Sulfation of Wheat Straw Soda Lignin with Sulfamic Acid over Solid Catalysts
Abstract
1. Introduction
2. Materials and Methods
Calculation Details
3. Results
3.1. Synthesis of the WSSL Sulfonic Derivatives
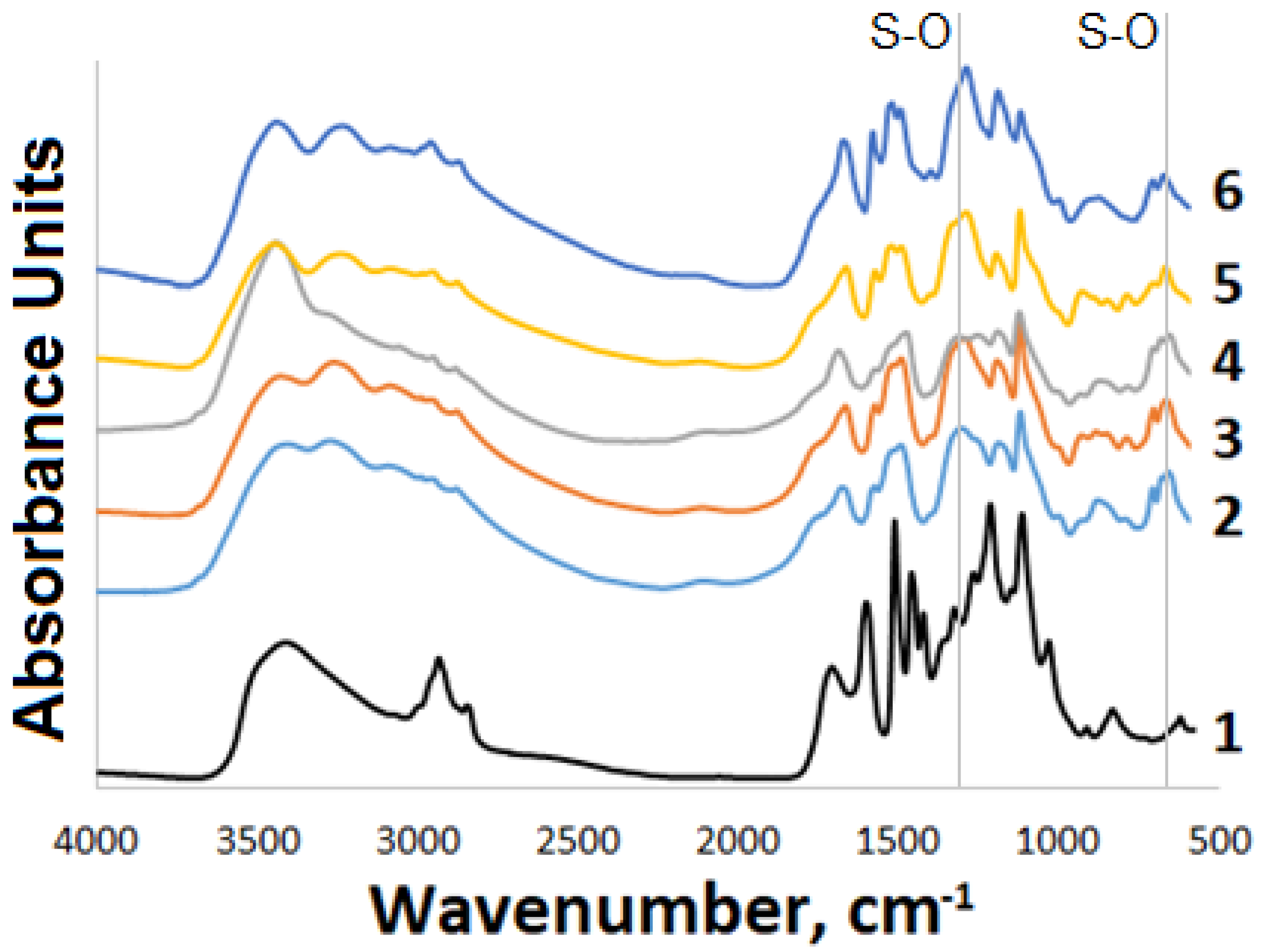
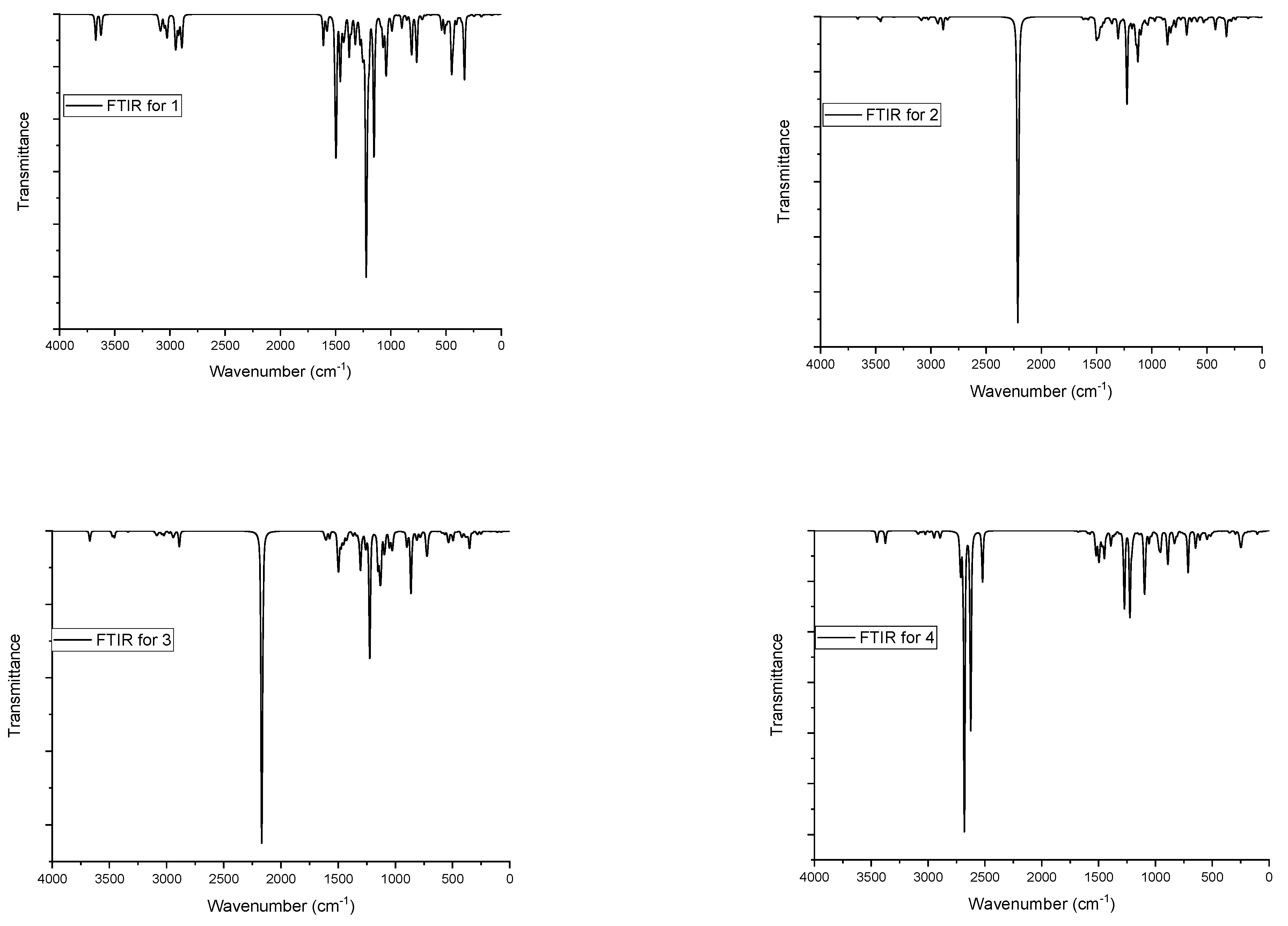
3.2. FTIR Study
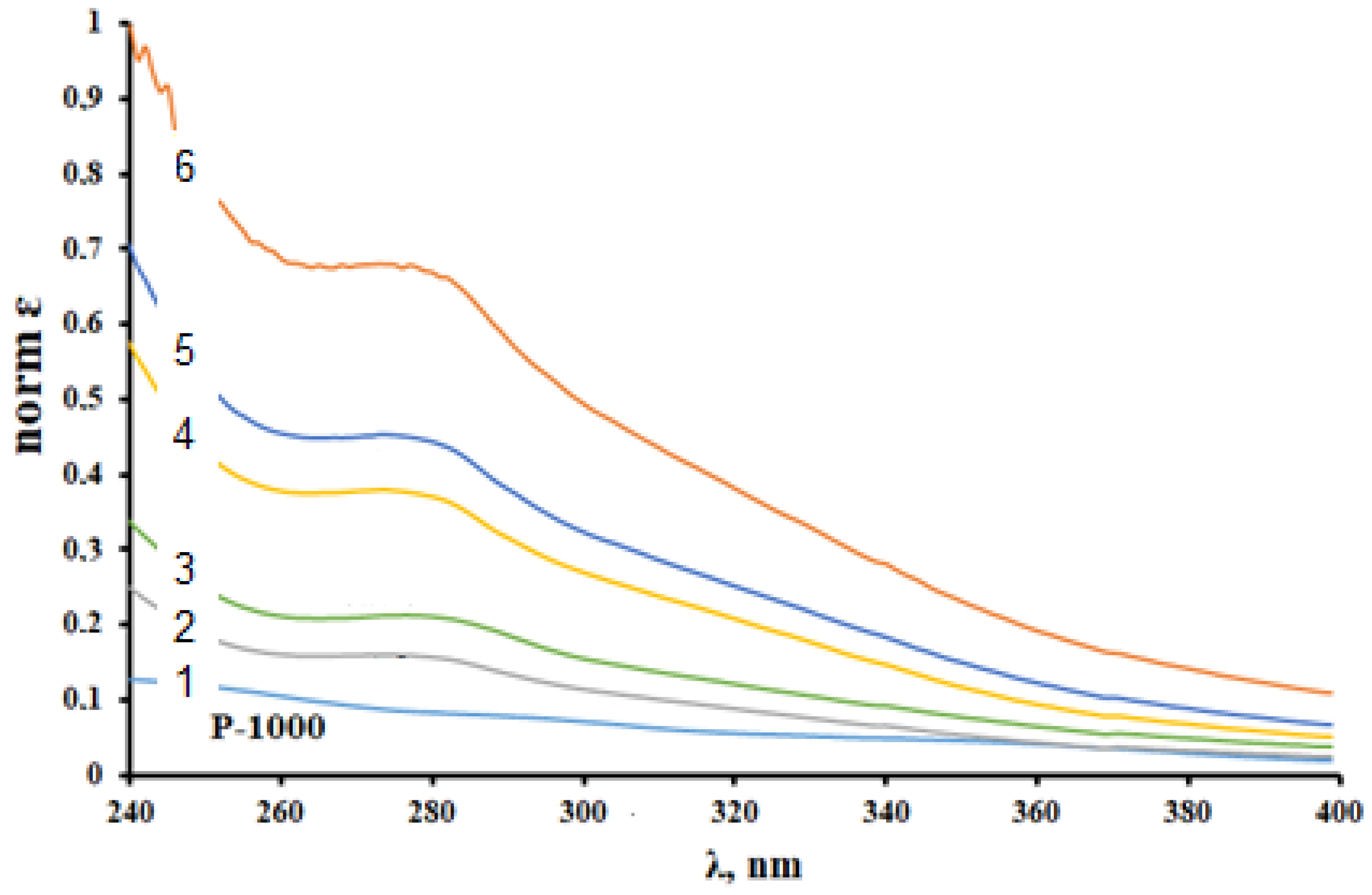
3.3. UV–Vis
3.4. GPC
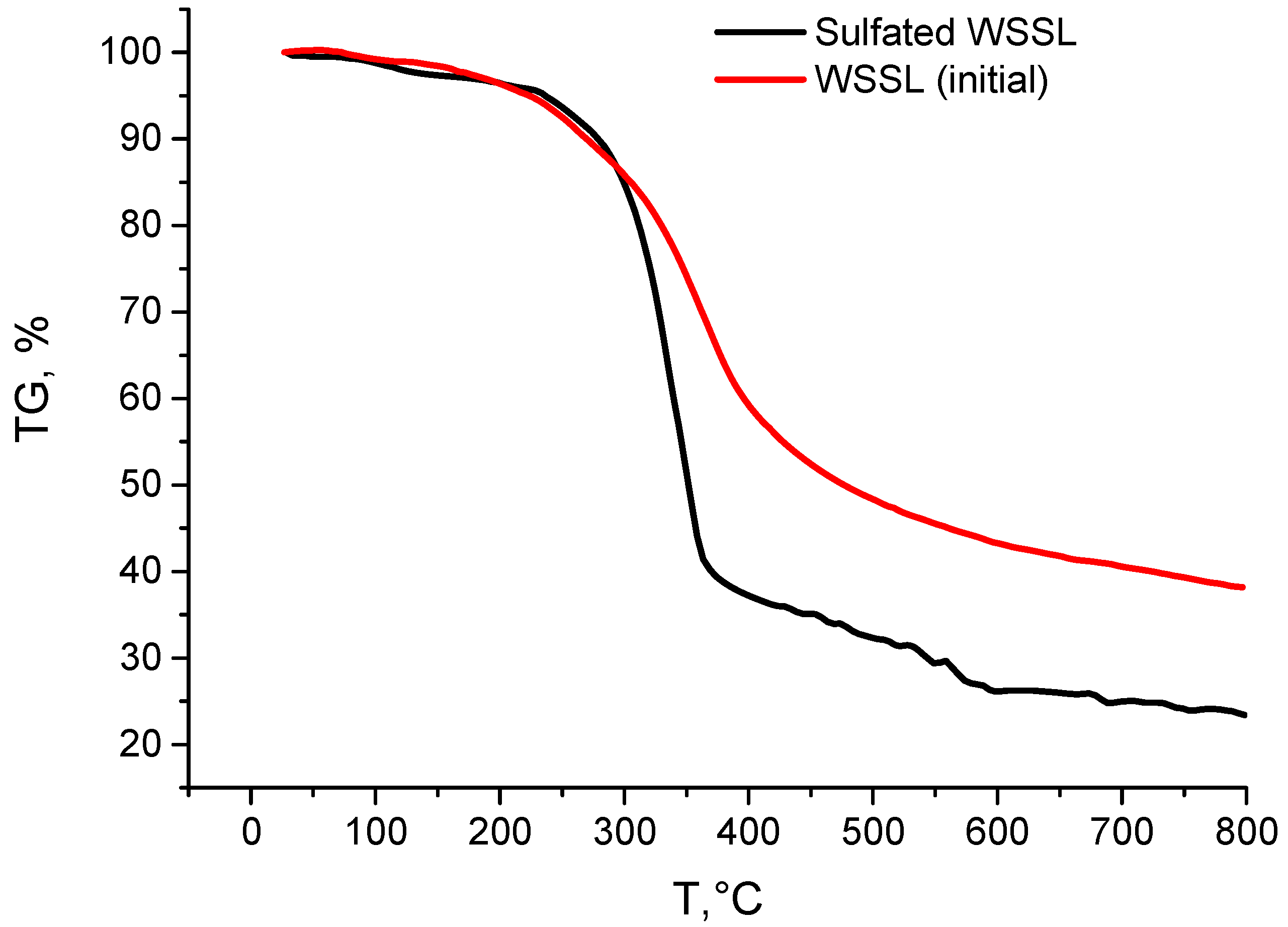
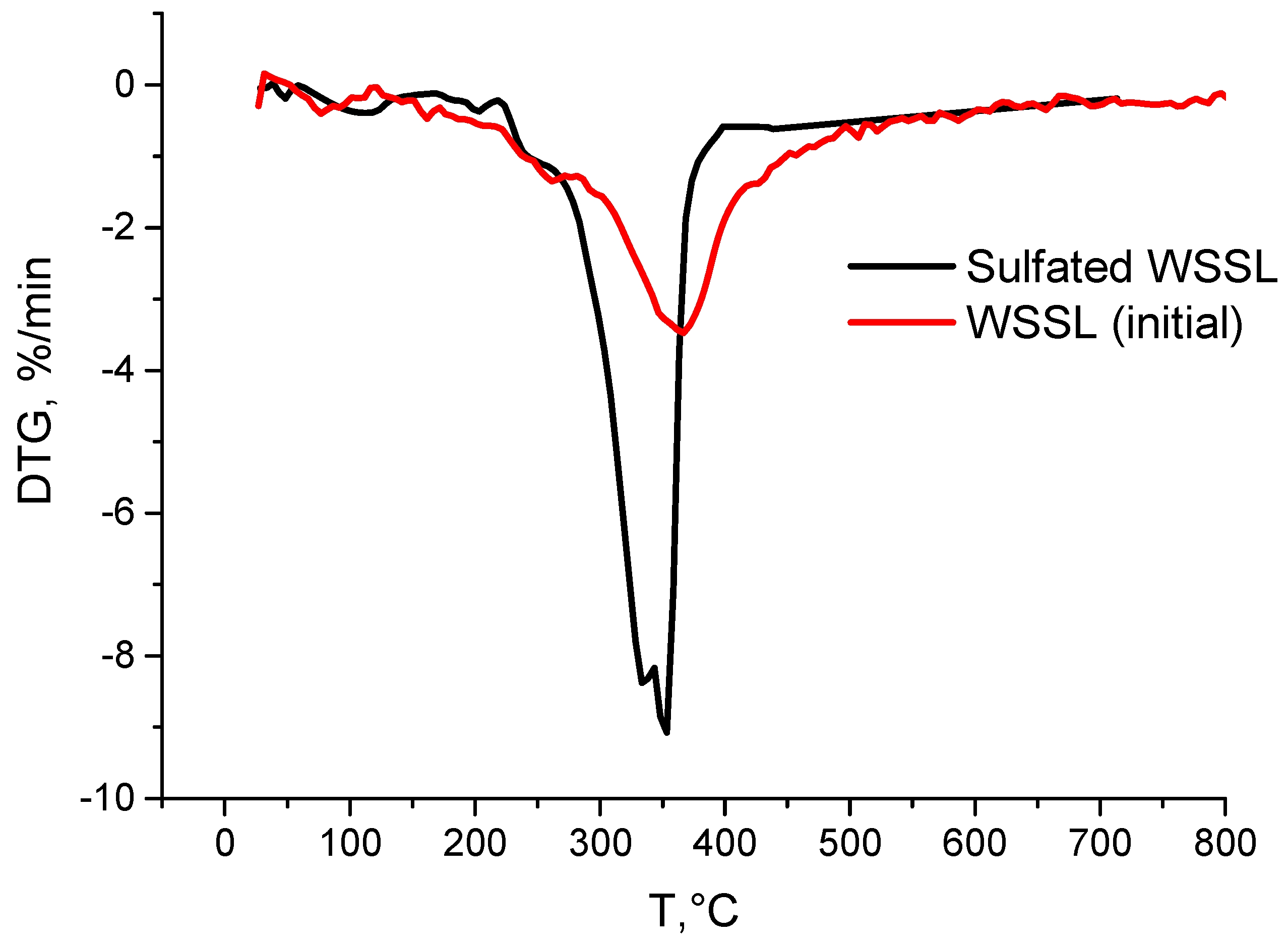
3.5. TGA/DSC
3.6. AFM
3.7. DFT Analysis
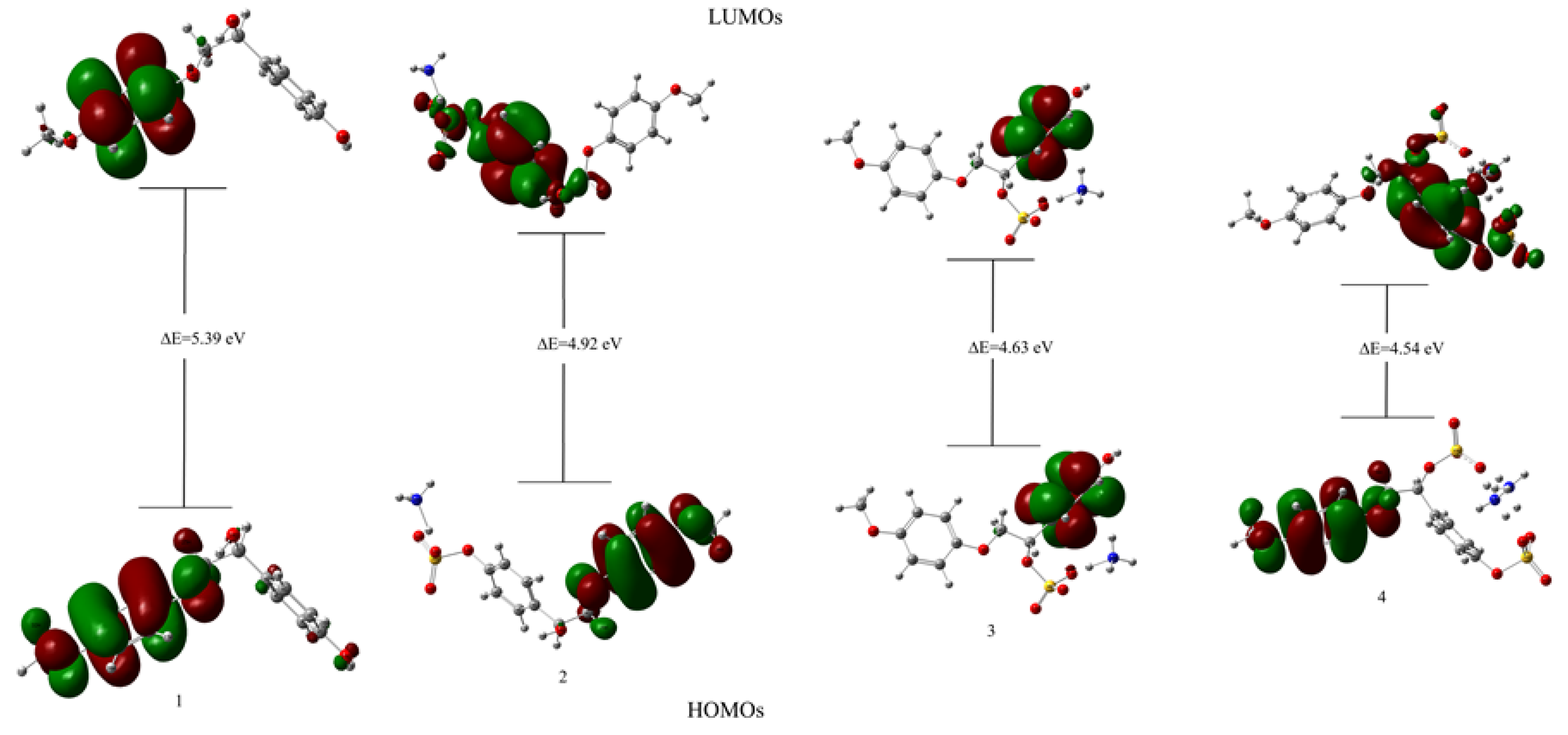
3.7.1. Structure Optimization and HOMO–LUMO Analysis
3.7.2. MEP Analysis
3.7.3. Mulliken Atomic Charge Analysis
3.7.4. Theoretical FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Alzate, C.A.C. The potential use of lignin as a platform product in biorefineries: A review. Renew. Sustain. Energy Rev. 2021, 138, 110688. [Google Scholar] [CrossRef]
- Spiridon, I. Extraction of lignin and therapeutic applications of lignin-derived compounds. A review. Environ. Chem. Lett. 2020, 18, 771–785. [Google Scholar] [CrossRef]
- Bajpai, P. Recent developments in cleaner production. In Environmentally Friendly Production of Pulp and Paper; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 264–344. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Malyar, Y.N.; Kuznetsova, S.A.; Grishechko, L.I.; Kazachenko, A.S.; Levdansky, A.V.; Pestunov, A.V.; Boyandin, A.N.; Celzar, A. Isolation, Study and Application of Organosolv Lignins (Review). J. Sib. Fed. Univ. Chem. 2016, 4, 454–482. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Bidgoli, N.S.S.; Shafiei, N.; Momenbeik, F. Biomass valorization: Sulfated lignin-catalyzed production of 5-hydroxymethylfurfural from fructose. Int. J. Biol. Macromol. 2021, 182, 59–64. [Google Scholar] [CrossRef]
- Cabral Almada, C.; Kazachenko, A.; Fongarland, P.; Da Silva Perez, D.; Kuznetsov, B.N.; Djakovitch, L. Supported-Metal Catalysts in Upgrading Lignin to Aromatics by Oxidative Depolymerization. Catalysts 2021, 11, 467. [Google Scholar] [CrossRef]
- Baryshnikov, S.V.; Miroshnikova, A.V.; Kazachenko, A.S.; Malyar, Y.N.; Taran, O.P.; Lavrenov, A.V.; Djakovitch, L.; Kuznetsov, B.N. Hydrogenation of abies wood and ethanol lignin by hydrogen in supercritical ethanol in the presence of bifunctional catalyst J. Sib. Fed. Univ. Chem. 2019, 12, 550–561. [Google Scholar] [CrossRef]
- Tarabanko, V.E. Catalytic Conversion of Lignins for Valuable Chemicals. Catalysts 2021, 11, 1254. [Google Scholar] [CrossRef]
- Deineko, I.L. Utilization of lignins: Achievements, problems and prospects. Chem. Plant Raw Mater. 2003, 5–20. (In Russian) [Google Scholar]
- Simonova, V.V.; Shendrik, T.G.; Kuznetsov, B.N. Methods of utilization of technical lignins. J. Sib. Fed. Univ. Chem. 2014, 4, 340–354. [Google Scholar]
- Agrawal, A.; Kaushik, N.; Biswas, S. Derivatives and Applications of Lignin—An Insight. SciTech J. 2014, 1, 30–36. [Google Scholar]
- Raghuraman, A.; Tiwari, V.; Zhao, Q.; Shukla, D.; Debnath, A.K.; Desai, U.R. Viral Inhibition Studies on Sulfated Lignin, a Chemically Modified Biopolymer and a Potential Mimic of Heparan Sulfate. Biomacromolecules 2007, 8, 1759–1763. [Google Scholar] [CrossRef]
- Henry, B.L.; Desai, U.R. Sulfated low molecular weight lignins, allosteric inhibitors of coagulation proteinases via the heparin binding site, significantly alter the active site of thrombin and factor xa compared to heparin. Thromb. Res. 2014, 134, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Malyar, Y.N.; Vasil’yeva, N.Y.; Kazachenko, A.S.; Skvortsova, G.P.; Korol’kova, I.V.; Kuznetsova, S.A. Sulfation of Abies Ethanol Lignin by Complexes of Sulfur Trioxide with 1,4-Dioxane and Pyridine. Russ. J. Bioorgan. Chem. 2021, 47, 1368–1375. [Google Scholar] [CrossRef]
- Leonov, V.V.; Fursova, E.Y.; Galochkin, A.I. Homogeneous synthesis of sulphoesters of ligno-carbohydrate materials as promising reagents for the preparation of drilling fluids. Bull. Ugra State Univ. 2006, 78–79. (In Russian) [Google Scholar]
- Kazachenko, A.S.; Medimagh, M.; Issaoui, N.; Al-Dossary, O.; Wojcik, M.J.; Kazachenko, A.S.; Miroshnokova, A.V.; Malyar, Y.N. Sulfamic acid/water complexes (SAA-H2O(1-8)) intermolecular hydrogen bond interactions: FTIR,X-ray, DFT and AIM analysis. J. Mol. Struct. 2022, 1265, 133394. [Google Scholar] [CrossRef]
- Caputo, H.E.; Straub, J.E.; Grinstaff, M.W. Design, synthesis, and biomedical applications of synthetic sulphated polysaccharides. Chem. Soc. Rev. 2019, 48, 2338–2365. [Google Scholar] [CrossRef] [PubMed]
- Al-Horani, R.A.; Desai, U.R. Chemical sulfation of small molecules—Advances and challenges. Tetrahedron 2010, 66, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Visanko, M. Lignin-rich sulfated wood nanofibers as high-performing adsorbents for the removal of lead and copper from water. J. Hazard. Mater. 2020, 383, 121174. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Fetisova, O.Y.; Chudina, A.I.; Sudakova, I.G.; Antonov, A.V.; Borovkova, V.S.; Kuznetsova, S.A. Isolation and sulfation of galactoglucomannan from larch wood (Larix sibirica). Wood Sci. Technol. 2021, 55, 1091–1107. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Vasilieva, N.Y.; Borovkova, V.S.; Fetisova, O.Y.; Issaoui, N.; Malyar, Y.N.; Elsuf’ev, E.V.; Karacharov, A.A.; Skripnikov, A.M.; Miroshnikova, A.V.; et al. Food Xanthan Polysaccharide Sulfation Process with Sulfamic Acid. Foods 2021, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Borovkova, V.S.; Issaoui, N. Optimization of guar gum galactomannan sulfation process with sulfamic acid. Biomass Convers. Biorefin. 2021, 1–10. [Google Scholar] [CrossRef]
- Malyar, Y.N.; Kazachenko, A.S.; Vasilyeva, N.Y.; Fetisova, O.Y.; Borovkova, V.S.; Miroshnikova, A.V.; Levdansky, A.V.; Skripnikov, A.M. Sulfation of wheat straw soda lignin: Role of solvents and catalysts. Catal. Today 2022, 397–399, 397–406. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Vasilyeva, N.Y.; Kazachenko, A.S.; Skvortsova, G.P.; Levdansky, V.A.; Lutoshkin, M.A. Development of a method for sulfation of ethanolignin of fir wood using sulfamic acid. J. Sib. Fed. Univ. Ser. Chem. 2018, 11, 122–130. [Google Scholar]
- Kuznetsov, B.N.; Vasilyeva, N.Y.; Kazachenko, A.S.; Levdansky, V.A.; Kondrasenko, A.A.; Malyar, Y.N.; Skvortsova, G.P.; Lutoshkin, M.A. Optimization of the process of abies ethanol lignin sulfation by sulfamic acid–urea mixture in 1,4-dioxane medium. Wood Sci. Technol. 2020, 54, 365–381. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Vasilyeva, N.Y.; Sudakova, I.G.; Levdansky, V.A.; Lutoshkin, M.A.; Kuznetsov, B.N. Numerical optimization of the abies ethanol lignin sulfation process with sulfamic acid in 1,4-dioxane medium in the presence of urea. J. Sib. Fed. Univ. Chem. 2020, 13, 232–246. [Google Scholar] [CrossRef]
- Levdansky, V.; Vasilyeva, N.; Malyar, Y.; Levdansky, A.; Kondrasenko, A.; Kazachenko, A.; Kuznetsov, B. Sulfation of ethanol lignin of abies wood by sulfamic acid in N,N-dimethylformamide medium. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Xie, M.; Chen, Z.; Xia, Y.; Lin, M.; Li, J.; Lan, W.; Zhang, L.; Yue, F. Influence of the Lignin Extraction Methods on the Content of Tricin in Grass Lignins. Front. Energy Res. 2021, 9, 756285. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, L.; Liu, D. Characterization and comparison of Acetosolv and Milox lignin isolated from crofton weed stem. J. Appl. Polym. Sci. 2009, 114, 1295–1302. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef]
- Taran, O.P.; Polyanskaya, E.M.; Ogorodnikova, O.L.; Descorme, C.; Besson, M.; Parmon, V.N. Sibunit-based catalytic materials for the deep oxidation of organic ecotoxicants in aqueous solution: I. Surface properties of the oxidized sibunit samples. Catal. Ind. 2010, 2, 381–386. [Google Scholar] [CrossRef]
- Gromov, N.V.; Taran, O.P.; Yatsenko, D.A.; Ayupov, A.B.; Loppinet-Serani, A.; Aymonier, C.; Agabekov, V.E. Development of Sulfonated Catalysts Based on Graphite-Like Carbon Sibunit for Cellulose Hydrolysis J. Agric. Food Chem. 2014, 1, 87–99. [Google Scholar]
- Koskin, A.P.; Larichev, Y.V.; Mishakov, I.V.; Mel’gunov, M.S.; Vedyagin, A.A. Synthesis and characterization of carbon nanomaterials functionalized by direct treatment with sulfonating agents. Microporous Mesoporous Mater. 2020, 299, 110130. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Vasilieva, N.Y.; Fetisova, O.Y.; Sychev, V.V.; Elsuf’ev, E.V.; Malyar, Y.N.; Issaoui, N.; Miroshnikova, A.V.; Borovkova, V.S.; Kazachenko, A.S.; et al. New reactions of betulin with sulfamic acid and ammonium sulfamate in the presence of solid catalysts. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Meng, X.; Bhagia, S.; Wang, Y.; Zhou, Y.; Pu, Y.; Dunlap, J.R.; Shuai, L.; Ragauskas, A.J.; Yoo, C.G. Effects of the advanced organosolv pretreatment strategies on structural properties of woody biomass. Ind. Crops. Prod. 2020, 146, 112144. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, C.01 ed.; Gaussian, Inc.: Wallingford, CT, UK, 2009. [Google Scholar]
- Dennington, R.; Keith, T. Millam J Gauss View, 5th ed.; Semichem Inc.: Shawnee Mission, KS, USA, 2010. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Bondarenko, G.N.; Korolkova, I.V.; Antonov, A.V.; Karacharov, A.A.; Fetisova, O.Y.; Skvortsova, G.P. «Green» synthesis and characterization of galactomannan sulfates obtained using sulfamic acid. Biomass Convers. Biorefin. 2020, 12, 2705–2714. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Ukkola, J.; Liimatainen, H. Direct sulfation of cellulose fibers using a reactive deep eutectic solvent to produce highly charged cellulose nanofibers. Cellulose 2019, 26, 2303–2316. [Google Scholar] [CrossRef]
- Akman, F.; Kazachenko, A.S.; Vasilyeva, N.Y.; Malyar, Y.N. Synthesis and characterization of starch sulfates obtained by the sulfamic acid-urea complex. J. Mol. Struct. 2020, 1208, 127899. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Vasilyeva, N.Y.; Mikova, N.M.; Zhizhaev, A.M. Synthesis and Sulfation with Sulfamic Acid of Aerogels Based on Birch-Wood and Cotton Celluloses. J. Sib. Fed. Univ. Chem. 2022, 15, 57–68. [Google Scholar] [CrossRef]
- Diebold, U. Structure and properties of TiO2 surfaces: A brief review. Appl. Phy. A 2003, 76, 681–687. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Tarabanko, V.E.; Miroshnikova, A.V.; Sychev, V.V.; Skripnikov, A.M.; Malyar, Y.N.; Mikhlin, Y.L.; Baryshnikov, S.V.; Taran, O.P. Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts. Catalysts 2021, 11, 42. [Google Scholar] [CrossRef]
- Polyanskaya, E.; Taran, O. Study of functional groups on the surface of the oxidized carbon material Sibunit by acid-base titration and XPS. Vestn. Тomskogo Gos. Universiteta. Khimiya 2017, 6–26. (In Russian) [Google Scholar] [CrossRef]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Titanium Dioxide as a Catalyst Support in Heterogeneous Catalysis. Sci. World J. 2014, 2014, 727496. [Google Scholar] [CrossRef]
- Romanova, R.G.; Petrova, E.V. Acid-base properties of the surface of aluminum oxides. Bull. Kazan Technol. Univ. 2006, 73–90. (In Russian) [Google Scholar]
- Shilova, O.A.; Panova, G.G.; Mjakin, S.V.; Kovalenko, A.S.; Nikolaev, A.M.; Chelibanov, V.P.; Chelibanov, I.V.; Yasenko, E.A.; Kornyukhin, D.L.; Artem’eva, A.M.; et al. Structure, Properties, and Phytoprotective Functions of Titanium Dioxide Nanopowders and Their Aqueous Suspensions. Russ. J. Inorg. Chem. 2021, 66, 765–772. [Google Scholar] [CrossRef]
- Nagasawa, K.; Yoshidome, H. Alcoholysis Reaction of Sulfamic Acids and Its Application to the Preparation of biochemically Related Sulfate Esters. Chem. Pharm. Bull. 1970, 18, 2023–2027. [Google Scholar] [CrossRef][Green Version]
- Huijgen, W.J.J.; Telysheva, G.; Arshanitsa, A.; Gosselink, R.J.A.; de Wild, P.J. Characteristics of wheat straw lignins from ethanol-based organosolv treatment. Ind. Crops. Prod. 2014, 59, 85–95. [Google Scholar] [CrossRef]
- Fisher, T.; Hajaligol, M.; Waymack, B.; Kellogg, D. Pyrolysis behavior and kinetics of biomass derived materials. J. Anal. Appl. Pyrolysis 2002, 62, 331–349. [Google Scholar] [CrossRef]
- Wittkowski, R.; Ruther, J.; Drinda, H.; Rafiei-Taghanaki, F. Formation of smoke flavor compounds by thermal lignin degradation. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992; pp. 232–243. [Google Scholar]
- Hansen, B.; Kusch, P.; Schulze, M.; Kamm, B. Qualitative and Quantitative Analysis of Lignin Produced from Beech Wood by Different Conditions of the Organosolv Process. J. Polym. Environ. 2016, 24, 85–97. [Google Scholar] [CrossRef]
- Kong, L.; Yu, L.; Feng, T.; Yin, X.; Liu, T.; Dong, L. Physicochemical characterization of the polysaccharide from Bletilla striata: Effect of drying method. Carbohydr. Polym. 2015, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Feng, D.; Liu, X.; Yang, T.; Guo, L.; Liang, J.; Liang, J.; Hu, F.; Cui, F.; Feng, S. Structure and antioxidant activity study of sulfated acetamido-polysaccharide from Radix Hedysari. Fitoterapia 2013, 89, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bao, A.; Meng, X.; Guo, H.; Zhang, Y.; Zhao, Y.; Kong, W.; Liang, J.; Yao, J.; Zhang, J. An efficient approach to prepare sulfated polysaccharide and evaluation of anti-tumor activities in vitro. Carbohydr. Polym. 2018, 184, 366–375. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Dong, K.; Fan, M.; Zhang, S. A DFT study on lignin dissolution in imidazolium-based ionic liquids. RSC Adv. 2017, 7, 12670–12681. [Google Scholar] [CrossRef]
- Ji, H.; Lv, P. Mechanistic insights into the lignin dissolution behaviors of a recyclable acid hydrotrope, deep eutectic solvent (DES), and ionic liquid (IL). Green Chem. 2020, 22, 1378–1387. [Google Scholar] [CrossRef]
- Mostaghni, F.; Teimouri, A.; Mirshokraei, S.A. Synthesis, spectroscopic characterization and DFT calculations of β-O-4 type lignin model compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 110, 430–436. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Gordobil, O.; Diaz, R.H.; Sandak, A.; Sandak, J. Fractionation of lignin using organic solvents: A combined experimental and theoretical study. Int. J. Biol. Macromol. 2021, 168, 792–805. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Akman, F.; Abdelmoulahi, H.; Issaoui, N.; Malyar, Y.N.; Al-Dossary, O.; Wojcik, M.J. Intermolecular hydrogen bonds interactions in water clusters of ammonium sulfamate: FTIR, X-ray diffraction, AIM, DFT, RDG, ELF, NBO analysis. J. Mol. Liq. 2021, 342, 117475. [Google Scholar] [CrossRef]
- Kazachenko, A.; Akman, F.; Abir, S.; Issaoui, N.; Malyar, Y.; Vasilieva, N.; Borovkova, V. Theoretical and experimental study of guar gum sulfation. J. Mol. Model. 2021, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Yonezawa, T.; Shingu, H. A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Pan, T.; Cheng, C. An ab initio molecular dynamics analysis of lignin as a potential antioxidant for hydrocarbons. J. Mol. Graph. Model. 2015, 62, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J. Frontier Orbitals and Organic Chemical Reactions. J. Für Prakt. Chem. 1978, 320, 879–880. [Google Scholar] [CrossRef]
- Griffith, J.S.; Orgel, L.E. Ligand-field theory. Q. Rev. Chem. Soc. 1957, 11, 381–393. [Google Scholar] [CrossRef]
- Rizwana, B.F.; Prasana, J.C.; Muthu, S.; Abraham, C.S. Vibrational spectroscopy, reactive site analysis and molecular docking studies on 2-[(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)methoxy]-3-hydroxypropyl (2S)-2-amino-3-methylbutanoate. J. Mol. Struct. 2020, 1202, 127274. [Google Scholar] [CrossRef]
- Lutoshkin, M.A.; Petrov, A.I.; Kuznetsov, B.N.; Kazachenko, A.S. Aqueous Complexation of Morin and Its Sulfonate Derivative with Lanthanum(III) and Trivalent Lanthanides. J. Solut. Chem. 2019, 48, 676–688. [Google Scholar] [CrossRef]
- Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978, 68, 3801–3807. [Google Scholar] [CrossRef]
- Gadre, S.R.; Suresh, C.H.; Mohan, N. Electrostatic Potential Topology for Probing Molecular Structure, Bonding and Reactivity. Molecules 2021, 26, 3289. [Google Scholar] [CrossRef]
- Suresh, C.H.; Remya, G.S.; Anjalikrishna, P.K. Molecular electrostatic potential analysis: A powerful tool to interpret and predict chemical reactivity. WIREs Comput. Mol. Sci. 2022, e1601. [Google Scholar] [CrossRef]
- Stewart, R.F. On the mapping of electrostatic properties from bragg diffraction data. Chem. Phys. Lett. 1979, 65, 335–342. [Google Scholar] [CrossRef]
- Drissi, M.; Benhalima, N.; Megrouss, Y.; Rachida, R.; Chouaih, A.; Hamzaoui, F. Theoretical and Experimental Electrostatic Potential around the m-Nitrophenol Molecule. Molecules 2015, 20, 4042–4054. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, S.; Jeyasingh, V.; Murugesan, K.; Selvapalam, N.; Dass, G. Molecular electrostatic potential (MEP) surface analysis of chemo sensors: An extra supporting hand for strength, selectivity & non-traditional interactions. J. Photochem. Photobiol. 2021, 6, 100022. [Google Scholar] [CrossRef]
- Noureddine, O.; Gatfaoui, S.; Brandan, S.A.; Sagaama, A.; Marouani, H.; Issaoui, N. Experimental and DFT studies on the molecular structure, spectroscopic properties, and molecular docking of 4-phenylpiperazine-1-ium dihydrogen phosphate. J. Mol. Struct. 2020, 1207, 127762. [Google Scholar] [CrossRef]
- Jomaa, I.; Noureddine, O.; Gatfaoui, S.; Issaoui, N.; Roisnel, T.; Marouani, H. Experimental, computational, and in silico analysis of (C8H14N2)2[CdCl6] compound. J. Mol. Struct. 2020, 1213, 128186. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. II. Overlap Populations, Bond Orders, and Covalent Bond Energies. J. Chem. Phys. 1955, 23, 1841–1846. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. III. Effects of Hybridization on Overlap and Gross AO Populations. J. Chem. Phys. 1955, 23, 2338–2342. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. IV. Bonding and Antibonding in LCAO and Valence-Bond Theories. J. Chem. Phys. 1955, 23, 2343–2346. [Google Scholar] [CrossRef]
- Mähner, C.; Lechner, M.; Nordmeier, E. Synthesis and characterisation of dextran and pullulan sulphate. Carbohydr. Res. 2001, 331, 203–208. [Google Scholar] [CrossRef]
- Khan, M.F.; Rashid, R.; Hossain, M.; Rashid, M. Computational Study of Solvation Free Energy, Dipole Moment, Polarizability, Hyperpolarizability and Molecular Properties of Betulin, a Constituent of Corypha taliera (Roxb.). Dhaka Univ. J. Pharm. Sci. 2017, 16, 1–9. [Google Scholar] [CrossRef]
- Carbó-Dorca, R.; Bultinck, P. Quantum Mechanical Basis for Mulliken Population Analysis. J. Math. Chem. 2004, 36, 231–239. [Google Scholar] [CrossRef]
- Sagaama, A.; Issaoui, N.; Al-Dossary, O.; Kazachenko, A.S.; Wojcik, M.J. Non covalent interactions and molecular docking studies on morphine compound. J. King Saud Univ.-Sci. 2021, 33, 101606. [Google Scholar] [CrossRef]
- Lutoshkin, M.A.; Kazachenko, A.S. Assessment of various density functionals and solvation models to describe acid-base, spectral and complexing properties of thiobarbituric and barbituric acids in aqueous solution. J. Comput. Methods Sci. Eng. 2017, 17, 851–863. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Tomilin, F.N.; Pozdnyakova, A.A.; Vasilyeva, N.Y.; Malyar, Y.N.; Kuznetsova, S.A.; Avramov, P.V. Theoretical DFT interpretation of infrared spectra of biologically active arabinogalactan sulphated derivatives. Chem. Pap. 2020, 74, 4103–4113. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Malyar, Y.N.; Ghatfaoui, S.; Issaoui, N.; Al-Dossary, O.; Wojcik, M.J.; Kazachenko, A.S.; Miroshnikova, A.V.; Berezhnaya, Y.D. A density functional theory calculations of infrared spectra of galactomannan butyl ether. J. Mol. Struct. 2022, 1251, 131998. [Google Scholar] [CrossRef]
- Akman, F. Spectroscopic investigation, HOMO–LUMO energies, natural bond orbital (NBO) analysis and thermodynamic properties of two-armed macroinitiator containing coumarin with DFT quantum chemical calculations. Can. J. Phys. 2016, 94, 583–593. [Google Scholar] [CrossRef]
- Issaoui, N.; Rekik, N.; Oujia, B.; Wójcik, M.J. Anharmonic effects on theoretical IR line shapes of medium strong H(D) bonds. Int. J. Quantum Chem. 2009, 109, 483–499. [Google Scholar] [CrossRef]
- Gatfaoui, S.; Sagaama, A.; Issaoui, N.; Roisnel, T.; Marouani, H. Synthesis, experimental, theoretical study and molecular docking of 1-ethylpiperazine-1,4-diium bis(nitrate). Solid State Sci. 2020, 106, 106326. [Google Scholar] [CrossRef]
- Vasilyeva, N.Y.; Kazachenko, A.S.; Malyar, Y.N.; Kuznetsov, B.N. Sulfation of betulin with chlorosulfonic acid in pyridine. J. Sib. Fed. Univ. Chem. 2020, 13, 447–459. [Google Scholar] [CrossRef]
- Malyar, Y.N.; Vasilyeva, N.Y.; Kazachenko, A.S.; Borovkova, V.S.; Skripnikov, A.M.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; Kazachenko, A.S.; Issaoui, N. Modification of Arabinogalactan Isolated from Larix sibirica Ledeb. into Sulfated Derivatives with the Controlled Molecular Weights. Molecules 2021, 26, 5364. [Google Scholar] [CrossRef] [PubMed]
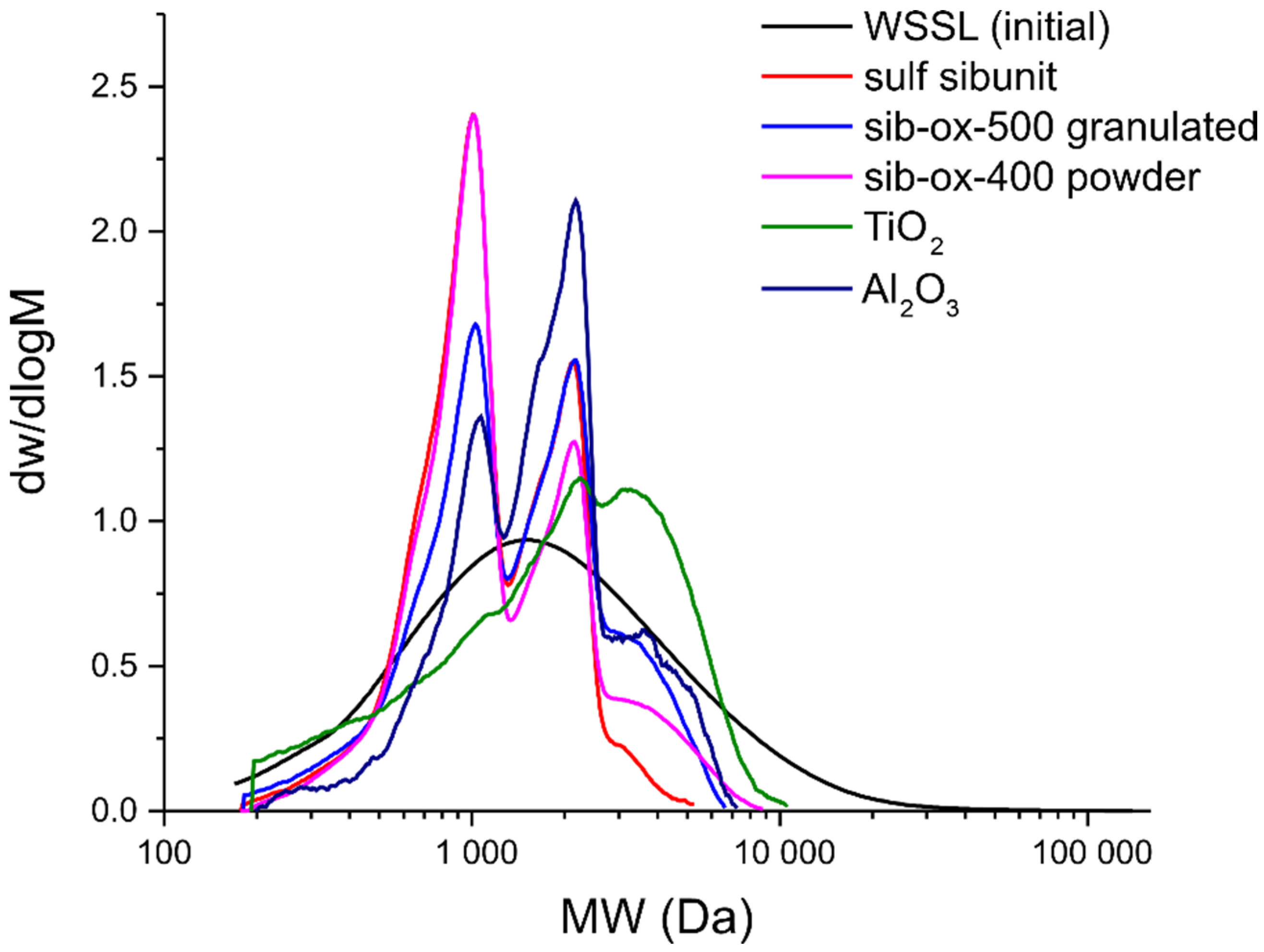

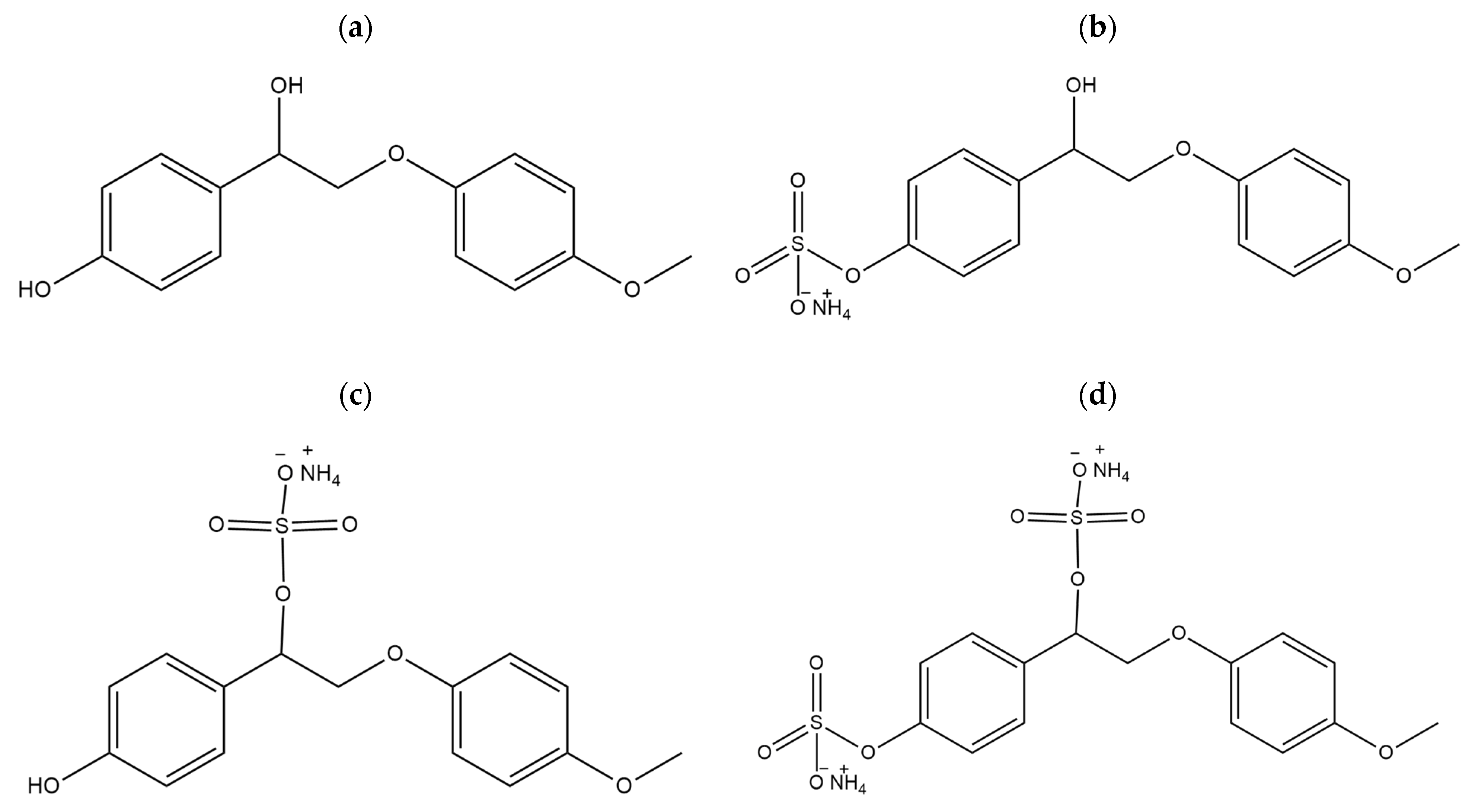
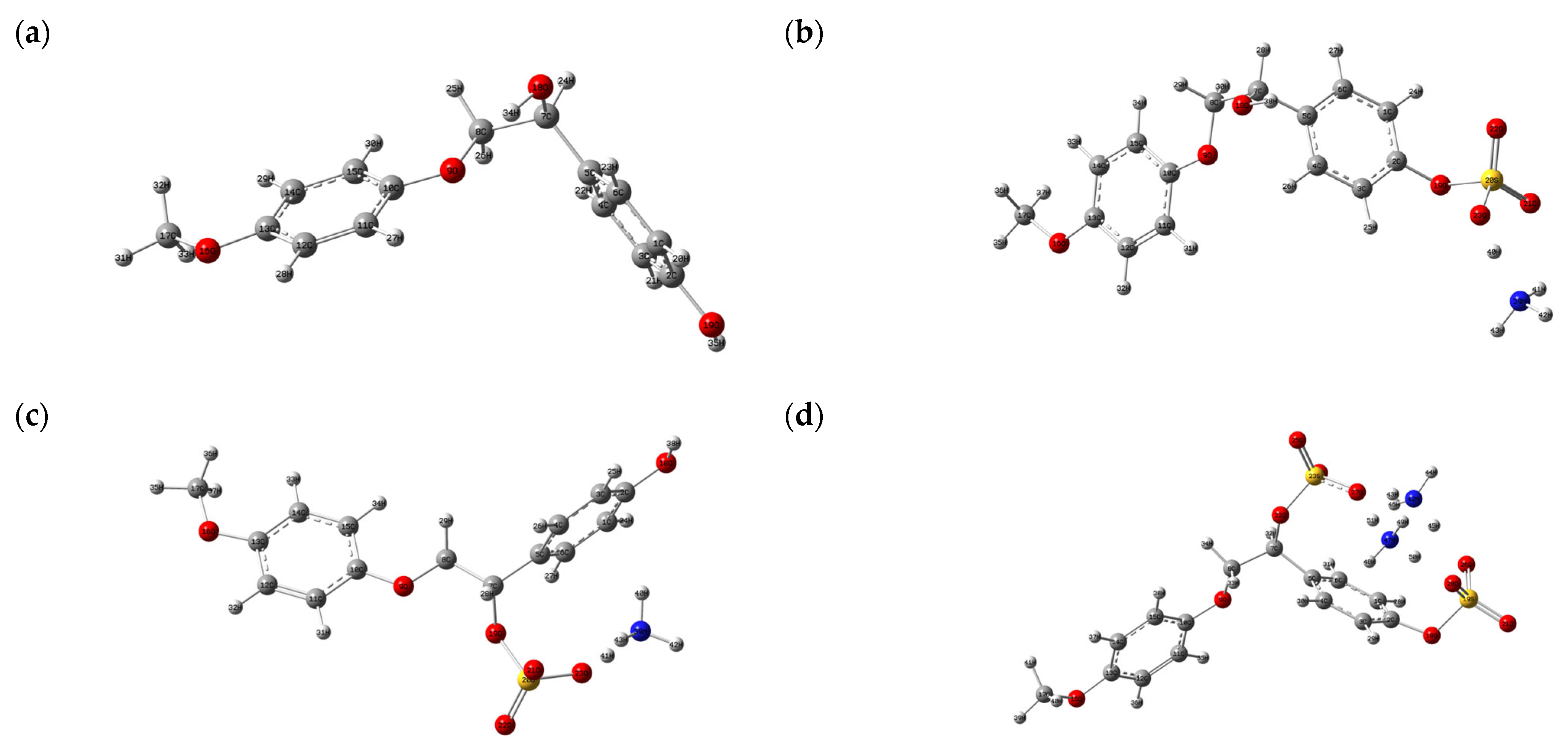
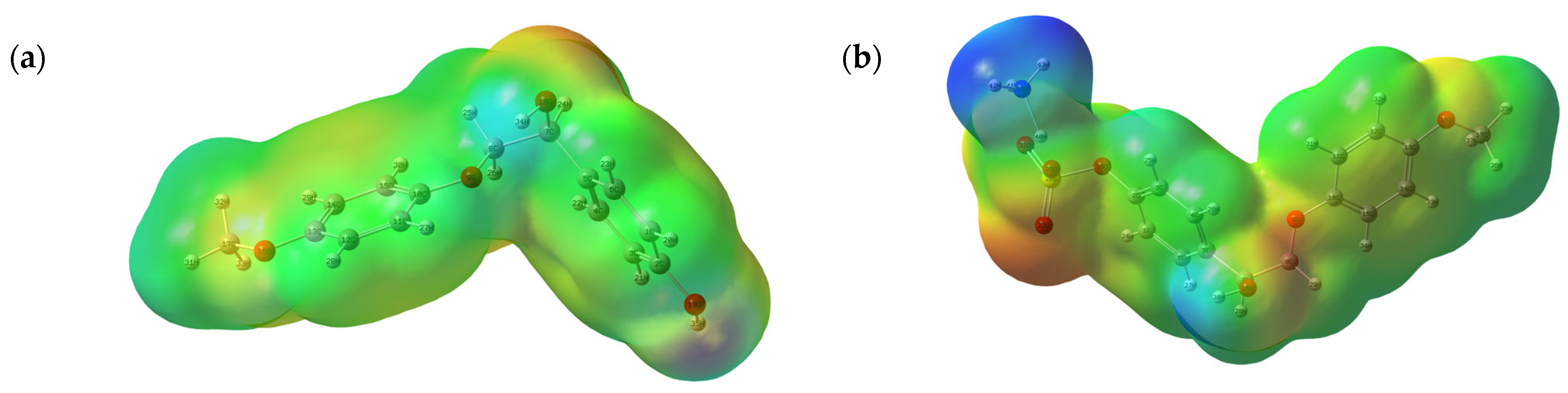
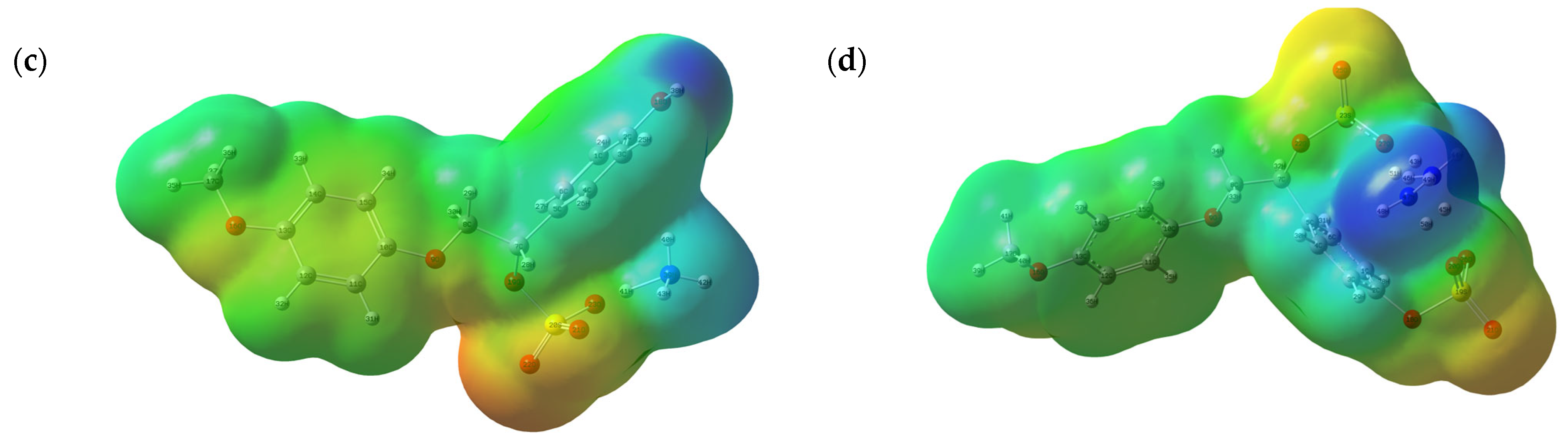
| № | Catalyst | pHpzc | Sulfur Content, wt.% | Mn (g/mol) | Mw (g/mol) | PDI |
|---|---|---|---|---|---|---|
| 1 | WSSL (initial) | - | - | 1067 | 2779 | 2.60 |
| 2 | Sibunit-ox-500 g | 3.34 | 10.7 | 1066 | 1680 | 1.58 |
| 3 | Sulfated Sibunit | 4.26 | 12.2 | 961 | 1290 | 1.34 |
| 4 | Sibunit-ox-400 | 6.88 | 16.5 | 1024 | 1536 | 1.50 |
| 5 | TiO2 | 3.75 | 8.1 | 1175 | 2424 | 2.06 |
| 6 | γ-Al2O3 | 6.71 | 5.5 | 1328 | 1946 | 1.47 |
| Sample | Weight Loss (%) at Different Temperatures (°C) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 250 | 300 | 350 | 400 | 450 | 500 | 550 | 600 | 650 | 700 | 750 | 800 | |
| Initial | 3.7 | 7.7 | 14.4 | 26.4 | 41.1 | 47.7 | 51.7 | 54.6 | 56.8 | 58.3 | 59.5 | 60.8 | 61.9 |
| Sulf. | 3.5 | 6.2 | 14.7 | 47.0 | 62.7 | 64.9 | 67.6 | 70.6 | 73.9 | 74.0 | 75.0 | 75.9 | 76.6 |
| Parameters | Values (eV) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| ELUMO | −0.0963 | −0.3720 | −0.6855 | −0.9706 |
| EHOMO | −5.4864 | −5.2970 | −5.3157 | −5.5109 |
| Energy gap (ΔE) | 5.3900 | 4.9250 | 4.6303 | 4.5402 |
| Electron affinity (A) | 0.0963 | 0.3720 | 0.6855 | 0.9706 |
| Ionization energy (I) | 5.4864 | 5.2970 | 5.3157 | 5.5109 |
| Chemical hardness (η) | 2.6950 | 2.4625 | 2.3151 | 2.2701 |
| Softness (ς) | 0.3711 | 0.4061 | 0.4319 | 0.4405 |
| Chemical potential (μ) | −2.7913 | −2.8345 | −3.0006 | −3.2407 |
| Electronegativity(χ) | 2.7913 | 2.8345 | 3.0006 | 3.2407 |
| Electrophilicity index (ω) | 1.4456 | 1.6313 | 1.9445 | 2.3132 |
| Nucleophilic index (N) | 0.6918 | 0.6130 | 0.5143 | 0.4323 |
| Maximum charge transfer index (ΔNmax) | 1.0357 | 1.1511 | 1.2961 | 1.4276 |
| 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|
| Atoms | Charges | Atoms | Charges | Atoms | Charges | Atoms | Charges |
| 1C | −0.1031 | 1C | −0.06686 | 1C | −0.10693 | 1C | −0.10445 |
| 2C | 0.32776 | 2C | 0.29177 | 2C | 0.32407 | 2C | 0.29368 |
| 3C | −0.13513 | 3C | −0.0774 | 3C | −0.15736 | 3C | −0.11439 |
| 4C | −0.12606 | 4C | −0.11321 | 4C | −0.13562 | 4C | −0.13892 |
| 5C | 0.10358 | 5C | 0.08089 | 5C | 0.06617 | 5C | 0.15057 |
| 6C | −0.12338 | 6C | −0.13989 | 6C | −0.10756 | 6C | −0.1529 |
| 7C | 0.09773 | 7C | 0.09447 | 7C | 0.06414 | 7C | 0.0546 |
| 8C | 0.05415 | 8C | 0.0841 | 8C | 0.08727 | 8C | 0.06606 |
| 9O | −0.55423 | 9O | −0.53056 | 9O | −0.53231 | 9O | −0.54192 |
| 10C | 0.3519 | 10C | 0.35404 | 10C | 0.3479 | 10C | 0.34901 |
| 11C | −0.12122 | 11C | −0.12083 | 11C | −0.11998 | 11C | −0.12101 |
| 12C | −0.11696 | 12C | −0.11686 | 12C | −0.11715 | 12C | −0.11679 |
| 13C | 0.34825 | 13C | 0.34628 | 13C | 0.34699 | 13C | 0.3484 |
| 14C | −0.14573 | 14C | −0.14569 | 14C | −0.14635 | 14C | −0.14668 |
| 15C | −0.13894 | 15C | −0.14184 | 15C | −0.14201 | 15C | −0.14166 |
| 16O | −0.52236 | 16O | −0.52371 | 16O | −0.52277 | 16O | −0.5222 |
| 17C | −0.07609 | 17C | −0.0748 | 17C | −0.07472 | 17C | −0.0762 |
| 18O | −0.55195 | 18O | −0.53871 | 18O | −0.55114 | 18O | −0.59512 |
| 19O | −0.56026 | 19O | −0.59243 | 19O | −0.54111 | 19S | 1.31849 |
| 20H | 0.09417 | 20S | 1.31194 | 20S | 1.32458 | 20O | −0.6431 |
| 21H | 0.07219 | 21O | −0.53093 | 21O | −0.57469 | 21O | −0.48016 |
| 22H | 0.07484 | 22O | −0.49368 | 22O | −0.46523 | 22O | −0.56656 |
| 23H | 0.09159 | 23O | −0.59405 | 23O | −0.59541 | 23S | 1.31134 |
| 24H | 0.11311 | 24H | 0.11232 | 24H | 0.10842 | 24O | −0.59864 |
| 25H | 0.10796 | 25H | 0.09538 | 25H | 0.08342 | 25O | −0.48728 |
| 26H | 0.09915 | 26H | 0.11256 | 26H | 0.09894 | 26O | −0.63249 |
| 27H | 0.09508 | 27H | 0.08687 | 27H | 0.12285 | 27O | −0.71167 |
| 28H | 0.09733 | 28H | 0.08226 | 28H | 0.14238 | 28H | 0.11397 |
| 29H | 0.08569 | 29H | 0.11075 | 29H | 0.10135 | 29H | 0.10509 |
| 30H | 0.08744 | 30H | 0.09728 | 30H | 0.11362 | 30H | 0.08854 |
| 31H | 0.12344 | 31H | 0.0926 | 31H | 0.10314 | 31H | 0.10441 |
| 32H | 0.10908 | 32H | 0.09329 | 32H | 0.09634 | 32H | 0.14052 |
| 33H | 0.10877 | 33H | 0.08342 | 33H | 0.08311 | 33H | 0.10006 |
| 34H | 0.31644 | 34H | 0.08511 | 34H | 0.08448 | 34H | 0.12339 |
| 35H | 0.31571 | 35H | 0.12139 | 35H | 0.12192 | 35H | 0.09645 |
| 36H | 0.10809 | 36H | 0.10677 | 36H | 0.09751 | ||
| 37H | 0.10781 | 37H | 0.10856 | 37H | 0.08618 | ||
| 38H | 0.30859 | 38H | 0.31997 | 38H | 0.08998 | ||
| 39N | −0.76959 | 39N | −0.77406 | 39N | 0.12368 | ||
| 40H | 0.42605 | 40H | 0.2893 | 40H | 0.10839 | ||
| 41H | 0.31383 | 41H | 0.43481 | 41H | 0.10962 | ||
| 42H | 0.28564 | 42H | 0.28011 | 42N | −0.68977 | ||
| 43H | 0.28433 | 43H | 0.30379 | 43H | 0.39359 | ||
| 44H | 0.3195 | ||||||
| 45H | 0.39914 | ||||||
| 46H | 0.33304 | ||||||
| 47N | −0.69366 | ||||||
| 48H | 0.3203 | ||||||
| 49H | 0.322 | ||||||
| 50H | 0.40576 | ||||||
| 51H | 0.40227 | ||||||
| 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|
| O–H | 3673 and 3623 | ʋO–H | 3662 | ʋO–H | 3670 | ʋN–H | 3452–3373 |
| ʋC–H (aromatic) | 3101–3034 | ʋN–H | 3475–3334 | ʋN–H | 3474–3336 | ʋC–H (aromatic) | 3099–3052 |
| ʋC–H (aliphatic) | 3022–2891 | ʋC–H (aromatic) | 3101–3052 | ʋC–H (aromatic) | 3100–3040 | ʋC–H (aliphatic) | 3022–2891 |
| ʋC=C | 1612–1574 | ʋC–H (aliphatic) | 3020–2848 | ʋC–H (aliphatic) | 3021–2887 | ʋC=C | 1611–1568 |
| ʋO–C | 1254–1220 | ʋC=C | 1612–1573 | 028BC=C | 1612–1574 | ʋO–C | 1241 and 1223 |
| ʋO–C | 1242 and 1224 | ʋO–C | 1239 and 1223 | ʋS–O | 1276, 1272, 1095, 1087, 955, 890 | ||
| ʋS–O | 1302, 1101, 858 | ʋS–O | 1303, 1098, 861 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazachenko, A.S.; Akman, F.; Vasilieva, N.Y.; Malyar, Y.N.; Fetisova, O.Y.; Lutoshkin, M.A.; Berezhnaya, Y.D.; Miroshnikova, A.V.; Issaoui, N.; Xiang, Z. Sulfation of Wheat Straw Soda Lignin with Sulfamic Acid over Solid Catalysts. Polymers 2022, 14, 3000. https://doi.org/10.3390/polym14153000
Kazachenko AS, Akman F, Vasilieva NY, Malyar YN, Fetisova OY, Lutoshkin MA, Berezhnaya YD, Miroshnikova AV, Issaoui N, Xiang Z. Sulfation of Wheat Straw Soda Lignin with Sulfamic Acid over Solid Catalysts. Polymers. 2022; 14(15):3000. https://doi.org/10.3390/polym14153000
Chicago/Turabian StyleKazachenko, Aleksandr S., Feride Akman, Natalya Yu. Vasilieva, Yuriy N. Malyar, Olga Yu. Fetisova, Maxim A. Lutoshkin, Yaroslava D. Berezhnaya, Angelina V. Miroshnikova, Noureddine Issaoui, and Zhouyang Xiang. 2022. "Sulfation of Wheat Straw Soda Lignin with Sulfamic Acid over Solid Catalysts" Polymers 14, no. 15: 3000. https://doi.org/10.3390/polym14153000
APA StyleKazachenko, A. S., Akman, F., Vasilieva, N. Y., Malyar, Y. N., Fetisova, O. Y., Lutoshkin, M. A., Berezhnaya, Y. D., Miroshnikova, A. V., Issaoui, N., & Xiang, Z. (2022). Sulfation of Wheat Straw Soda Lignin with Sulfamic Acid over Solid Catalysts. Polymers, 14(15), 3000. https://doi.org/10.3390/polym14153000










