Graft Polymerization of Stearyl Methacrylate on PET Track-Etched Membranes for Oil–Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Modification of Track-Etched Membranes (TeMs)
2.3. Methods of Characterization
2.4. Performance of Membranes in Oil–Water Separation
3. Results
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halder, J.; Islam, N. Water Pollution and Its Impact on the Human Health. J. Environ. Hum. 2015, 2, 36–46. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Wang, Y.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A Review on Oil/Water Emulsion Separation Membrane Material. J. Environ. Chem. Eng. 2022, 10, 107257. [Google Scholar] [CrossRef]
- Ali, N.; Bilal, M.; Khan, A.; Ali, F.; Iqbal, H.M.N. Design, Engineering and Analytical Perspectives of Membrane Materials with Smart Surfaces for Efficient Oil/Water Separation. TrAC Trends Anal. Chem. 2020, 127, 115902. [Google Scholar] [CrossRef]
- Qiao, A.; Huang, R.; Penkova, A.; Qi, W.; He, Z.; Su, R. Superhydrophobic, Elastic and Anisotropic Cellulose Nanofiber Aerogels for Highly Effective Oil/Water Separation. Sep. Purif. Technol. 2022, 295, 121266. [Google Scholar] [CrossRef]
- Dou, Y.-L.; Yue, X.; Lv, C.-J.; Yasin, A.; Hao, B.; Su, Y.; Ma, P.-C. Dual-Responsive Polyacrylonitrile-Based Electrospun Membrane for Controllable Oil-Water Separation. J. Hazard. Mater. 2022, 438, 129565. [Google Scholar] [CrossRef]
- Kasemset, S.; Lee, A.; Miller, D.J.; Freeman, B.D.; Sharma, M.M. Effect of Polydopamine Deposition Conditions on Fouling Resistance, Physical Properties, and Permeation Properties of Reverse Osmosis Membranes in Oil/Water Separation. J. Memb. Sci. 2013, 425–426, 208–216. [Google Scholar] [CrossRef]
- Jamshidi Gohari, R.; Korminouri, F.; Lau, W.J.; Ismail, A.F.; Matsuura, T.; Chowdhury, M.N.K.; Halakoo, E.; Jamshidi Gohari, M.S. A Novel Super-Hydrophilic PSf/HAO Nanocomposite Ultrafiltration Membrane for Efficient Separation of Oil/Water Emulsion. Sep. Purif. Technol. 2015, 150, 13–20. [Google Scholar] [CrossRef]
- Prince, J.A.; Bhuvana, S.; Anbharasi, V.; Ayyanar, N.; Boodhoo, K.V.K.; Singh, G. Ultra-Wetting Graphene-Based PES Ultrafiltration Membrane—A Novel Approach for Successful Oil-Water Separation. Water Res. 2016, 103, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Dudchenko, A.; Gu, X.; Jassby, D. Surfactant-Stabilized Oil Separation from Water Using Ultrafiltration and Nanofiltration. J. Memb. Sci. 2017, 529, 159–169. [Google Scholar] [CrossRef]
- Muppalla, R.; Jewrajka, S.K.; Reddy, A.V.R. Fouling Resistant Nanofiltration Membranes for the Separation of Oil–Water Emulsion and Micropollutants from Water. Sep. Purif. Technol. 2015, 143, 125–134. [Google Scholar] [CrossRef]
- Kalla, S. Use of Membrane Distillation for Oily Wastewater Treatment—A Review. J. Environ. Chem. Eng. 2021, 9, 104641. [Google Scholar] [CrossRef]
- Dong, B.B.; Wang, F.H.; Yang, M.Y.; Yu, J.L.; Hao, L.Y.; Xu, X.; Wang, G.; Agathopoulos, S. Polymer-Derived Porous SiOC Ceramic Membranes for Efficient Oil-Water Separation and Membrane Distillation. J. Memb. Sci. 2019, 579, 111–119. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O.; Bessbousse, H.; Wade, T.L.; Beuneu, F.; Clochard, M.-C. Nanopore Size Tuning of Polymeric Membranes Using the RAFT-Mediated Radical Polymerization. J. Memb. Sci. 2013, 445, 135–145. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Gorin, Y.G.; Kozlovskiy, A.L.; Zdorovets, M.V.; Zhidkov, I.S.; Cholach, S.O. Electron/Gamma Radiation-Induced Synthesis and Catalytic Activity of Gold Nanoparticles Supported on Track-Etched Poly(Ethylene Terephthalate) Membranes. Mater. Chem. Phys. 2018, 217, 31–39. [Google Scholar] [CrossRef]
- Ma, T.; Janot, J.; Balme, S. Track-Etched Nanopore/Membrane: From Fundamental to Applications. Small Methods 2020, 4, 2000366. [Google Scholar] [CrossRef]
- Apel, P.Y. Fabrication of Functional Micro- and Nanoporous Materials from Polymers Modified by Swift Heavy Ions. Radiat. Phys. Chem. 2019, 159, 25–34. [Google Scholar] [CrossRef]
- Husaini, S.N.; Zaidi, J.H.; Malik, F.; Arif, M. Application of Nuclear Track Membrane for the Reduction of Pollutants in the Industrial Effluent. Radiat. Meas. 2008, 43 (Suppl. S1), S607–S611. [Google Scholar] [CrossRef]
- Yamazaki, I.M.; Paterson, R.; Geraldo, L.P. A New Generation of Track Etched Membranes for Microfiltration and Ultrafiltration. Part I. Preparation and Characterisation. J. Memb. Sci. 1996, 118, 239–245. [Google Scholar] [CrossRef]
- Yeszhanov, A.B.; Korolkov, I.V.; Dosmagambetova, S.S.; Zdorovets, M.V.; Güven, O. Recent Progress in the Membrane Distillation and Impact of Track-Etched Membranes. Polymers 2021, 13, 2520. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Grafting in Confined Spaces: Functionalization of Nanochannels of Track-Etched Membranes. Radiat. Phys. Chem. 2014, 105, 26–30. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zou, C.; Shao, J. Study of Plasma-Induced Graft Polymerization of Stearyl Methacrylate on Cotton Fabric Substrates. Appl. Surf. Sci. 2015, 357, 2327–2332. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Gorin, Y.G.; Zdorovets, M.V. Protein Fouling of Modified Microporous PET Track-Etched Membranes. Radiat. Phys. Chem. 2018, 151, 141–148. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, C.; Zheng, L.; Shan, F.; Chen, G. Aqueous Broadband Photopolymerization on Microreactor Arrays: From High Throughput Polymerization to Fabricating Artificial Cells. Polym. Chem. 2022, 13, 989–996. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Miller, K.A.; Zhu, H.; Egap, E. Lead Halide Perovskite Nanocrystals as Photocatalysts for PET-RAFT Polymerization under Visible and Near-Infrared Irradiation. ACS Macro Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Korolkov, I.V.; Narmukhamedova, A.R.; Melnikova, G.B.; Muslimova, I.B.; Yeszhanov, A.B.; Zhatkanbayeva, Z.K.; Chizhik, S.A.; Zdorovets, M.V. Preparation of Hydrophobic PET Track-Etched Membranes for Separation of Oil–Water Emulsion. Membranes 2021, 11, 637. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Borgekov, D.; Kenzhina, I.; Zdorovets, M.; Korolkov, I.; Kaniukov, E.; Kutuzau, M.; Shumskaya, A. PET Ion-Track Membranes: Formation Features and Basic Applications. Springer Proc. Phys. 2019, 221, 461–479. [Google Scholar] [CrossRef]
- He, D.; Susanto, H.; Ulbricht, M. Photo-Irradiation for Preparation, Modification and Stimulation of Polymeric Membranes. Prog. Polym. Sci. 2009, 34, 62–98. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Springer Series; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Mulder, M. Transport in Membranes. In Basic Principles of Membrane Technology; Springer: Dordrecht, The Netherlands, 1996; pp. 210–279. [Google Scholar]
- Tung, N.T.; Duc, N.T.; Thu Ha, P.T.; Van Khoi, N.; Son, N.T. Graft Polymerization of Lauryl Methacrylate onto Bamboo Fiber—A Potential Material for Oil Spills. Polym. Polym. Compos. 2022, 30, 096739112210931. [Google Scholar] [CrossRef]
- Daugaard, A.E.; Jankova, K.; Hvilsted, S. Poly(Lauryl Acrylate) and Poly(Stearyl Acrylate) Grafted Multiwalled Carbon Nanotubes for Polypropylene Composites. Polymer 2014, 55, 481–487. [Google Scholar] [CrossRef]
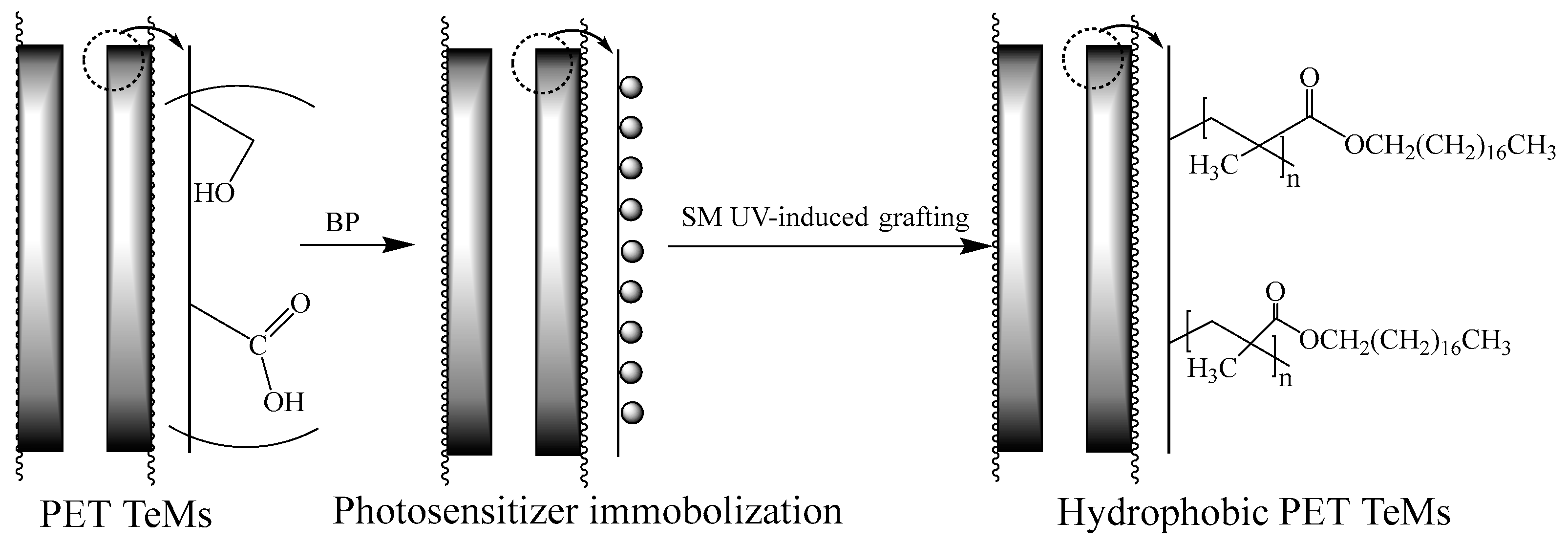

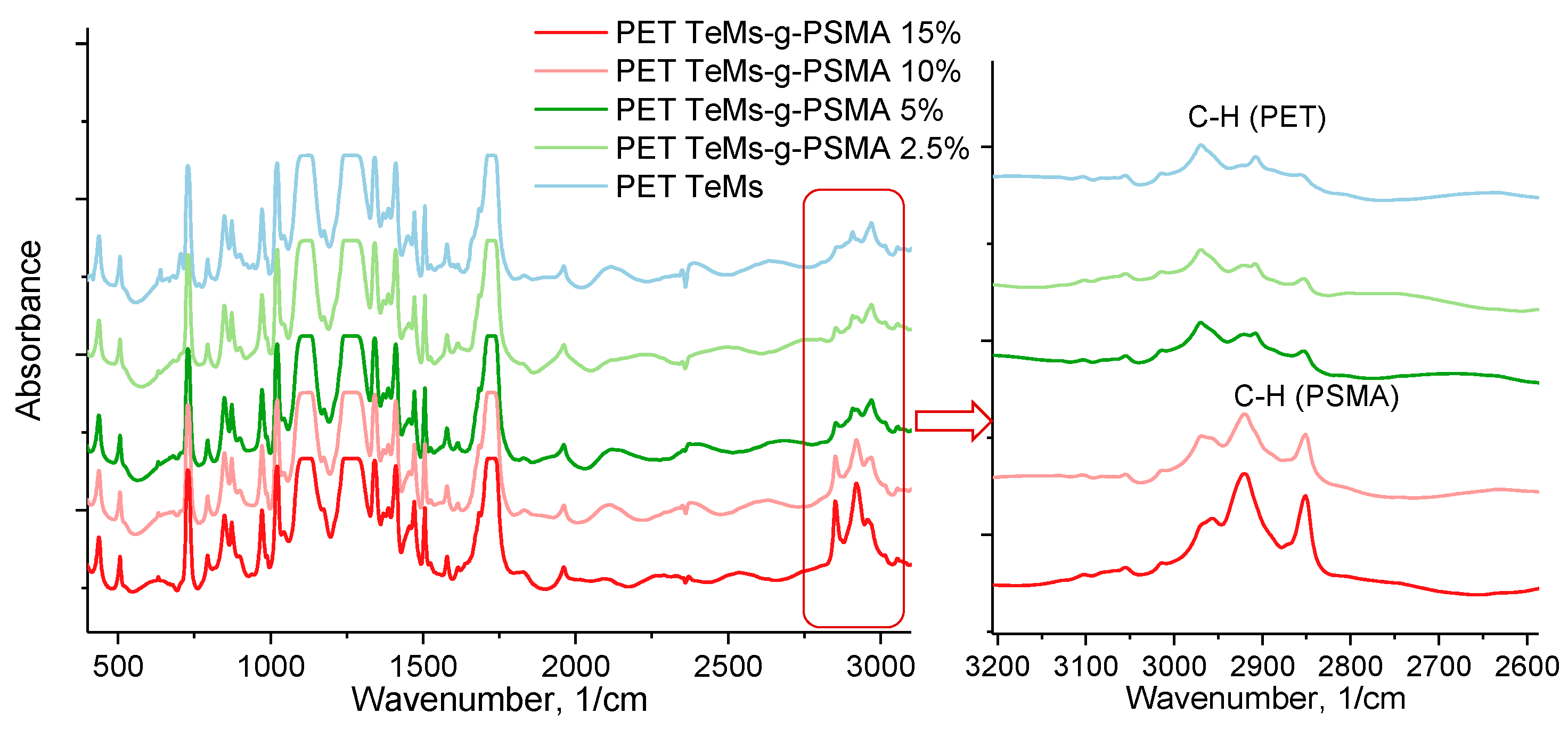



| Time of Irradiation, Min | Monomer Concentration, % | Degree of Grafting, % | Effective Pore Diameter, nm |
|---|---|---|---|
| 0 | - | - | 350 ± 30 * |
| 30 | 5 | 2.4 | 326 ± 5 |
| 45 | 5 | 2.9 | 322 ± 4 |
| 60 | 5 | 3.6 | 319 ± 5 |
| 90 | 5 | 7.1 | 283 ± 4 |
| 120 | 5 | 15.1 | 226 ± 2 |
| 60 | 1 | 0 | 345 ± 5 |
| 60 | 2.5 | 2.8 | 321 ± 5 |
| 60 | 10 | 10.7 | 216 ± 6 |
| 60 | 15 | 53.1 | - |
| 0 | - | - | 3050 ± 30 ** |
| 30 | 5 | 2.3 | 3055 ± 25 |
| 45 | 5 | 2.7 | 2995 ± 28 |
| 60 | 5 | 3.4 | 2984 ± 26 |
| 90 | 5 | 6.5 | 2910 ± 25 |
| 120 | 5 | 14.0 | 2920 ± 32 |
| 60 | 1 | 0 | 3040 ± 28 |
| 60 | 2.5 | 2.4 | 2990 ± 25 |
| 60 | 10 | 10.8 | 2935 ± 29 |
| 60 | 15 | 45.2 | 2746 ± 35 |
| 60 | 5 | 3.40 | 2095 ± 25 *** |
| 60 | 5 | 3.35 | 2465 ± 32 *** |
| 60 | 5 | 3.51 | 2785 ± 30 *** |
| Time, Min | Concentration, % | Degree of Grafting, % | θ, Water | θ, Diiodomethane | γ, mJ/m2 | γp, mJ/m2 |
|---|---|---|---|---|---|---|
| 0 | - | - | 80.1 | 25.7 | 48.3 | 2.4 |
| 30 | 5% | 2.3 | 99.6 | 53.0 | 32.9 | 0.3 |
| 60 | 5% | 3.4 | 109.0 | 71.1 | 22.4 | 0.1 |
| 90 | 5% | 6.5 | 99.1 | 54.8 | 32.0 | 0.4 |
| 120 | 5% | 14.0 | 99.4 | 39.7 | 39.8 | 0.1 |
| 60 | 10% | 10.8 | 99.7 | 61.1 | 28.6 | 0.6 |
| 60 | 15% | 45.2 | 94.0 | 30.3 | 28.8 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeszhanov, A.B.; Muslimova, I.B.; Melnikova, G.B.; Petrovskaya, A.S.; Seitbayev, A.S.; Chizhik, S.A.; Zhappar, N.K.; Korolkov, I.V.; Güven, O.; Zdorovets, M.V. Graft Polymerization of Stearyl Methacrylate on PET Track-Etched Membranes for Oil–Water Separation. Polymers 2022, 14, 3015. https://doi.org/10.3390/polym14153015
Yeszhanov AB, Muslimova IB, Melnikova GB, Petrovskaya AS, Seitbayev AS, Chizhik SA, Zhappar NK, Korolkov IV, Güven O, Zdorovets MV. Graft Polymerization of Stearyl Methacrylate on PET Track-Etched Membranes for Oil–Water Separation. Polymers. 2022; 14(15):3015. https://doi.org/10.3390/polym14153015
Chicago/Turabian StyleYeszhanov, Arman B., Indira B. Muslimova, G. B. Melnikova, A. S. Petrovskaya, Aibek S. Seitbayev, S. A. Chizhik, Nariman K. Zhappar, Ilya V. Korolkov, Olgun Güven, and Maxim V. Zdorovets. 2022. "Graft Polymerization of Stearyl Methacrylate on PET Track-Etched Membranes for Oil–Water Separation" Polymers 14, no. 15: 3015. https://doi.org/10.3390/polym14153015
APA StyleYeszhanov, A. B., Muslimova, I. B., Melnikova, G. B., Petrovskaya, A. S., Seitbayev, A. S., Chizhik, S. A., Zhappar, N. K., Korolkov, I. V., Güven, O., & Zdorovets, M. V. (2022). Graft Polymerization of Stearyl Methacrylate on PET Track-Etched Membranes for Oil–Water Separation. Polymers, 14(15), 3015. https://doi.org/10.3390/polym14153015








