Characterization and Antibacterial Activities of Carboxymethylated Paramylon from Euglena gracilis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of EGP
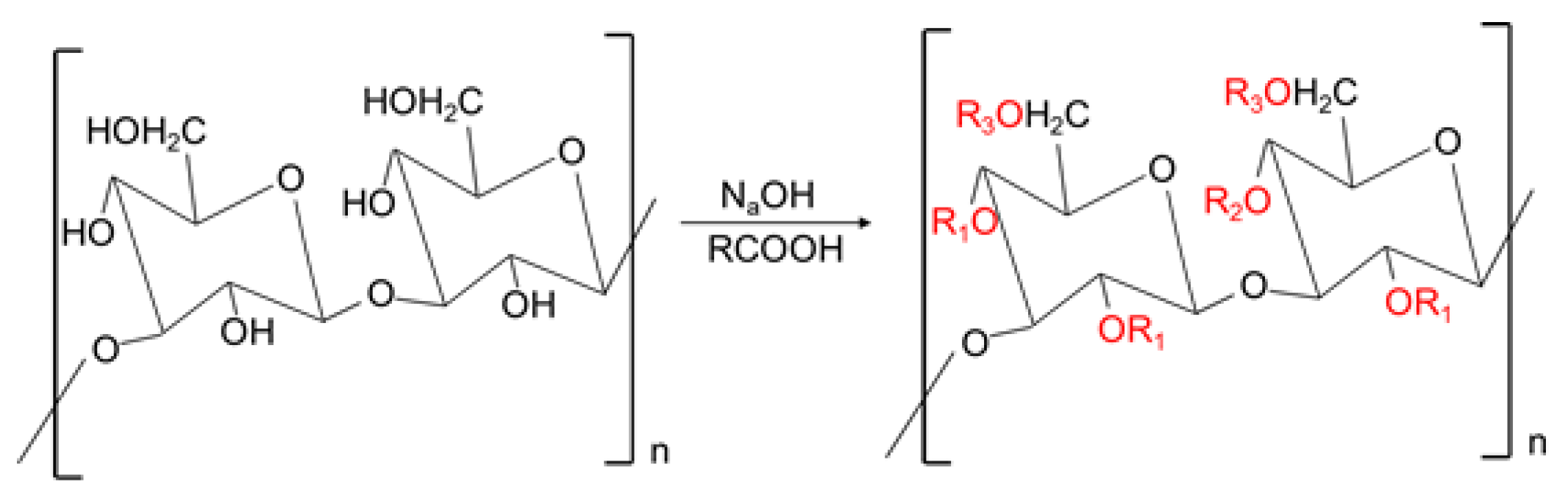
2.3. Preparation of Carboxymethylated EGP (C-EGP)
2.4. Characterization
2.4.1. Determination of the DS
2.4.2. Component Analysis of the EGP and Its Modified Derivatives
2.4.3. Determination of Molecular Weight
2.4.4. FTIR Spectroscopy
2.4.5. NMR Spectroscopy Measurements
2.4.6. Congo Red Experiment
2.4.7. Morphological Characteristics
2.4.8. Crystalline Characteristics
2.4.9. TG Analysis
2.5. Water Solubility (WS) Test
2.6. Antibacterial Activities
2.6.1. Inhibition Zone Experiment
2.6.2. MIC Assay
2.6.3. Dynamic Antibacterial Activity
2.7. Statistical Analysis
3. Result and Discussion
3.1. Chemical Composition, DS and Mw Analysis
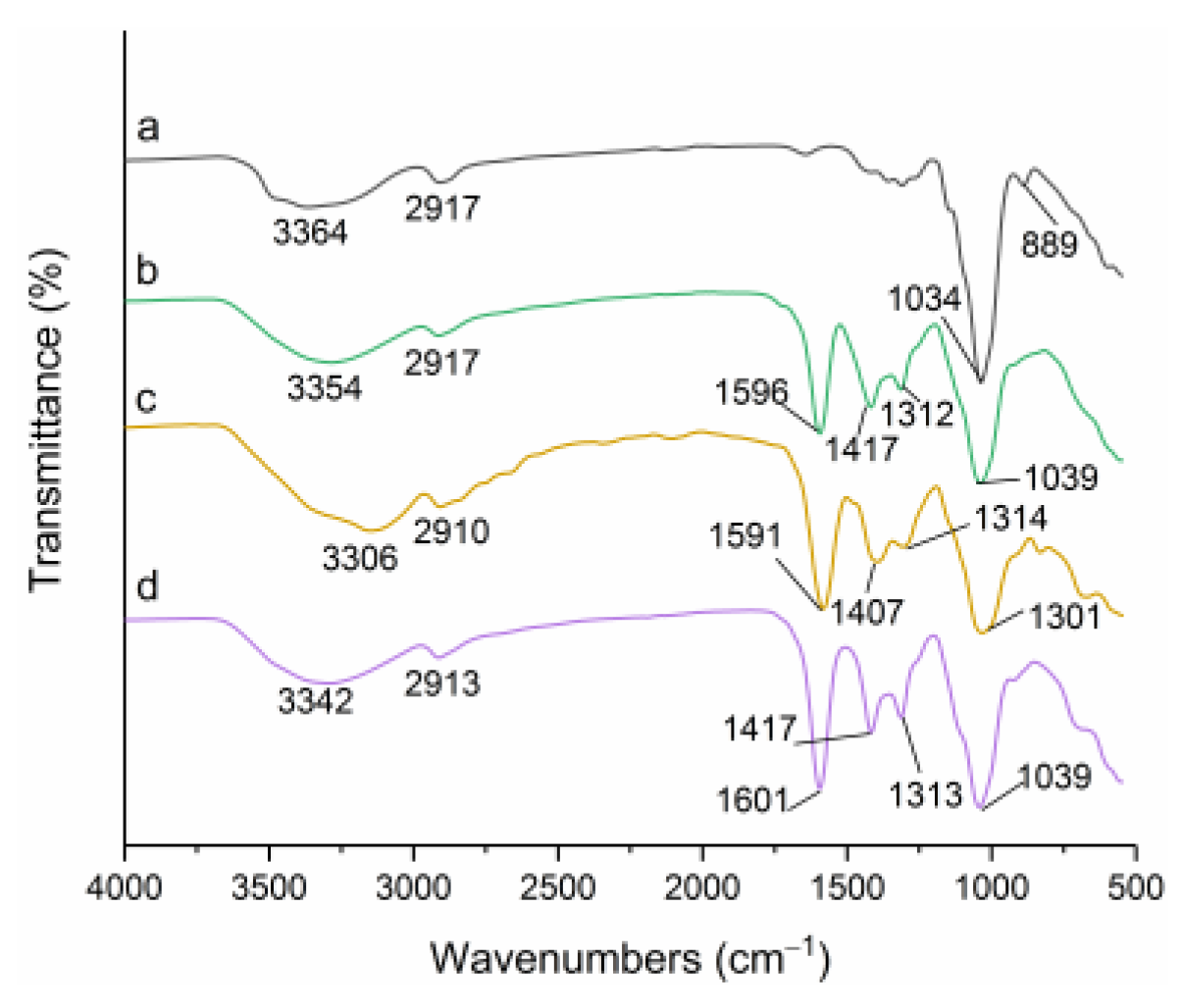
3.2. FTIR Spectroscopy Analysis
3.3. NMR Analysis
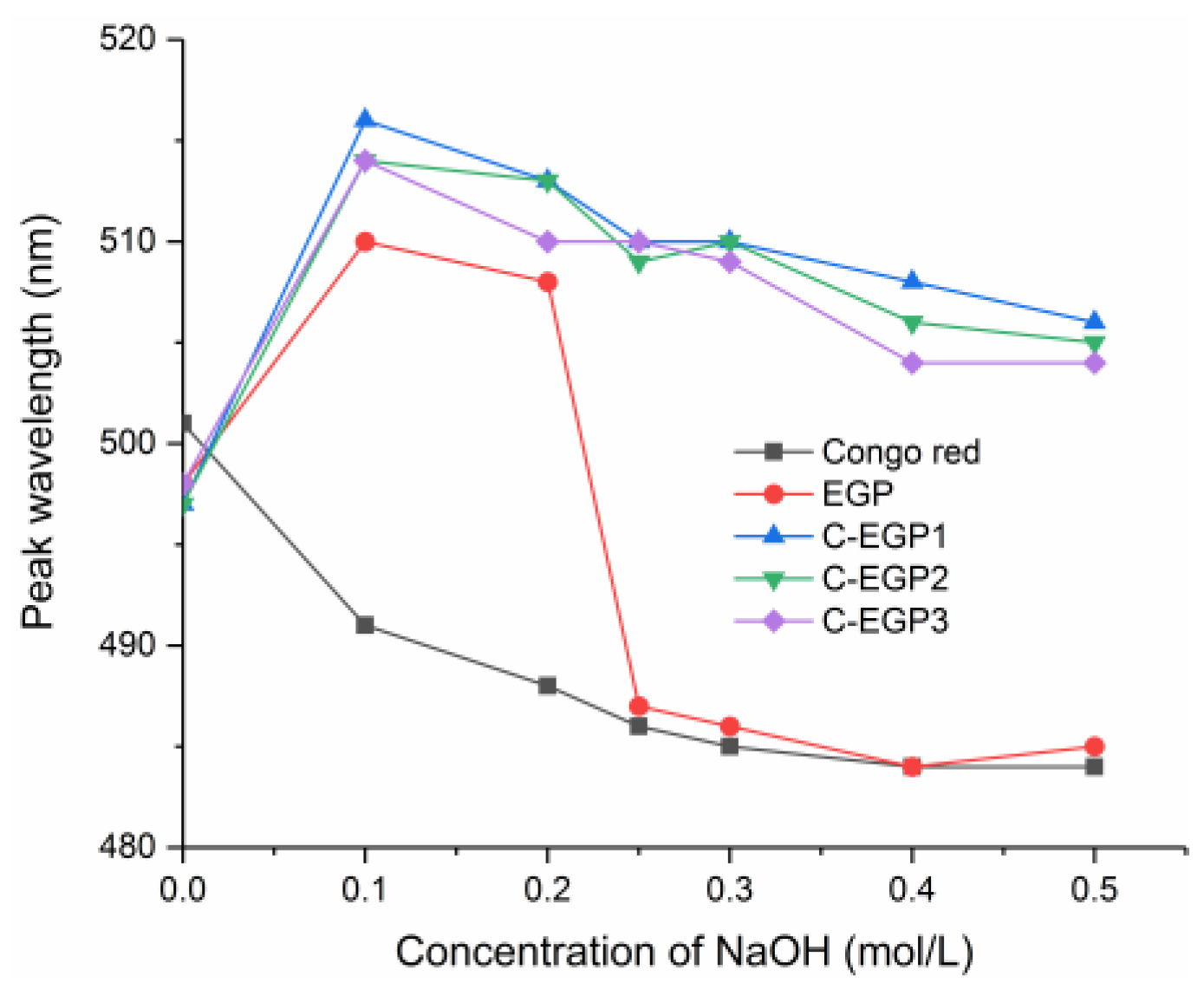
3.4. CR Analysis
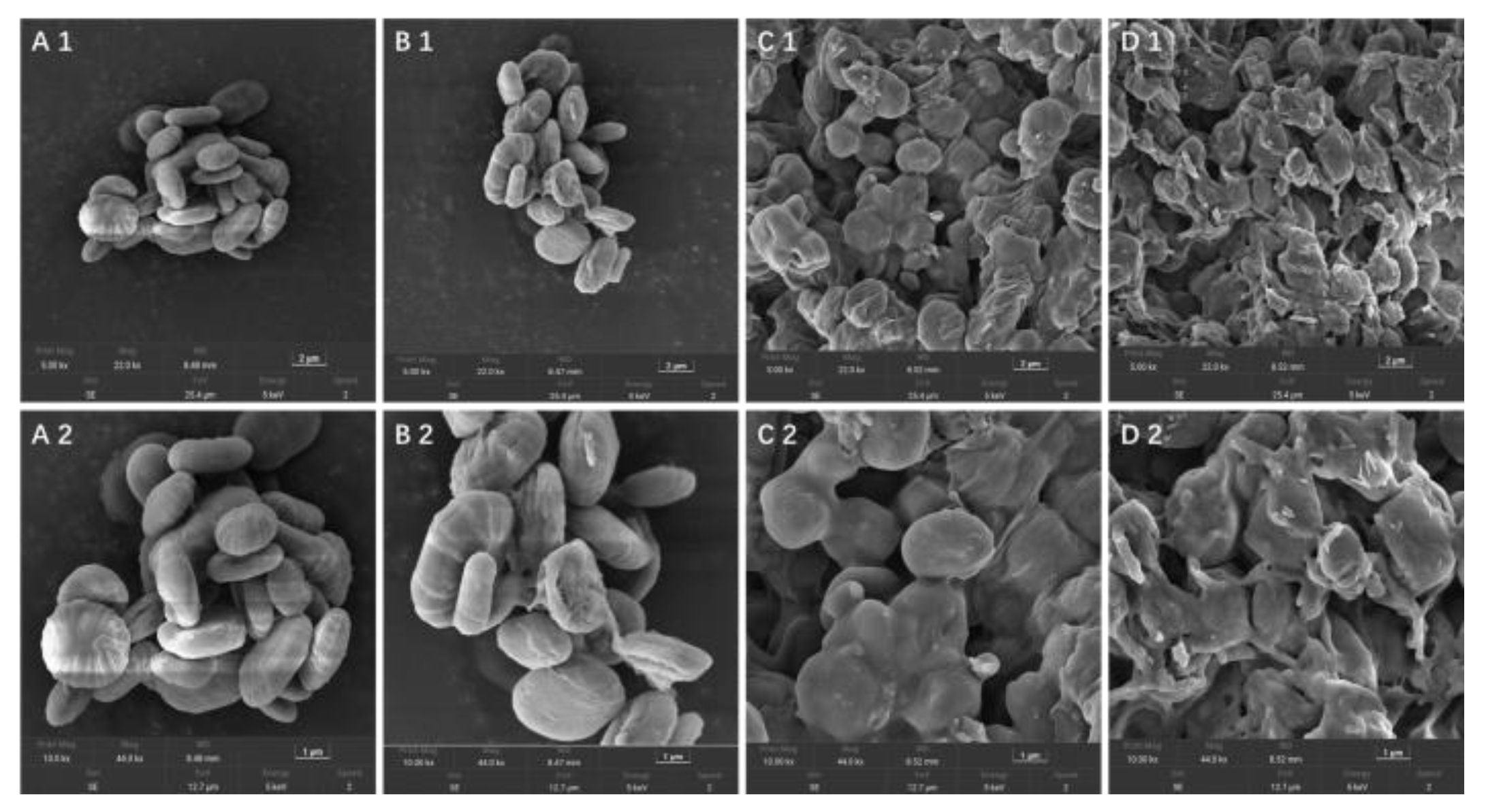
3.5. Surface Morphology Analysis
3.6. XRD Analysis
3.7. Thermogravimetric Analysis
3.8. Water Solubility Analysis
3.9. Antibacterial Activity
3.9.1. Bacterial Inhibition Zone Essay Analysis
3.9.2. MIC Analysis
3.9.3. Dynamic Antibacterial Activity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shibakami, M.; Tsubouchi, G.; Nakamura, M.; Hayashi, M. Polysaccharide nanofiber made from euglenoid alga. Carbohydr. Polym. 2013, 93, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Shimada, R.; Matsuyama, A.; Yuasa, M.; Sawamura, H.; Yoshida, E.; Suzuki, K. Antitumor activity of the beta-glucan paramylon from Euglena against preneoplastic colonic aberrant crypt foci in mice. Food Funct. 2013, 4, 1685–1690. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kimura, S.; Togawa, E.; Wada, M.; Kuga, S. Crystal transition of paramylon with dehydration and hydration. Carbohydr. Polym. 2010, 80, 491–497. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Paramylon, a Potent Immunomodulator from WZSL Mutant of Euglena gracilis. Molecules 2019, 24, 3114. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Suzuki, K.; Mitra, S.; Arashida, R.; Yoshida, E.; Nakano, R.; Yabuta, Y.; Takeuchi, T. Hepatoprotective Effects of Paramylon, a beta-1, 3-D-Glucan Isolated from Euglena gracilis Z, on Acute Liver Injury Induced by Carbon Tetrachloride in Rats. J. Vet. Med. Sci. 2009, 71, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Suzuki, K.; Asayama, Y.; Konno, M.; Saito, K.; Yamazaki, N.; Takimoto, H. Oral administration of Euglena gracilis Z and its carbohydrate storage substance provides survival protection against influenza virus infection in mice. Biochem. Biophys. Res. Commun. 2017, 494, 379–383. [Google Scholar] [CrossRef]
- Yamamoto, F.Y.; Sutili, F.J.; Hume, M.; Gatlin III, D.M. The effect of beta-1,3-glucan derived from Euglena gracilis (Algamune™) on the innate immunological responses of Nile tilapia (Oreochromis niloticus L.). J. Fish Dis. 2018, 41, 1579–1588. [Google Scholar] [CrossRef]
- Feng, R.; Kou, J.; Chen, S.; Wang, N.; Wang, W.; Wang, L.; Wang, H.; Ference, C.; Liu, M.; Ao, C.; et al. Effects of sulfated, phosphorylated and carboxymethylated modifications on the antioxidant activities in-vitro of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2020, 146, 887–896. [Google Scholar] [CrossRef]
- Russo, R.; Barsanti, L.; Evangelista, V.; Frassanito, A.M.; Longo, V.; Pucci, L.; Penno, G.; Gualtieri, P. Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food Sci. Nutr. 2017, 5, 205–214. [Google Scholar] [CrossRef]
- Bezerra, L.S.; Magnani, M.; Castro-Gomez, R.J.H.; Cavalcante, H.C.; da Silva, T.A.F.; Vieira, R.L.P.; de Medeiros, I.A.; Veras, R.C. Modulation of vascular function and anti-aggregation effect induced by (1→3) (1→6)-(β-D-glucan of & ITSaccharomyces cerevisiae & IT and its carboxymethylated derivative in rats. Pharmacol. Rep. 2017, 69, 448–455. [Google Scholar] [CrossRef]
- Li, H.; Wang., X.; Xiong, Q.; Peng, L. Sulfated modification, characterization, and potential bioactivities of polysaccharide from the fruiting bodies of Russula virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447. [Google Scholar] [CrossRef]
- Huang, S.; Chen, F.; Cheng, H.; Huang, G. Modification and application of polysaccharide from traditional Chinese medicine such as Dendrobium officinale. Int. J. Biol. Macromol. 2020, 157, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Y.; Sun, P.P.; Ji, Y.P.; Wang, X.T.; Dai, S.H.; Zhu, Z.Y. Carboxymethylation and acetylation of the polysaccharide from Cordyceps militaris and their alpha-glucosidase inhibitory activities. Nat. Prod. Res. 2020, 34, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Zhao, M. Carboxymethylation of polysaccharides from Tremella fuciformis for antioxidant and moisture-preserving activities. Int. J. Biol. Macromol. 2015, 72, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.J.; Wei, X.; Xu, J.; Chen, B.J.; Zhao, D.Y.; Cui, S.; Zhou, T. Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: Preparation and in vitro antioxidant activity. Food Chem. 2017, 215, 76–83. [Google Scholar] [CrossRef]
- Miyatake, K.; Takenaka, S.; Yamaji, R.; Nakano, Y. Characteristics and the Utilization of Bid-particles, Paramylon, Formed by Protist. J. Soc. Powder Technol. Jpn. 1995, 32, 566–572. [Google Scholar] [CrossRef][Green Version]
- Bhattad, T.; Koradiya, A.; Prakash, G. Prebiotic activity of paramylon isolated from heterotrophically grown Euglena gracilis. Heliyon 2021, 7, e07884. [Google Scholar] [CrossRef]
- Shibakami, M.; Tsubouchi, G.; Sohma, M.; Hayashi, M. Synthesis of nanofiber-formable carboxymethylated Euglena-derived beta-1,3-glucan. Carbohydr. Polym. 2016, 152, 468–478. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, M.; Du, Z.; Jing, L.; Surhio, M.M.; Yang, L. Carboxymethylation of an exopolysaccharide from Lachnum and effect of its derivatives on experimental chronic renal failure. Carbohydr. Polym. 2014, 114, 190–195. [Google Scholar] [CrossRef]
- Cheng, H.; Huang G, L. The antioxidant activities of carboxymethylated garlic polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 140, 1054–1063. [Google Scholar] [CrossRef]
- Jain, V.M.; Karibasappa, G.N.; Dodamani, A.S.; Mali, G.V. Estimating the carbohydrate content of various forms of tobacco by phenol-sulfuric acid method. J. Educ. Health Promot. 2017, 6, 90. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G. Characterisation of cell wall polysaccharides from okra (Abelmoschus esculentus (L.) Moench). Carbohydr. Res. 2009, 344, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ye, Z.; Liu, S.; Yang, Y.; Dong, J.; Wang, K.; Zhang, S.; Shen, Q.; Li, X.; Liu, D. Effects of crude Sphallerocarpus gracilis polysaccharides as potential prebiotics on acidifying activity and growth of probiotics in fermented milk. Lwt.-Food Sci. Technol. 2021, 149, 111882. [Google Scholar] [CrossRef]
- Wu, D.T.; He, Y.; Fu, M.X.; Gan, R.Y.; Hu, Y.C.; Peng, L.X.; Zhao, G.; Zou, L. Structural characteristics and biological activities of a pectic-polysaccharide from okra affected by ultrasound assisted metal-free Fenton reaction. Food Hydrocoll. 2022, 122, 107085. [Google Scholar] [CrossRef]
- Hou, C.; Liu, L.; Ren, J.; Huang, M.; Yuan, E. Structural characterization of two Hericium erinaceus polysaccharides and their protective effects on the alcohol-induced gastric mucosal injury. Food Chem. 2022, 375, 131896. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, J.; Yuan, Y.; Gui, Y.; Zou, F.; Lu, L.; Cui, B. Structural and functional modification of kudzu starch using a-amylase and transglucosidase. Int. J. Biol. Macromol. 2021, 169, 67–74. [Google Scholar] [CrossRef]
- Gui, Y.; Zou, F.; Li, J.; Tang, J.; Guo, L.; Cui, B. Corn starch modification during endogenous malt amylases: The impact of synergistic hydrolysis time of alpha-amylase and beta-amylase and limit dextrinase. Int. J. Biol. Macromol. 2021, 190, 819–826. [Google Scholar] [CrossRef]
- Shibakami, M.; Tsubouchi, G.; Sohma, M.; Hayashi, M. One-pot synthesis of thermoplastic mixed paramylon esters using trifluoroacetic anhydride. Carbohydr. Polym. 2015, 119, 1–7. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, H.; Cai, G.; Guan, S.; Tong, H.; Yang, X.; Liu, J. Sulfated modification of the water-soluble polysaccharides from Polyporus albicans mycelia and its potential biological activities. Int. J. Biol. Macromol. 2009, 44, 14–17. [Google Scholar] [CrossRef]
- Liu, Y.; You, Y.; Li, Y.; Zhang, L.; Tang, T.; Duan, X.; Li, C.; Liu, A.; Hu, B.; Chen, D. Characterization of carboxymethylated polysaccharides from Catathelasma ventricosum and their antioxidant and antibacterial activities. J. Funct. Foods 2017, 38, 355–362. [Google Scholar] [CrossRef]
- Shankar, N.; Baghdayan, A.S.; Gilmore, M.S. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 2002, 417, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shen, M.; Wen, P.; Hong, Y.; Liu, X.; Xie, J. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of phosphorylated polysaccharide from Cyclocarya paliurus. Food Chem. Toxicol. 2020, 145, 111754. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Sulfated modification, characterization and bioactivities of an acidic polysaccharide fraction from an edible mushroom Pleurotus eous (Berk.) Sacc. Heliyon 2021, 7, e05964. [Google Scholar] [CrossRef]
- Yu, Y.; Song, Q.; Huang, L.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. Immunomodulatory activities of sulfated Cyclocarya paliurus polysaccharides with different degrees of substitution on mouse spleen lymphocytes. J. Funct. Foods 2020, 64, 103706. [Google Scholar] [CrossRef]
- Saha, S.; Galhardi, L.C.; Yamamoto, K.A.; Linhares, R.E.C.; Bandyopadhyay, S.S.; Sinha, S.; Nozawa, C.; Ray, B. Water-extracted polysaccharides from Azadirachta indica leaves: Structural features, chemical modification and anti-bovine herpesvirus type 1 (BoHV-1) activity. Int. J. Biol. Macromol. 2010, 47, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, H.; Tian, J.; Wang, Y.; Xing, L.; Wang, J. Chemical modification, antioxidant and alpha-amylase inhibitory activities of corn silk polysaccharides. Carbohydr. Polym. 2013, 98, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ai, L.; Hang, F.; Ding, S.; Liu, Y. Composition and antioxidant activity of polysaccharides from jujuba by classical and ultrasound extraction. Int. J. Biol. Macromol. 2014, 63, 150–153. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.; Chen, X.; Tong, C.; Pang, J. Synthesis and characteristics of konjac glucomannan films incorporated with functionalized microcrystalline cellulose. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 563, 237–245. [Google Scholar] [CrossRef]
- Su, J.F.; Huang, Z.; Yuan, X.Y.; Wang, X.Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Production and physicochemical properties of carboxymethyl cellulose films enriched with spent coffee grounds polysaccharides. Int. J. Biol. Macromol. 2018, 106, 647–655. [Google Scholar] [CrossRef]
- Daglio, Y.; Rodríguez, M.C.; Prado, H.J.; Matulewicz, M.C. Paramylon and synthesis of its ionic derivatives: Applications as pharmaceutical tablet disintegrants and as colloid flocculants. Carbohydr. Res. 2019, 484, 107779. [Google Scholar] [CrossRef] [PubMed]
- Shibakami, M.; Tsubouchi, G.; Nakamura, M.; Hayashi, M. Preparation of carboxylic acid-bearing polysaccharide nanofiber made from euglenoid beta-1,3-glucans. Carbohydr. Polym. 2013, 98, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.M.; Nie, S.P.; Zhou, H.L.; Huang, D.F.; Xie, M.Y. Carboxymethylation enhances the maturation-inducing activity in dendritic cells of polysaccharide from the seeds of Plantago asiatica L. Int. Immunopharmacol. 2014, 22, 324–331. [Google Scholar] [CrossRef]
- Liu, W.; Hu, C.; Liu, Y.; Dai, S.; Lu, W.; Yao, W.; Gao, X. Preparation, characterization, and alpha-glycosidase inhibition activity of a carboxymethylated polysaccharide from the residue of Sarcandra glabra (Thunb.) Nakai. Int. J. Biol. Macromol. 2017, 99, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Xu, D.; Cui, B.; Wang, Y. Gelation of kappa-carrageenan/Konjac glucommanan compound gel: Effect of cyclodextrins. Food Hydrocoll. 2019, 87, 158–164. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mazumder, B. Crosslinking of Isabgol husk polysaccharides for microspheres development and its impact on particle size, swelling kinetics and thermal behavior. Polym. Bull. 2014, 71, 735–757. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation properties of salt-extracted pea protein induced by heat treatment. Food Res. Int. 2010, 43, 509–515. [Google Scholar] [CrossRef]
- Sun, Q.; Gong, M.; Li, Y.; Xiong, L. Effect of retrogradation time on preparation and characterization of proso millet starch nanoparticles. Carbohydr. Polym. 2014, 111, 133–138. [Google Scholar] [CrossRef]
- Erum, A.; Bashir, S.; Saghir, S. Modified and unmodified arabinoxylans from Plantago ovata husk: Novel excipients with antimicrobial potential. Bangladesh J. Pharmacol. 2015, 10, 765–769. [Google Scholar] [CrossRef]
- Song, J.; Chen, H.; Wei, Y.; Liu, J. Synthesis of carboxymethylated beta-glucan from naked barley bran and its antibacterial activity and mechanism against Staphylococcus aureus. Carbohydr. Polym. 2020, 242, 116418. [Google Scholar] [CrossRef]
- Panja, S.; Bharti, R.; Dey, G.; Lynd, N.A.; Chattopadhyay, S. Coordination-Assisted Self-Assembled Polypeptide Nanogels to Selectively Combat Bacterial Infection. ACS Appl. Mater. Interfaces 2019, 11, 33599–33611. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.L.; Xu, J.; Shi, M.J.; Wang, X.L.; Li, Y.T.; Kong, L.M.; Hider, R.C.; Zhou, T. Preparation, antioxidant and antimicrobial evaluation of hydroxamated degraded polysaccharides from Enteromorpha prolifera. Food Chem. 2017, 237, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Hemar, Y.; Perera, C.O.; Lewis, G.; Krissansen, G.W.; Buchanan, P.K. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact. Carbohydr. Diet. Fibre 2014, 3, 41–51. [Google Scholar] [CrossRef]




| Samples | EGP | C-EGP1 | C-EGP2 | C-EGP3 |
|---|---|---|---|---|
| Total sugar (%) | 55.06 ± 0.992 a | 44.72 ± 0.121 b | 42.39 ± 0.866 c | 38.91 ± 0.651 d |
| Uronic acid (%) | 19.76 ± 1.348 a | 17.67 ± 1.332 b | 16.25 ± 0.265 b | 13.08 ± 0.291 c |
| Protein (%) | 0.74 ± 0.045 a | 0.72 ± 0.021 a | 0.50 ± 0.067 b | 0.37 ± 0.047 c |
| Mw (×105) | 1.094 | 1.153 | 1.330 | 1.482 |
| DS | – | 0.14 ± 0.005 c | 0.55 ± 0.01 b | 0.78 ± 0.015 a |
| Sample | First-Stage Weight Loss (30~150 °C) | Second-Stage Weight Loss (150~750 °C) | Residue (%) | ||
|---|---|---|---|---|---|
| Td1 (°C) | Δw1 (%) | Td2 (°C) | Δw2 (%) | ||
| EGP | 38.44 ± 0.52 b | 6.1 ± 1.26 d | 324.88 ± 2.81 a | 84.81 ± 1.81 a | 9.08 ± 0.60 d |
| C-EGP1 | 44.95 ± 0.63 a | 12.9 ± 2.28 bc | 244.72 ± 0.84 b | 46.19 ± 0.56 b | 40.25 ± 0.58 b |
| C-EGP2 | 44.07 ± 0.79 a | 15.07 ± 1.5 b | 231.53 ± 1.05 c | 40.33 ± 0.49 c | 44.6 ± 1.034 a |
| C-EGP3 | 37.15 ± 0.7 b | 19.85 ± 2.78 a | 220.50 ± 1.06 d | 48.12 ± 1.07 b | 32.03 ± 1.72 c |
| Samples | EGP | C-EGP1 | C-EGP2 | C-EGP3 |
|---|---|---|---|---|
| Water solubility (%) | 0.52 ± 0.27 d | 53.31 ± 1.03 c | 75.52 ± 2.95 b | 80.96 ± 1.45 a |
| Item (30 mg/mL) | Diameters of Inhibition Zone (mm) | |
|---|---|---|
| E. coli | S. aureus | |
| EGP | 6.08 ± 0.100 d | 6.08 ± 0.055 d |
| C-EGP1 | 8.01 ± 0.121 c | 10.10 ± 0.187 c |
| C-EGP2 | 9.30 ± 0.239 b | 11.19 ± 0.137 b |
| C-EGP3 | 11.24 ± 0.151 a | 12.05 ± 0.092 a |
| Physiological saline | 6.00 | 6.00 |
| Ampicillin * | 18.23 ± 0.226 | 20.39 ± 0.539 |
| Concentration (mg/mL) | Growth Status | |
|---|---|---|
| E. coli | S. aureus | |
| 100 | – | – |
| 50 | – | – |
| 25 | – | – |
| 12.5 | – | – |
| 6.25 | + | – |
| 3.13 | + + | + |
| 1.56 | + + | + + |
| Physiological saline | + + | + + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Zhao, X.; Liu, M.; Zhao, X. Characterization and Antibacterial Activities of Carboxymethylated Paramylon from Euglena gracilis. Polymers 2022, 14, 3022. https://doi.org/10.3390/polym14153022
Gao L, Zhao X, Liu M, Zhao X. Characterization and Antibacterial Activities of Carboxymethylated Paramylon from Euglena gracilis. Polymers. 2022; 14(15):3022. https://doi.org/10.3390/polym14153022
Chicago/Turabian StyleGao, Liwei, Xinjie Zhao, Meng Liu, and Xiangzhong Zhao. 2022. "Characterization and Antibacterial Activities of Carboxymethylated Paramylon from Euglena gracilis" Polymers 14, no. 15: 3022. https://doi.org/10.3390/polym14153022
APA StyleGao, L., Zhao, X., Liu, M., & Zhao, X. (2022). Characterization and Antibacterial Activities of Carboxymethylated Paramylon from Euglena gracilis. Polymers, 14(15), 3022. https://doi.org/10.3390/polym14153022







