Nano-composite coatings as corrosion inhibitors: A quick review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 983, 12016. [Google Scholar] [CrossRef]
- Valipour, M.N.; Birjandi, F.C.; Sargolzaei, J. Super-non-wettable surfaces: A review. Colloids Surf. A Physicochem. Eng. Asp. 2014, 448, 93–106. [Google Scholar] [CrossRef]
- Manasa, S.; Jyothirmayi, A.; Siva, T.; Sarada, B.V.; Ramakrishna, M.; Sathiyanarayanan, S.; Gobi, K.V.; Subasri, R. Nanoclay-based self-healing, corrosion protection coatings on aluminum, A356.0 and AZ91 substrates. J. Coatings Technol. Res. 2017, 14, 1195–1208. [Google Scholar] [CrossRef]
- Maity, S.; Chatterjee, A. Preparation and characterization of electro-conductive rotor yarn by in situ chemical polymerization of pyrrole. Fibers Polym. 2013, 14, 1407–1413. [Google Scholar] [CrossRef]
- Verma, C.; Haque, J.; Quraishi, M.; Ebenso, E.E. Aqueous phase environmental friendly organic corrosion inhibitors derived from one step multicomponent reactions: A review. J. Mol. Liq. 2019, 275, 18–40. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Ahamad, I.; Quraishi, M. Bis (benzimidazol-2-yl) disulphide: An efficient water soluble inhibitor for corrosion of mild steel in acid media. Corros. Sci. 2009, 51, 2006–2013. [Google Scholar] [CrossRef]
- Obot, I.; Obi-Egbedi, N. Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: Experimental and theoretical investigation. Corros. Sci. 2010, 52, 198–204. [Google Scholar] [CrossRef]
- Shukla, S.K.; Quraishi, M. 4-Substituted anilinomethylpropionate: New and efficient corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2009, 51, 1990–1997. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.; Soltani, N.; Salavati-Niasari, M.; Hamadanian, M.; Gandomi, A. Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corros. Sci. 2008, 50, 2172–2181. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Wu, Y.; Wang, Y.-M.; Jiang, X. Synergistic effect between cationic gemini surfactant and chloride ion for the corrosion inhibition of steel in sulphuric acid. Corros. Sci. 2008, 50, 576–582. [Google Scholar] [CrossRef]
- Hosseini, S.; Azimi, A. The inhibition of mild steel corrosion in acidic medium by 1-methyl-3-pyridin-2-yl-thiourea. Corros. Sci. 2009, 51, 728–732. [Google Scholar] [CrossRef]
- Ali, S.; Saeed, M.; Rahman, S. The isoxazolidines: A new class of corrosion inhibitors of mild steel in acidic medium. Corros. Sci. 2003, 45, 253–266. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Liu, F.; Hou, B. Investigation on inhibition behavior of S-triazole–triazole derivatives in acidic solution. Corros. Sci. 2008, 50, 3261–3266. [Google Scholar] [CrossRef]
- Obot, I.; Obi-Egbedi, N.; Umoren, S. The synergistic inhibitive effect and some quantum chemical parameters of 2,3-diaminonaphthalene and iodide ions on the hydrochloric acid corrosion of aluminium. Corros. Sci. 2009, 51, 276–282. [Google Scholar] [CrossRef]
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H.; Bentiss, F. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010, 52, 3042–3051. [Google Scholar] [CrossRef]
- Raman, R.S.; Siew, W. Role of nitrite addition in chloride stress corrosion cracking of a super duplex stainless steel. Corros. Sci. 2010, 52, 113–117. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green Corrosion Inhibitors from Natural Sources and Biomass Wastes. Molecules 2018, 24, 48. [Google Scholar] [CrossRef] [Green Version]
- Popoola, L.T. Organic green corrosion inhibitors (OGCIs): A critical review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R. Plant extracts as sustainable and green corrosion inhibitors for protection of ferrous metals in corrosive media: A mini review. Corros. Commun. 2022, 5, 25–38. [Google Scholar] [CrossRef]
- Sekine, I.; Nakahata, Y.; Tanabe, H. The corrosion inhibition of mild steel by ascorbic and folic acids. Corros. Sci. 1988, 28, 987–1001. [Google Scholar] [CrossRef]
- Wang, X. Rose, Gardenia, and Solanum Violaceum Extracts as Inhibitors of Steel Corrosion. Int. J. Electrochem. Sci. 2019, 14, 8405–8418. [Google Scholar] [CrossRef]
- Mohammed, H.; Sobri, S.B. Corrosion inhibition studies of cashew nut (Anacardium occidentale) on carbon steel in 1.0 M hydrochloric acid environment. Mater. Lett. 2018, 229, 82–84. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Giacomelli, F.C.; Spinelli, A. Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 2004, 83, 129–134. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H.; Mu, G. Inhibition effect of 6-benzylaminopurine on the corrosion of cold rolled steel in H2SO4 solution. Corros. Sci. 2009, 51, 620–634. [Google Scholar] [CrossRef]
- Bello, M.; Ochoa, N.; Balsamo, V.; López-Carrasquero, F.; Coll, S.; Monsalve, A.; González, G. Modified cassava starches as corrosion inhibitors of carbon steel: An electrochemical and morphological approach. Carbohydr. Polym. 2010, 82, 561–568. [Google Scholar] [CrossRef]
- Solomon, M.; Umoren, S.; Udosoro, I.; Udoh, A. Inhibitive and adsorption behaviour of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros. Sci. 2010, 52, 1317–1325. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Tantawy, A.H.; Soliman, K.A.; El-Lateef, H.M.A. Cationic gemini-surfactants based on waste cooking oil as new ‘green’ inhibitors for N80-steel corrosion in sulphuric acid: A combined empirical and theoretical approaches. J. Mol. Struct. 2020, 1203, 127442. [Google Scholar] [CrossRef]
- Lavanya, M.; Murthy, V.R.; Rao, P. Erosion corrosion control of 6061 aluminum alloy in multi-phase jet impingement conditions with eco-friendly green inhibitor. Chin. J. Chem. Eng. 2020, 28, 340–347. [Google Scholar] [CrossRef]
- Mobin, M.; Rizvi, M. Inhibitory effect of xanthan gum and synergistic surfactant additives for mild steel corrosion in 1 M HCl. Carbohydr. Polym. 2016, 136, 384–393. [Google Scholar] [CrossRef]
- Umoren, S.A.; Banera, M.J.; Alonso-Garcia, T.; Gervasi, C.A.; Mirífico, M.V. Inhibition of mild steel corrosion in HCl solution using chitosan. Cellulose 2013, 20, 2529–2545. [Google Scholar] [CrossRef]
- Bentrah, H.; Rahali, Y.; Chala, A. Gum Arabic as an eco-friendly inhibitor for API 5L X42 pipeline steel in HCl medium. Corros. Sci. 2014, 82, 426–431. [Google Scholar] [CrossRef]
- Fares, M.M.; Maayta, A.; Al-Mustafa, J.A. Corrosion inhibition of iota-carrageenan natural polymer on aluminum in presence of zwitterion mediator in HCl media. Corros. Sci. 2012, 65, 223–230. [Google Scholar] [CrossRef]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose—Inhibition of cast iron corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, A.H.; Soliman, K.A.; El-Lateef, H.M.A. Novel synthesized cationic surfactants based on natural piper nigrum as sustainable-green inhibitors for steel pipeline corrosion in CO2-3.5%NaCl: DFT, Monte Carlo simulations and experimental approaches. J. Clean. Prod. 2020, 250, 119510. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Albokheet, W.A.; Gouda, M. Carboxymethyl cellulose/metal (Fe, Cu and Ni) nanocomposites as non-precious inhibitors of C-steel corrosion in HCl solutions: Synthesis, characterization, electrochemical and surface morphology studies. Cellulose 2020, 27, 8039–8057. [Google Scholar] [CrossRef]
- Tian, Y.; Su, B.; Jiang, L. Interfacial Material System Exhibiting Superwettability. Adv. Mater. 2014, 26, 6872–6897. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Gouda, M.; Shalabi, K.; Al-Omair, M.A.; Khalaf, M.M. Superhydrophobic films-based nonanyl carboxy methylcellulose grafted polyacrylamide for AISI-stainless steel corrosion protection: Empirical explorations and computational models. J. Mol. Liq. 2022, 356, 119063. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2009, 367, 1487–1509. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- El-Lateef, H.M.A.; Sayed, A.R.; Shalabi, K. Synthesis and theoretical studies of novel conjugated polyazomethines and their application as efficient inhibitors for C1018 steel pickling corrosion behavior. Surf. Interfaces 2021, 23, 101037. [Google Scholar] [CrossRef]
- El-Lateef, H.M.; Abdallah, Z.A.; Ahmed, M.S. Solvent-free synthesis and corrosion inhibition performance of Ethyl 2-(1,2,3,6-tetrahydro-6-oxo-2-thioxopyrimidin-4-yl)ethanoate on carbon steel in pickling acids: Experimental, quantum chemical and Monte Carlo simulation studies. J. Mol. Liq. 2019, 296, 111800. [Google Scholar] [CrossRef]
- Dehghani, A.; Mostafatabar, A.H.; Bahlakeh, G.; Ramezanzadeh, B. A detailed study on the synergistic corrosion inhibition impact of the Quercetin molecules and trivalent europium salt on mild steel; electrochemical/surface studies, DFT modeling, and MC/MD computer simulation. J. Mol. Liq. 2020, 316, 113914. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Guo, W.; Zhang, Y.; Liu, G. Inhibition of iron corrosion by 5,10,15,20-tetraphenylporphyrin and 5,10,15,20-tetra-(4-chlorophenyl)porphyrin adlayers in 0.5M H2SO4 solutions. J. Electroanal. Chem. 2007, 602, 115–122. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Beltagi, H.S.; Mohamed, M.E.M.; Kandeel, M.; Bakir, E.; Toghan, A.; Shalabi, K.; Tantawy, A.H.; Khalaf, M.M. Novel Natural Surfactant-Based Fatty Acids and Their Corrosion-Inhibitive Characteristics for Carbon Steel-Induced Sweet Corrosion: Detailed Practical and Computational Explorations. Front. Mater. 2022, 9, 843438. [Google Scholar] [CrossRef]
- Karkhani, R.; Javanbakht, V. A polyurethane foam membrane filled with double cross-linked chitosan/carboxymethyl cellulose gel and decorated with ZSM-5 nano zeolite: Simultaneous dye removal. Int. J. Biol. Macromol. 2022, 213, 699–717. [Google Scholar] [CrossRef]
- Culica, M.E.; Chibac-Scutaru, A.L.; Melinte, V.; Coseri, S. Cellulose Acetate Incorporating Organically Functionalized CeO2 NPs: Efficient Materials for UV Filtering Applications. Materials 2020, 13, 2955. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, H. Recent advances in electrochemical techniques for characterizing surface properties of minerals. Adv. Colloid Interface Sci. 2021, 288, 102346. [Google Scholar] [CrossRef] [PubMed]
- Hamadi, L.; Kareche, A.; Mansouri, S.; Benbouta, S. Corrosion inhibition of Fe-19Cr stainless steel by glutamic acid in 1M HCl. Chem. Data Collect. 2020, 28, 100455. [Google Scholar] [CrossRef]
- Rammelt, U.; Duc, L.M.; Plieth, W. Improvement of protection performance of polypyrrole by dopant anions. J. Appl. Electrochem. 2005, 35, 1225–1230. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A. Synergistic effect of polyethylene glycols and rare earth Ce4+ on the corrosion inhibition of carbon steel in sulfuric acid solution: Electrochemical, computational, and surface morphology studies. Res. Chem. Intermed. 2016, 42, 3219–3240. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Ismael, M.; Mohamed, I. Novel Schiff base amino acid as corrosion inhibitors for carbon steel in CO2-saturated 3.5% NaCl solution: Experimental and computational study. Corrosion Rev. 2015, 33, 77–97. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A. Experimental and Computational Investigation on the Corrosion Inhibition Characteristics of mild steel by some novel synthesized imines in Hydrochloric acid Solutions. Corros. Sci. 2015, 92, 104–117. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Tantawy, A.H. Synthesis and evaluation of novel series of Schiff base cationic surfactants as corrosion inhibitors for carbon steel in acidic/chloride media: Experimental and theoretical investigations. RSC Adv. 2016, 6, 8681–8700. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Abo-Riya, M.A.; Tantawy, A.H. Empirical and quantum chemical studies on the corrosion inhibition performance of some novel synthesized cationic gemini surfactants on carbon steel pipelines in acid pickling processes. Corros. Sci. 2016, 108, 94–110. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Soliman, K.A.; Tantawy, A.H. Novel synthesized Schiff Base-based cationic Gemini surfactants: Electrochemical investigation, theoretical modeling and applicability as biodegradable inhibitors for mild steel against acidic corrosion. J. Mol. Liq. 2017, 232, 478–498. [Google Scholar] [CrossRef]
- Aly, K.I.; Moustafa, A.H.; Ahmed, E.K.; El-lateef, H.M.A.; Mohamed, M.G.; Mohamed, S.M. New Polymer Syntheses Part 60: A Facile Synthetic Route to Polyamides Based on Thieno [2,3-b]thiophene and Their Corrosion Inhibition Behavior. Chin. J. Polym. Sci. 2018, 36, 835–847. [Google Scholar] [CrossRef]
- Saleh, M.M.; Mahmoud, M.G.; El-Lateef, H.M.A. Comparative study of synergistic inhibition of mild steel and pure iron by 1- hexadecylpyridinium chloride and bromide ions. Corros. Sci. 2019, 154, 70–79. [Google Scholar] [CrossRef]
- Sayed, A.R.; El-lateef, H.M.A.; Mohamad, A.D.M. Polyhydrazide Incorporated with Thiadiazole Moiety as Novel and Effective Corrosion Inhibitor for C-Steel in Pickling Solutions of HCl and H2SO4. Macromol. Res. 2018, 26, 882–891. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Khalaf, M.M. Novel dispersed Tl2O3-SiO2/polyaniline nanocomposites: In-sit polymerization, characterization and enforcement as a corrosion protective layer for carbon-steel in acidic chloride medium. Colloids Surf. A 2019, 573, 95–111. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A. Corrosion inhibition characteristics of a novel salycilidene isatin hydrazine sodium sulfonate on carbon steel in HCl and a synergistic nickel ions additive: A combined experimental and theoretical perspective. Appl. Surf. Sci. 2020, 501, 144237. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Alnajjar, A.O. Enhanced the protection capacity of poly(o-toluidine) by synergism with zinc or lanthanum additives at C-steel/HCl interface: A combined DFT, molecular dynamic simulations and experimental methods. J. Mol. Liq. 2020, 303, 112641. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Khalaf, M.M.; El-Lateef, H.M.A.; Khalaf, M.M. Fabrication and characterization of alumina-silica/poly(o-toluidine) nanocomposites as novel anticorrosive epoxy coatings films on carbon steel. Microchem. J. 2020, 158, 105129. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Sayed, A.R.; Shalabi, K. Studying the effect of two isomer forms thiazole and thiadiazine on the inhibition of acidic chloride-induced steel corrosion: Empirical and Computer simulation explorations. J. Mol. Liq. 2022, 356, 119044. [Google Scholar] [CrossRef]
- Tantawy, A.H.; Soliman, K.A.; El-Lateef, H.M.A. Experimental and computational approaches of sustainable quaternary bisammonium fluorosurfactants for corrosion inhibition as protective films at mild steel/H2SO4 interface. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126141. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. Development of metal-organic framework (MOF) decorated graphene oxide nanoplatforms for anti-corrosion epoxy coatings. Carbon 2020, 161, 231–251. [Google Scholar] [CrossRef]
- Cao, K.; Yu, Z.; Yin, D.; Chen, L.; Jiang, Y.; Zhu, L. Fabrication of BTA-MOF-TEOS-GO nanocomposite to endow coating systems with active inhibition and durable anticorrosion performances. Prog. Org. Coat. 2020, 143, 105629. [Google Scholar] [CrossRef]
- Palaniappan, N.; Cole, I.S.; Kuznetsov, A.E. Experimental and computational studies of graphene oxide covalently functionalized by octylamine: Electrochemical stability, hydrogen evolution, and corrosion inhibition of the AZ13 Mg alloy in 3.5% NaCl. RSC Adv. 2020, 10, 11426–11434. [Google Scholar] [CrossRef] [Green Version]
- Yesudass, S.; Olasunkanmi, L.O.; Bahadur, I.; Kabanda, M.M.; Obot, I.; Ebenso, E.E. Experimental and theoretical studies on some selected ionic liquids with different cations/anions as corrosion inhibitors for mild steel in acidic medium. J. Taiwan Inst. Chem. Eng. 2016, 64, 252–268. [Google Scholar] [CrossRef]
- Debab, H.; Douadi, T.; Daoud, D.; Issaadi, S.; Chafaa, S. Electrochemical and quantum chemical studies of adsorption and corrosion inhibition of two new schiff bases on carbon steel in hydrochloric acid media. Int. J. Electrochem. Sci. 2018, 13, 6958–6977. [Google Scholar] [CrossRef]
- Goyal, M.; Vashist, H.; Kumar, S.; Bahadur, I.; Benhiba, F.; Zarrouk, A. Acid corrosion inhibition of ferrous and non-ferrous metal by nature friendly Ethoxycarbonylmethyltriphenylphosphonium Bromide (ECMTPB): Experimental and MD simulation evaluation. J. Mol. Liq. 2020, 315, 113705. [Google Scholar] [CrossRef]
- El-Lateef, H.M.; Shaaban, S.; Shalabi, K.; Khalaf, M.M. Novel organoselenium-based N-mealanilic acids as efficacious corrosion inhibitors for 6061 aluminum alloy in molar HCl: In-silico modeling, electrochemical, and surface morphology studies. J. Taiwan Inst. Chem. Eng. 2022, 133, 104258. [Google Scholar] [CrossRef]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Liang, C. Electrochemical and DFT studies of β-amino-alcohols as corrosion inhibitors for brass. Electrochim. Acta 2007, 52, 4554–4559. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Shalabi, K.; Tantawy, A.H. Corrosion inhibition of carbon steel in hydrochloric acid solution using newly synthesized urea-based cationic fluorosurfactants: Experimental and computational investigations. New J. Chem. 2020, 44, 17791–17814. [Google Scholar] [CrossRef]
- Oyebamiji, A.K.; Adeleke, B.B. Quantum chemical studies on inhibition activities of 2,3-dihydroxypropyl-sulfanyl derivative on carbon steel in acidic media. Int. J. Corros. Scale Inhib. 2018, 7, 498–508. [Google Scholar] [CrossRef]
- Toghan, A.; Gouda, M.; Shalabi, K.; El-Lateef, H. Preparation, Characterization, and Evaluation of Macrocrystalline and Nanocrystalline Cellulose as Potential Corrosion Inhibitors for SS316 Alloy during Acid Pickling Process: Experimental and Computational Methods. Polymers 2021, 13, 2275. [Google Scholar] [CrossRef]
- Shalabi, K.; Helmy, A.; El-Askalany, A.; Shahba, M. New pyridinium bromide mono-cationic surfactant as corrosion inhibitor for carbon steel during chemical cleaning: Experimental and theoretical studies. J. Mol. Liq. 2019, 293, 111480. [Google Scholar] [CrossRef]
- Özcan, M.; Dehri, I.; Erbil, M. Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: Correlation between inhibition efficiency and chemical structure. Appl. Surf. Sci. 2004, 236, 155–164. [Google Scholar] [CrossRef]




| Sample Description | Ecor/ V vs. (Ag/AgCl) | icor/ µA cm−2 ±SD | βa/ V dec −1 | −βc/ V dec −1 | ηPc/% |
|---|---|---|---|---|---|
| Blank MS | −0.447 | 765.3 ± 54.1 | 0.135 | 0.318 | - |
| Pure CMC | −0.453 | 278.6 ± 16.7 | 0.153 | 0.334 | 63.6 |
| 1.0%CeO2-CMC | −0.445 | 179.1 ± 15.4 | 0.156 | 0.355 | 76.6 |
| 2.0%CeO2-CMC | −0.461 | 79.6 ± 4.6 | 0.149 | 0.359 | 89.6 |
| 3.0%CeO2-CMC | −0.456 | 28.3 ± 2.1 | 0.152 | 0.330 | 96.3 |
| 4.0%CeO2-CMC | −0.469 | 44.4 ± 3.6 | 0.145 | 0.345 | 94.2 |
| 5.0%CeO2-CMC | −0.470 | 56.6 ± 4.3 | 0.155 | 0.361 | 92.6 |
| 10.0%CeO2-CMC | −0.465 | 107.9 ± 8.5 | 0.145 | 0.352 | 85.9 |
| Sample Description | Rs/ Ω cm2 | CPEcoat (Qcoat) | Rpo/ Ω cm2 ±SD | Rp/ Ω cm2 ±SD | CPEdl (Qdl) | θ | ηe/% | ||
|---|---|---|---|---|---|---|---|---|---|
| Y0/ µ Ω−1 sn cm−2 | n | Y0/ µΩ−1 sn cm−2 | n | ||||||
| Blank M-steel | 0.95 | - | - | - | 27.7 ± 1.7 | 123.61 | 0.746 | - | - |
| 1.0%CeO2-CMC | 4.71 | 221.72 | 0.859 | 23.1 ± 2.1 | 663.8 ± 40.6 | 45.24 | 0.874 | 0.958 | 95.8 |
| 2.0%CeO2-CMC | 5.10 | 137.62 | 0.877 | 62.2 ± 4.5 | 1014.4 ± 60.3 | 34.65 | 0.855 | 0.972 | 97.2 |
| 3.0%CeO2-CMC | 1.35 | 65.16 | 0.868 | 88.2 ± 7.4 | 1765.1 ± 116.4 | 21.37 | 0.905 | 0.984 | 98.4 |
| 4.0%CeO2-CMC | 1.92 | 70.62 | 0.839 | 81.3 ± 5.5 | 1459.9 ± 78.8 | 24.34 | 0.911 | 0.981 | 98.1 |
| 5.0%CeO2-CMC | 2.43 | 78.54 | 0.865 | 74.5 ± 4.8 | 1385.6 ± 76.1 | 26.89 | 0.915 | 0.980 | 98.0 |
| 10.0%CeO2-CMC | 2.88 | 108.5 | 0.883 | 68.7 ± 5.1 | 1227.5 ± 65.2 | 31.23 | 0.899 | 0.977 | 97.7 |
| Parameters | CeO2 | CMC | CeO2–CMC |
|---|---|---|---|
| EHOMO (eV) | −8.48 | −5.75 | −5.28 |
| ELUMO (eV) | −1.71 | −5.04 | −5.11 |
| ΔE = ELUMO − EHOMO (eV) | 6.77 | 0.71 | 0.18 |
| Electronegativity (χ) | 5.10 | 5.39 | 5.20 |
| Global hardness (η) | 3.39 | 0.35 | 0.09 |
| Global softness (σ) | 0.30 | 2.83 | 11.24 |
| The number of electrons transferred (ΔN) | 0.28 | 2.27 | 10.14 |
| ∆Eback-donation | −0.85 | −0.09 | −0.02 |
| Dipole moments (µ) Debye | 2.99 | 5.88 | 18.56 |
| Molecular surface area (Å2) | 427.35 | 1368.24 | 2741.52 |
| Corrosion Systems | Adsorption Energy/ kcal mol−1 | Rigid Adsorption Energy/ kcal mol−1 | Deformation Energy/ kcal mol−1 | dEads/dNi: Inhibitor kcal mol−1 | dEads/dNi: Hydronium kcal mol−1 | dEads/dNi: Cl− ions kcal mol−1 | dEads/dNi: Water kcal mol−1 |
|---|---|---|---|---|---|---|---|
| Fe(110) | −2868.29 | −2168.86 | −699.42 | −372.13 | −31.07 | −61.03 | −15.26 |
| CeO2-CMC | |||||||
| Water | |||||||
| Hydronium | |||||||
| Cl− ions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouda, M.; Khalaf, M.M.; Al-Shuaibi, M.A.A.; Mohamed, I.M.A.; Shalabi, K.; El-Shishtawy, R.M.; El-Lateef, H.M.A. Facile Synthesis and Characterization of CeO2-Nanoparticle-Loaded Carboxymethyl Cellulose as Efficient Protective Films for Mild Steel: A Comparative Study of Experiential and Computational Findings. Polymers 2022, 14, 3078. https://doi.org/10.3390/polym14153078
Gouda M, Khalaf MM, Al-Shuaibi MAA, Mohamed IMA, Shalabi K, El-Shishtawy RM, El-Lateef HMA. Facile Synthesis and Characterization of CeO2-Nanoparticle-Loaded Carboxymethyl Cellulose as Efficient Protective Films for Mild Steel: A Comparative Study of Experiential and Computational Findings. Polymers. 2022; 14(15):3078. https://doi.org/10.3390/polym14153078
Chicago/Turabian StyleGouda, Mohamed, Mai M. Khalaf, Manal A. A. Al-Shuaibi, Ibrahim M. A. Mohamed, Kamal Shalabi, Reda M. El-Shishtawy, and Hany M. Abd El-Lateef. 2022. "Facile Synthesis and Characterization of CeO2-Nanoparticle-Loaded Carboxymethyl Cellulose as Efficient Protective Films for Mild Steel: A Comparative Study of Experiential and Computational Findings" Polymers 14, no. 15: 3078. https://doi.org/10.3390/polym14153078
APA StyleGouda, M., Khalaf, M. M., Al-Shuaibi, M. A. A., Mohamed, I. M. A., Shalabi, K., El-Shishtawy, R. M., & El-Lateef, H. M. A. (2022). Facile Synthesis and Characterization of CeO2-Nanoparticle-Loaded Carboxymethyl Cellulose as Efficient Protective Films for Mild Steel: A Comparative Study of Experiential and Computational Findings. Polymers, 14(15), 3078. https://doi.org/10.3390/polym14153078










