Abstract
The medicinal administration of Aloe vera gel has become promising in pharmaceutical and cosmetic applications particularly with the development of the nanotechnology concept. Nowadays, effective H. pylori treatment is a global problem; therefore, the development of natural products with nanopolymers such as chitosan nanoparticles (CSNPs) could represent a novel strategy for the treatment of gastric infection of H. pylori. HPLC analysis of A. vera gel indicated the presence of chlorogenic acid as the main constituent (1637.09 µg/mL) with other compounds pyrocatechol (1637.09 µg/mL), catechin (1552.92 µg/mL), naringenin (528.78 µg/mL), rutin (194.39 µg/mL), quercetin (295.25 µg/mL), and cinnamic acid (37.50 µg/mL). CSNPs and A. vera gel incorporated with CSNPs were examined via TEM, indicating mean sizes of 83.46 nm and 36.54 nm, respectively. FTIR spectra showed various and different functional groups in CSNPs, A. vera gel, and A. vera gel incorporated with CSNPs. Two strains of H. pylori were inhibited using A. vera gel with inhibition zones of 16 and 16.5 mm, while A. vera gel incorporated with CSNPs exhibited the highest inhibition zones of 28 and 30 nm with resistant and sensitive strains, respectively. The minimal inhibitory concentration (MIC) was 15.62 and 3.9 µg/mL, while the minimal bactericidal concentration (MBC) was 15.60 and 7.8 µg/mL with MBC/MIC 1 and 2 indexes using A. vera gel and A. vera gel incorporated with CSNPs, respectively, against the resistance strain. DPPH Scavenging (%) of the antioxidant activity exhibited an IC50 of 138.82 μg/mL using A.vera gel extract, and 81.7 μg/mL when A.vera gel was incorporated with CSNPs. A.vera gel incorporated with CSNPs enhanced the hemolysis inhibition (%) compared to using A.vera gel alone. Molecular docking studies through the interaction of chlorogenic acid and pyrocatechol as the main components of A. vera gel and CSNPs with the crystal structure of the H. pylori (4HI0) protein supported the results of anti-H. pylori activity.
1. Introduction
In the last decade, natural products are becoming vital resources in numerous applications such as cosmetics industries, biopesticides, and the prevention and treatment of microbial and nonmicrobial diseases. As mentioned in numerous studies, Aloe vera is a tropical succulent plant that belongs to the liliaceous family; it is considered an important medicinal plant, which contains vital ingredients including amino acids, glycoproteins, phenolic compounds, polysaccharides, organic acids, lignin, hormones, vitamins, and saponins, as well as enzymes [1]. These components give this plant many important medicinal properties such as immune-boosting, antioxidant, anti-cancer, intestinal health promotion, anti-inflammatory, bone proliferation promotion, antimicrobial, neuroprotection, and hypoglycemic properties [2]. A strong anti-oxidative capacity of A. vera gel was reported previously due to the existence of various contents of phenolic compounds. The anti-oxidative activity reduces hydroperoxides, catalase, superoxide dismutase, and glutathione peroxidase [3]. The viscosity of A. vera gel may be due to its content of the sugar glucomannan, in addition to approximately 200 active components that were detected in A. vera gel among proteins and its monomers, lipids, vitamins, and polysaccharides [4]. The extracted gel of A. vera leaves exhibited a fungistatic activity against Candida paraprilosis, Candida krusei, and C. albicans [5,6]. As described previously, Helicobacter pylori inhabits the stomach of humans early in life; however, the related pathology may be expressed later. Surprisingly, 50% of the people worldwide are carriers of H. pylori, but infection symptoms appeared only in approximately 15%, with the increase in peptic ulcer, gastritis, and gastric adenocarcinoma [7]. Bacterial resistance, particularly H. pylori, to several antibiotics is considered a global problem for public health, and it has been of interest to many scientists to find a solution of this problem. Furthermore, significant challenges have emerged as a result of the failure to treat H. pylori infection; therefore, the discovery of a novel strategy combining nanoparticles and natural compounds has become a medical requirement for infection control.
A. vera inner gel was tested against H. pylori and the treatment of peptic ulcers [8]. Moreover, a synergistic efficacy was recorded in vitro using plant extracts with diverse antibiotics toward H. pylori strains [9]. Both susceptible and resistant H. pylori strains were influenced by A. vera gel according to a previous study [10].
Nanoparticles (NPs) have played and still play an important role in many medical, industrial, agricultural, environmental, and food fields [11,12,13,14,15,16,17,18,19,20,21,22,23]. From the applied NPs, chitosan nanoparticles (CSNPs) have been lately used at large scales for medical applications such as drug carriers and cosmetics [24].
The chemical structure of chitosan (cationic polymer) is composed of monomers of β-(1-4)-linked d-glucosamine and N-acetyl-d-glucosamine. The advantages of chitosan were recorded in the literature as biocompatible, nontoxic, bioavailable, and biodegradable; therefore, it was applied in wound healing, to inhibit the development of scar tissue, and for protein delivery, as well as other biomedical applications [25,26].
The synthesis of CSNPs has attracted the attention of many researchers due to the many applications of chitosan. Pharmaceuticals administration was reported in recent years using CSNPs due to their high activity level [27]. CSNPs are used as a drug carrier to support the medicinal features of drugs such as drug release regulation leading to drug solubility and stability enhancement. As a result of the incorporation of A. vera gel with CSNPs, the gel has gained several pharmacological properties that are of great interest to pharmaceutical fabrications [1]. The combination of CSNPs with A. vera extract exhibited an enhancement in wound healing caused by microbial pathogens [4]. Electrostatic attraction among cationic amino groups of chitosan with negative charges on the microbial cell wall is the main reason of microbial cell destruction. The activity of CSNPs loaded by Satureja hortensis essential oils had superior effects compared to CSNPs alone [28]. Other biological activities of A. vera gel were enhanced when it was incorporated with CSNPs such as their stability, in vitro release, and antioxidant potential [29]. Incorporation of Byrsonima crassifolia extract into CSNPs enhanced the control of some pathogenic fungi including Colletotrichum gloeosporioides and Alternaria species [30]. Phytoconstituents and its concentrations of A. vera as well as other plants may differ according to cultivation soil, climatic changes, type of fertilizers, and extraction methods. In addition, most of the studies on the incorporation of A. vera gel with CSNPs focused on wound healing and antimicrobial activity against certain microorganisms but not against H. pylori. Therefore, the aim of this study was to enhance the biological activities of A. vera gel with CSNPs as a natural and safe compound. We also studied the effects of this incorporation on H. pylori growth, antioxidant activity, and anti-inflammatory activity. Moreover, a docking study of the main components of A. vera gel on H. pylori was conducted.
2. Materials and Methods
2.1. Chemicals Used
Chitosan nanoparticles (CSNPs) were purchased from Primex, Siglufjordur, Iceland. Other chemicals including buffers, reagents, solvents, and bacterial growth medium were obtained from Sigma-Aldrich (St. Louis, MS, USA). All chemicals used in the current experiments were of analytical grade.
2.2. Plant Sample Used and Extraction Process
Aloe vera gel was collected from mature and healthy leaves to obtain 400 mL of slime of the gel through the cutting of leaves transversely into pieces. The gel was concentrated using a rotary evaporator at 50 °C. The final A. vera gel was concentrated to obtain 12 g. A voucher herbarium specimen had been deposited in the Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt.
2.3. Flavonoid and Phenolic Contents Analysis by High-Performance Liquid Chromatography (HPLC)
One gram of dried gel was extracted with 10 mL of methyl alcohol via a rotary evaporator to obtain concentrated extract. Five microliters of the extracted gel was injected in HPLC (Agilent 1260 series, Agilent Technologies, Santa Clara, CA, USA) characterized with the following brief conditions: The separation was performed using an Eclipse C18 column (4.6 mm × 250 mm i.d., 5 μm). Water and 0.05% trifluoroacetic acid in acetonitrile were applied as the mobile phase with a flow rate of 0.9 mL/min. The mobile phase consisted of water and 0.05% trifluoroacetic acid in acetonitrile (B) at a flow rate 0.9 mL/min, and the column temperature was adjusted at 40 °C. The mobile phase was programmed successively in a linear gradient with 60% at 0 min; 82% at 0–5, 5–8, 8–12, 12–15, 15–16, and 16–20 min. The UV-detector was set to 280 nm. The qualitative detection of phenolic and flavonoids was performed according to Abdelghany et al. [31] compared to the injected standards of phenolic and flavonoids in HPLC.
2.4. Preparation of Coating Solutions of CSNPs with Aloe vera Gel
The CSNPs solution was prepared by dissolving chitosan in an aqueous solution (1%, v/v) of acetic acid, and then the addition of CSNPs obtained a final concentration of 2% (w/v). Glycerol was added to the prepared solution as a plasticizer at the concentration of 2% (w/v), followed by the addition of Tween 20 to the prepared solution at a concentration of 0.05% (v/v) in order to increase its wettability and adhesion properties. To obtain a CSNPs-A. vera gel composite coating solution, A. vera gel was incorporated into CSNPs solution (stirred for 25 min and then ultrasonicated for 45 min) to obtain the final concentration of A. vera gel concentrate/CSNPs in the solution at 10% by weight [32].
2.5. Characterization of CSNPs, Aloe vera Gel, and CSNPs Incorporated with Aloe vera Gel
2.5.1. UV–Vis
The UV–vis absorption spectrum of the tested samples was scanned via a spectrophotometer (JASCO V-670, Thermo Fisher Scientific, Waltham, USA) at a range of 200–800 nm.
2.5.2. Transmission Electron Microscopy
The diameters and shape of CSNPs and CSNPs incorporated with A. vera gel were examined via Transmission Electron Microscopy (TEM) (JEOL-JEM-1200, JEOL, Tokyo, Japan). A drop of colloidal solution of each sample was loaded on copper grids (400 mesh) coated by amorphous carbon film.
2.5.3. Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR) (FT-IR; FTIR 8400S, Shimadzu, Tokyo, Japan) was applied to detect specific chemical groups of the tested samples. The CSNPs, dried Aloe vera gel extract, and CSNPs incorporated with A. vera gel were ground with KBr powder and pressed into pellets for FT-IR spectra measurement at a range of 400 to 4000 cm−1 of frequency.
2.6. Antimicrobial Activity of CSNPs, with Aloe vera Gel and CSNPs Incorporated with A. vera Gel
The agar diffusion method using Brain Heart Infusion medium with 7% serum was used to detect the activity of A. vera gel and A. vera gel incorporated with CSNPs against H. pylori (Hospital of Ain Shams University, Cairo, Egypt). Briefly, 100 μL of H. pylori suspension containing 108 colony-forming units (CFUs)/mL was spread on the medium surface. Via a sterile cork borer with a diameter of 6 mm, a hole was punched from agar, and then 100 μL of the tested compounds at the desired quantity was introduced into the well. DMSO (solvent of gel) as a negative control while antibiotics including clarithromycin (CLR, 0.05 mg/mL), amoxicillin (AMX, 0.05 mg/mL), and metronidazole (MTZ, 0.8 mg/mL) as a positive control were applied. The plates were kept in a refrigerator for 30 min for appropriate diffusion of the tested compounds and control samples, and then transferred to an incubator for 3 days at 37 °C under microaerophilic conditions. At end of the incubation period, the diameter of clear zones around the well was measured [16,33].
2.7. Minimal Inhibitory Concentration and Minimal Bactericidal Concentration
The minimal inhibitory concentration (MIC) of A. vera gel and A. vera gel incorporated with CSNPs was detected via the micro-dilution broth technique. The Mueller–Hinton (MH) broth provided with lysed horse blood was used for cultivation of H. pylori. Serial two-fold dilutions were performed to gain different concentrations ranging from 0.98 to 1000 μg/mL of the tested compounds. The sterile 96-well polystyrene microtitrate plates were prepared by distributing 200 μL of each dilution in broth medium per well. Fresh inocula of H. pylori were suspended in sterile NaCl (0.85%) to match the turbidity of 1.0 McFarland standard, and then 2 µL was transferred to the wells to attain 3.0 × 106 colony forming units (CFU)/mL, followed by incubation under microaerophilic conditions with a limited amount of CO2 (15%) at 35 °C for 3 days. At the end of the incubation period, the MIC was assessed visually as the lowest concentration of each tested compound with the viewing of the complete inhibition of H. pylori growth. Microtitrate plates containing bacterial inoculum without the tested compounds (positive control) and tested compounds without bacterial inoculum (negative control) were applied in the current experiment. The minimal bactericidal concentration (MBC) was assayed by sub-culturing 100 mL of culture of the H. pylori from each well that showed thorough growth inhibition, from the last positive and from the growth control, onto the plates of MH agar provided with horse blood (5%), followed by incubation under microaerophilic conditions with a limited amount of CO2 (15%) at 35 °C for 3 days. MBC was determined at the lowest concentration of the tested compounds without the appearance of any H. pylori growth. The bactericidal or bacteriostatic effect of the tested compounds was evaluated via calculation of the MBC/MIC ratio. According to French [34], if the MBC/MIC ratio is no more than four times the MIC, the effect of the tested compounds is considered as bactericidal. Each trial was repeated in triplicate.
2.8. Antioxidant Activity
The antioxidant activity of A. vera gel and A. vera gel incorporated with CSNPs was evaluated via a 1,1-diphenyl-2-picryl hydrazyl (DPPH) radical scavenging assay. The free radical scavenging activity of different extracts of leaves plant were measured by 1,1-diphenyl-2-picryl hydrazyl (DPPH). One milliliter of 0.1 mM solution of DPPH in ethanol was mixed with 3 mL of the dissolved tested compounds in ethanol at various doses (3.9, 7.8, 15.62, 31.25, 62.5, 125, 250, 500, and 1000 μg/mL) that were prepared by sequential dilution. At room temperature (22 °C), the mixture was shaken for 30 min; then, via a spectrophotometer (UV-VIS milton roy), the absorbance of the reaction mixture was measured at 517 nm [31]. The dose of tested compounds necessary to inhibit 50% of the DPPH free radical (IC50) was recorded using a log dose inhibition curve. Ascorbic acid as a standard antioxidant agent was applied.
where CA and TCA are the absorbance of the control reaction and tested compound reaction, respectively.
2.9. Preparation of Erythrocyte Suspension and Hypotonicity-Induced Hemolysis
The collected blood sample (3 mL) from a healthy volunteer (corresponding author of current paper) was transferred to a heparinized tube, then centrifuged for 10 min at 3000 rpm. The obtained supernatant was mixed with an equivalent volume of normal saline that needed to dissolve the red blood pellets. The liquefied red blood pellets were reconstituted with isotonic sodium phosphate buffer (10 mM, pH 7.4) solution as 40/60% v/v. The used buffer consisted of 0.002 g of NaH2PO4, 0.016 g of Na2HPO4, and 0.09 g of NaCl per 100 mL of distilled water. The reconstituted red blood cells (re-suspended supernatant) were used as such.
The dissolved test compounds in distilled water represent the hypotonic solution. Different doses (100, 200, 400, 600, 800, and 1000 μg/mL) of tested compounds were added to 5 mL of hypotonic solution in centrifuge tubes. The same doses were added to 5 mL of isotonic solution in centrifuge tubes. Five milliliters of the vehicle (distilled water) and 5 mL of 200 μg/mL of indomethacin in each tube alone were used as a control. The suspended erythrocyte (0.1 mL) was added to each tube, followed by incubation at 37 °C for 60 min, then centrifuged for 3 min at 1300 g. Via a spectrophotometer (UV-VIS milton roy), the absorbance of the supernatant containing hemoglobin was estimated at 540 nm. The % of hemolysis was designed by supposing the hemolysis formed in the existence of distilled water as 100%. The % of hemolysis inhibition by the tested compounds was calculated:
where OD1 is the absorbance of tested compounds in isotonic solution; OD2 is the absorbance of tested compounds in hypotonic solution; OD3 is the absorbance of the control sample in hypotonic solution.
2.10. Molecular Docking
We studied the interaction between chlorogenic acid, chitosan, and pyrocatechol with the crystal structure of the H. pylori (4HI0) protein. A molecular modeling study using the Molecular Operating Environment (MOE) module was conducted to explain the observed antibacterial effect of the main detected compounds with the crystal structure of the H. pylori (4HI0) protein. MOE’s BUILDER module was used to create the structural model, and optimization conformational evaluations of the generated molecules were performed in two steps. The geometry of the compounds was optimized using the semiempirical PM3 Hamiltonian with the Restricted Hartree–Fock (RHF) and RMS gradient of 0.05 Kcal/mol, as well as the integrated MOPAC 7.0 energy minimization tool.
The resultant model was then used for the MOE’s ‘Systematic Conformational Search’. To rank the compounds’ binding affinity to the 4HI0 protein, the binding free energy and bonds of hydrogen among the compounds and amino acid into the 4HI0 protein were utilized. Estimation of the bonds of hydrogen was performed by determining the length of the hydrogen bond; moreover, the RMSD (Root-Mean-Square Deviation) of the co-crystal ligand position compared to the docking pose was utilized in ranking. Both RMSD as well as the mode of interaction of the native ligands within the crystal structure of the H. pylori (4HI0) protein receptor was utilized as a standard docked model.
2.11. Statistical Analysis
Assessments were performed in triplicate; therefore, it was estimated as mean ± standard deviation (SD). GraphPad Prism® software (version 5.0, GraphPad Software, San Diego, CA, U.S.) was applied to obtain the IC50 value of DPPH radical scavenging potential graphs.
3. Result and Discussion
3.1. Characterization of A. vera Gel and Phytochemical Analysis
Two major parts were observed in the A. vera leaf including the outer green rind composed of vascular tissues and the interior colorless parenchyma tissues containing viscous clear liquid known as gel (Figure 1). Different terms were reported to describe the inner part of the A. vera leaf including the inner pulp, mucilaginous gel, inner gel, and mucilage tissue. According to previous investigators, approximately 99.5% water is the main content of the gel, while numerous contents represent 0.5–1% of the gel constituents [1].

Figure 1.
A. vera leaf showing outer green rind and viscous clear liquid (gel).
Based on HPLC analysis, different flavonoids and phenolic acids were detected in the extract of A. vera gel with different concentrations and retention time (Table 1 and Figure 2). Chlorogenic acid was the main constituent followed by pyrocatechol and catechin with the concentrations of 1637.09, 1552.92, and 1100.43 µg/mL, respectively. Other compounds were recognized, namely naringenin (528.78 µg/mL), rutin (194.39 µg/mL), and quercetin (295.25 µg/mL) (Table 1). Cinnamic acid was also detected but with the lowest concentration (37.50 µg/mL). HPLC analysis of the A. vera gel extract showed the existence of 13 identified compounds in addition to unknowns with different areas (Figure 2), indicating its richness with phenolic and flavonoids contents. Our results agree with another study with some exceptions, for example, gallic acid and daidzein were detected in the A. vera gel under study but not detected in A. vera gel according to López et al. [35]. Guo and Mei [36] reported that the A. vera gel possesses diverse constituents and, therefore, it is applied in various medical purposes. The variation in concentration of these chemical constituents is based on the plant part used, extraction process, solvent, stage of growth, and plant source. All the detected compounds possess multiple biological functions in the medicinal fields as well as cosmetics and food industry. Our data match with some results by Hassan et al. [37] who observed that caffeic, coumaric, syringic, sinapic acid, cinnamic, and ferulic acid are the main acids of A. vera gel. Numan [38] confirmed the existence of phenolic compounds such as catechin, quercetin, aloe emodin, aloin, and sinapic acid in A. vera gel.

Table 1.
Flavonoids and Phenolic acids content of M. pulegium extract identified by HPLC.
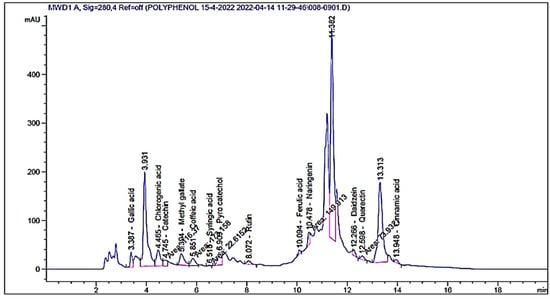
Figure 2.
HPLC chromatograms of detected flavonoids and phenolic acids content of A. vera gel extract.
3.2. Characterization of CSNPs and CSNPs Incorporated with A. vera Gel
The UV–vis spectrum of CSNPs is shown in Figure 3. Broad absorption bands were observed at 270 nm. According to Thamilarasan et al. [39], the UV–Visible spectrum presented an absorption peak at 250 nm of CSNPs. Another absorption peak for CSNPs was obtained at 226 nm [40], while the absorption peak at 360 was observed with A. vera gel incorporated with CSNPs. TEM images indicated that the mean diameter of CSNPs was 83.46 nm with irregular shape, while the mean diameter of CSNPs incorporated with gel was 36.54 nm with similar forms of particles (Figure 4A,B). The diameters of some randomly selected CSNPs and CSNPs incorporated with gel were recorded (Figure 4C). The size of A. vera gel incorporated with CSNPs decreased as a result of the reaction of A. vera gel content with CSNPs or may be due to sonication before TEM examination. According to a recent study performed, CSNPs particles had a size ranging from 15 nm to 150 nm [41]. Spherical CSNPs with a size from 20 to 100 nm were visualized via TEM [39].
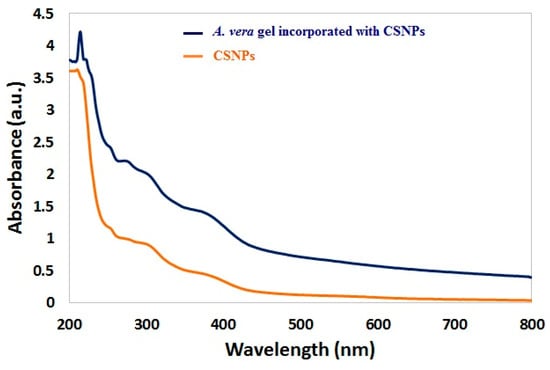
Figure 3.
UV–vis spectra of CSNPs and A. vera gel incorporated with CSNPs.
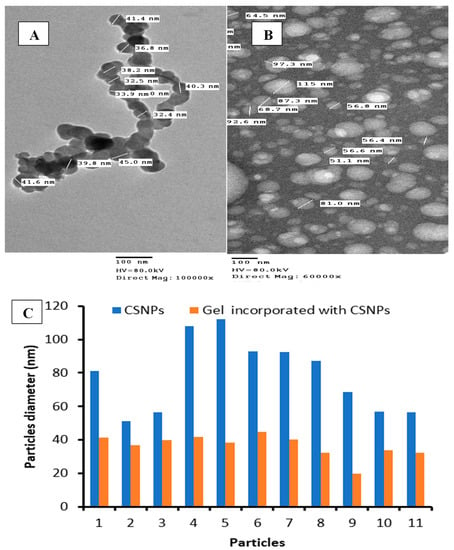
Figure 4.
TEM of synthesized A. vera gel incorporated with CSNPs (A), CSNPs (B), and diameter of some particles (C).
FTIR spectroscopy was performed to characterize the chemical structure of CSNPs, A. vera gel, and A. vera gel incorporated with CSNPs (Figure 5, Figure 6 and Figure 7). In a narrow range, peaks were observed at 3417.80, 3496.42, and 3442.24 cm−1 for A. vera gel, CSNPs, and A. vera gel incorporated with CSNPs, respectively, where these peaks are attributed to stretching vibrations of –NH2 and –OH groups.
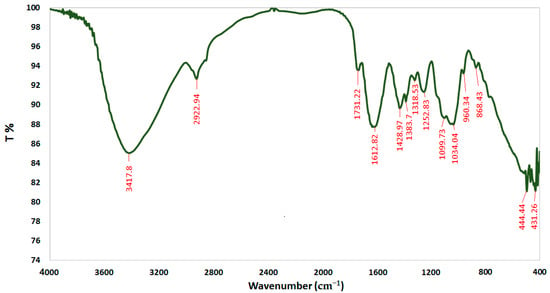
Figure 5.
FTIR spectra of A. vera gel.
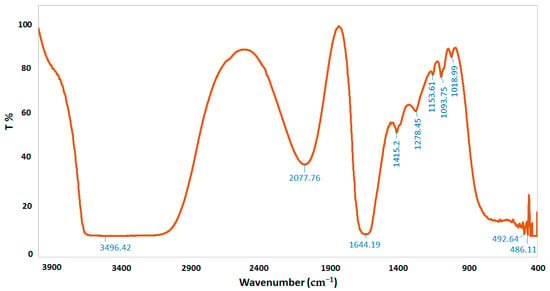
Figure 6.
FTIR spectra of CSNPs.
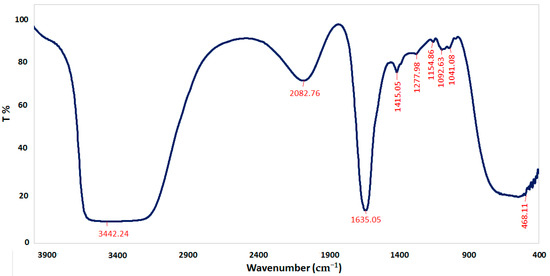
Figure 7.
FTIR spectra of A. vera gel incorporated to CSNPs.
A characteristic band of A. vera gel at 2922.94 cm−1 is associated with C–H or -CH3, these peaks are not detected in CSNPs alone or in A. vera gel incorporated with CSNPs, and they may be due to the reaction between chemical groups of A. vera gel with the chemical groups of CSNPs resulting from the appearance of new groups or the disappearance of detected groups.
The band at 1644.19 cm−1 indicates the presence of N-acetyl groups or the C=O stretching of amide I in CSNPs. In A. vera gel incorporated with CSNPs, the band at 1635.05 cm−1 that appeared may be due to the NH primary amine, while the band at 1731.22 cm−1 appearing in A. vera gel alone may be due to the C=O stretching vibration associated with acids, ketones, and aldehydes. The same approximate bands 1415.20 cm−1 and 1415.05 cm−1 appeared in CSNPs and A. vera gel incorporated with CSNPs, respectively, that may confirm the CH2 bending. In addition, peaks at 1153.61 and 1154.86 were observed for CSNPs and A. vera gel incorporated with CSNPs, respectively, that may be associated with the C–OH bending vibration and may detect the percentage of the crystalline phase. Characteristic bands in the range of 1383.70–1318.53 cm−1 are attributed to C–N stretching (amide III) in A. vera gel; however, Branca et al. [42] reported the presence of the group amide III at 1317 cm−1 CSNPs. The peak at 1252.83 cm-1 is characteristic of R=C-O-C and belongs to ethers in A. vera gel. The characteristic of the pyranoside ring absorption peak at 868.43 cm−1 (C-H ring vibration) was recognized in A. vera gel (Figure 5) as already identified by Nejatzadeh-Barandozi and Enferadi [43].
The appearance of a high-intensity band at 1018.99 cm−1 in CSNPs (Figure 6) may be attributed to the C−O and C−OH bonds in polysaccharides, as reported in a previous study [44] (Torres-Giner et al., 2017). The intense band at 1041.08 cm−1 is assigned to the existence of C–O and C–OH bonds in glucan units of A. vera after being incorporated with CSNPs (Figure 7). It can be presumed that this type of bond is designed among the various molecules (polysaccharides of A. vera gel and chitosan) interactions. CSNPs characterization with FTIR gave results parallel to those obtained in the reports conducted earlier [1,27].
3.3. Biological Activities of A. vera Gel and A. vera Gel Incorporated to CSNPs
3.3.1. Anti-Helicobacter pylori
The current study demonstrates that the A. vera gel exhibited a moderate antibacterial potential against both resistant and susceptible H. pylori strains with inhibition zones of 16 and 16.5 mm, while CSNPs increased the antibacterial activity of A. vera gel to 28 and 30 mm, compared with using standard antibiotics that caused 20 and 25 mm inhibition zones, respectively (Table 2 and Figure 8). The inhibitor potential of A. vera gel was reported against H. pylori that could be attributed to the existence of anthraquinones [8]. In addition to the inhibition of H. pylori, Nostro et al. [9] mentioned that A. vera gel may prevent the H. pylori adhesion on gastric cells. A previous study on rats infected by H. pylori revealed that A. vera gel reduced the inflammation of gastric mucosa caused by H. pylori [45]. As reported in another study, not only H. pylori but other bacteria and fungi were inhibited by A. vera gel such as Escherchia coli, Salmonella typhi, Bacillus subtilis, Staphylococcus aureus [46], Candida paraprilosis, Candida krusei [5], and C. albicans [6]. MIC (15.62 and 3.9 µg/mL) and MBC (15.60 and 7.8 µg/mL) were assayed for both A. vera gel and A. vera gel incorporated with CSNPs, respectively, against a resistant strain of H. pylori (Table 3). The MBC/MIC Index was 1 and 2 for both A. vera gel and A. vera gel incorporated with CSNPs, respectively, indicating its bactericidal properties, while an MBC/MIC index of the current samples more than 4 revealed their bacteriostatic activity. These findings may well have an impact on the antimicrobial resistance phenomenon of H. pylori, proposing the A. vera inner gel as a promising effective natural agent with CSNPs for the treatment of H. pylori infection. Antibacterial properties of A. vera inner gel were demonstrated against both susceptible and resistant H. pylori strains [8]. These results may resolve the multi-drug-resistance phenomenon particularly in cases of H. pylori. Furthermore, as proposed by Pandey and Mishra [47], the inner gel of A. vera could be greatly effective when taken orally, because, inside a living human as well as an animal, both anthraquinones and acemannans as phytoconstituents of A. vera gel were able to guarantee its complete activity.

Table 2.
Anti-Helicobacter pylori using A. vera gel and A. vera gel incorporated with CSNPs.
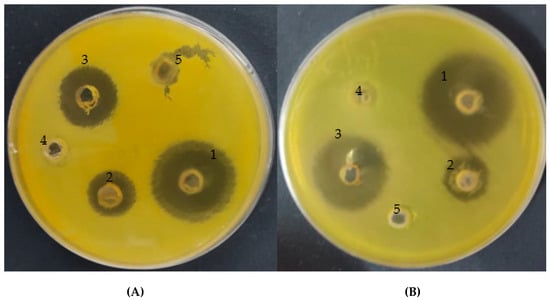
Figure 8.
Inhibitory action of A. vera gel incorporated with CSNPs (1), A. vera gel (2), positive control (3), CSNPs (4), and acetic acid (5) against resistant strain (A) and sensitive strain (B) of Helicobacter pylori.

Table 3.
MIC and MBC of A. vera gel and A. vera gel incorporated with CSNPs against resistance strain of H. pylori.
3.3.2. Antioxidant Activity
A vital role of antioxidants was reported regarding the prevention of numerous diseases associated with oxidative stress; therefore, the antioxidant activity of A. vera gel alone and incorporated with CSNPs was determined. Data in the table showed that the antioxidant activity increased with the concentration increment in a dependent manner. As noticed, A. vera gel incorporated with CSNPs encouraged the antioxidant potential compared with A. vera gel alone particularly at high concentrations (Table 4 and Figure 9). DPPH Scavenging (%) was 68.1 and 75.2% at 1000 μg/mL using A. vera gel extract and A. vera gel incorporated with CSNPs, respectively. IC50 was 138.82 μg/mL using A. vera gel extract, while it became 81.7 μg/mL incorporated with CSNPs. In the current study, we identified and quantified 13 phenolic and flavonoid compounds (Table 3), confirming that the A. vera gel is a rich source of well-known antioxidant constituents [35]. The previous literature indicated that there is a correlation among the antioxidant properties and the content of phenolic compounds [48]. Therefore, the antioxidant potential of A.vera can be influenced according to development stages that are associated with changes in active compounds ingredients as well, as reported previously [46]. Kesharwani et al. [29] reported the enhancement of the A. vera gel antioxidant and good stability properties when loaded with CSNPs.

Table 4.
Antioxidant activity of A. vera gel and A. vera gel incorporated with CSNPs.
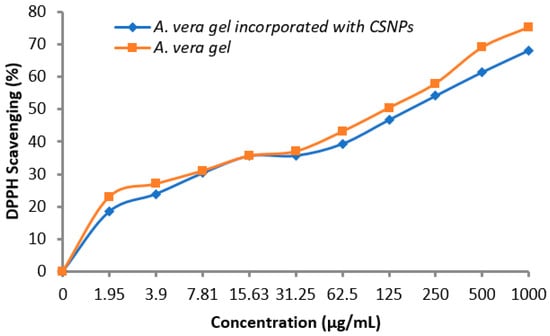
Figure 9.
Antioxidant activity of A. vera gel and A. vera gel incorporated with CSNPs.
3.3.3. Anti-Hemolytic Activity
The anti-hemolytic activity increased as a result of A. vera gel incorporated with CSNPs compared with using A. vera gel alone (Figure 10), where the hemolysis inhibition was 40.0 and 75.4%, while it was 29.2 and 63.9% at 100 and 1000 mg/mL, respectively.
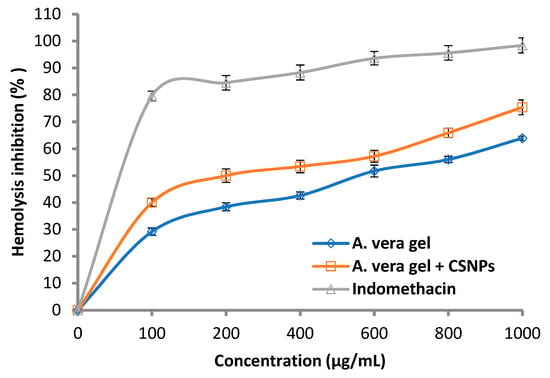
Figure 10.
Hemolysis inhibition (%) of A. vera gel, A. vera gel incorporated with CSNPs, and indomethacin.
Generally, the hemolysis inhibition increased with the concentration of A. vera gel alone or A. vera gel incorporated with CSNPs in a dose-dependent mode. The results were compared with the hemolysis inhibition caused by the standard indomethacin where lysis of erythrocytes was shown to decrease with the increase in concentration. Sharifi-Rad et al. [16] according to a literature review concluded that pre-clinical and clinical investigations promote the application of CSNPs in nanomedicine. The effects of A. vera with CSNPs on inflammation and wound healing were studied [4], where A. vera gel was effective and becoming more effective when incorporated with CSNPs.
3.4. Molecular Docking Study
Molecular docking is a method in molecular modeling that predicts the preferred orientation of one molecule to another when they are bonded in order to create a stable complex. Using scoring functions, the preferred orientation is utilized to determine the strength of connection or binding affinity between two molecules.
The goal of molecular docking is to create an optimum conformation for both the protein and the ligand, as well as a relative orientation between the two, in order to reduce the total system’s free energy. Molecular recognition is important for enhancing basic biomolecular interactions including enzyme–substrate, drug–protein, and drug–nucleic acid interactions.
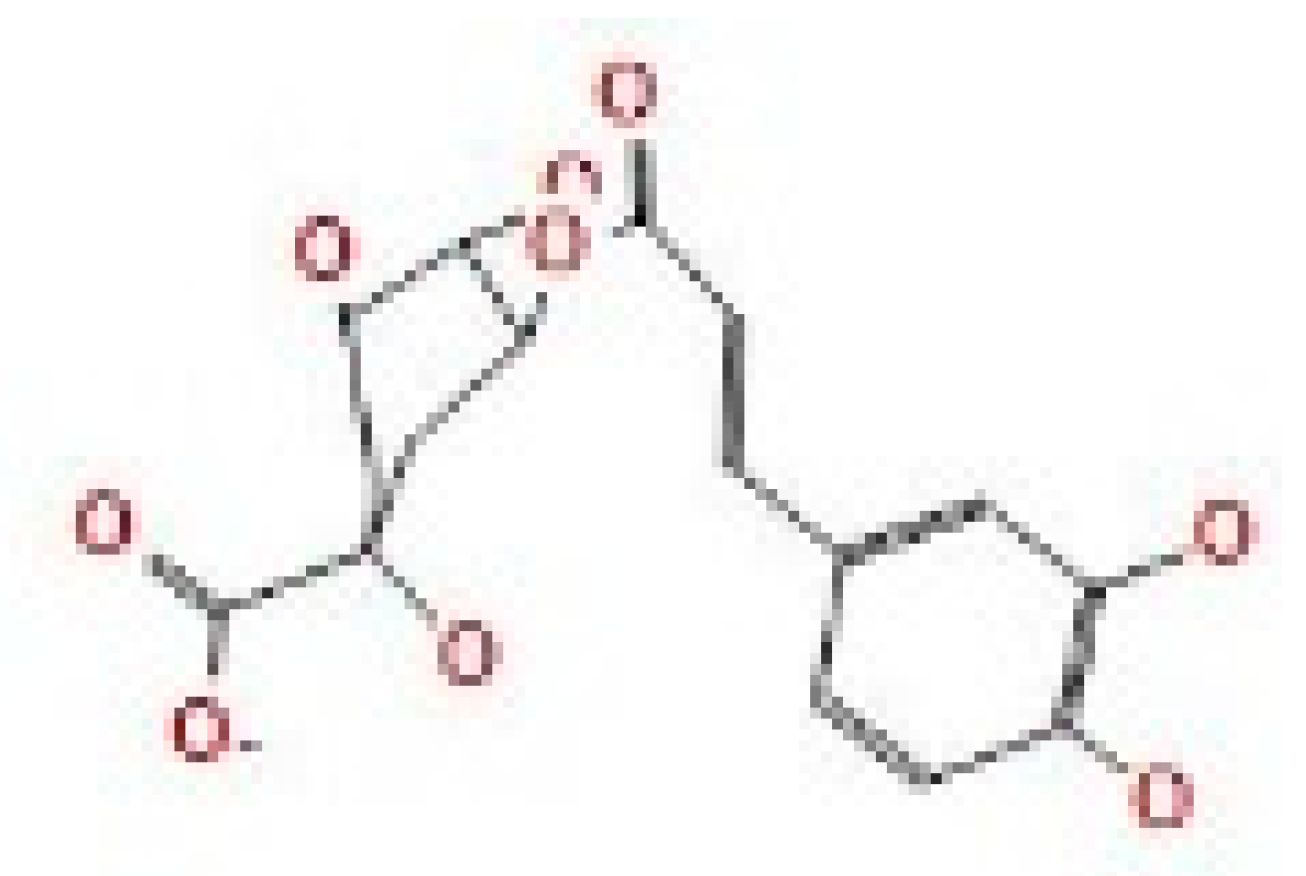
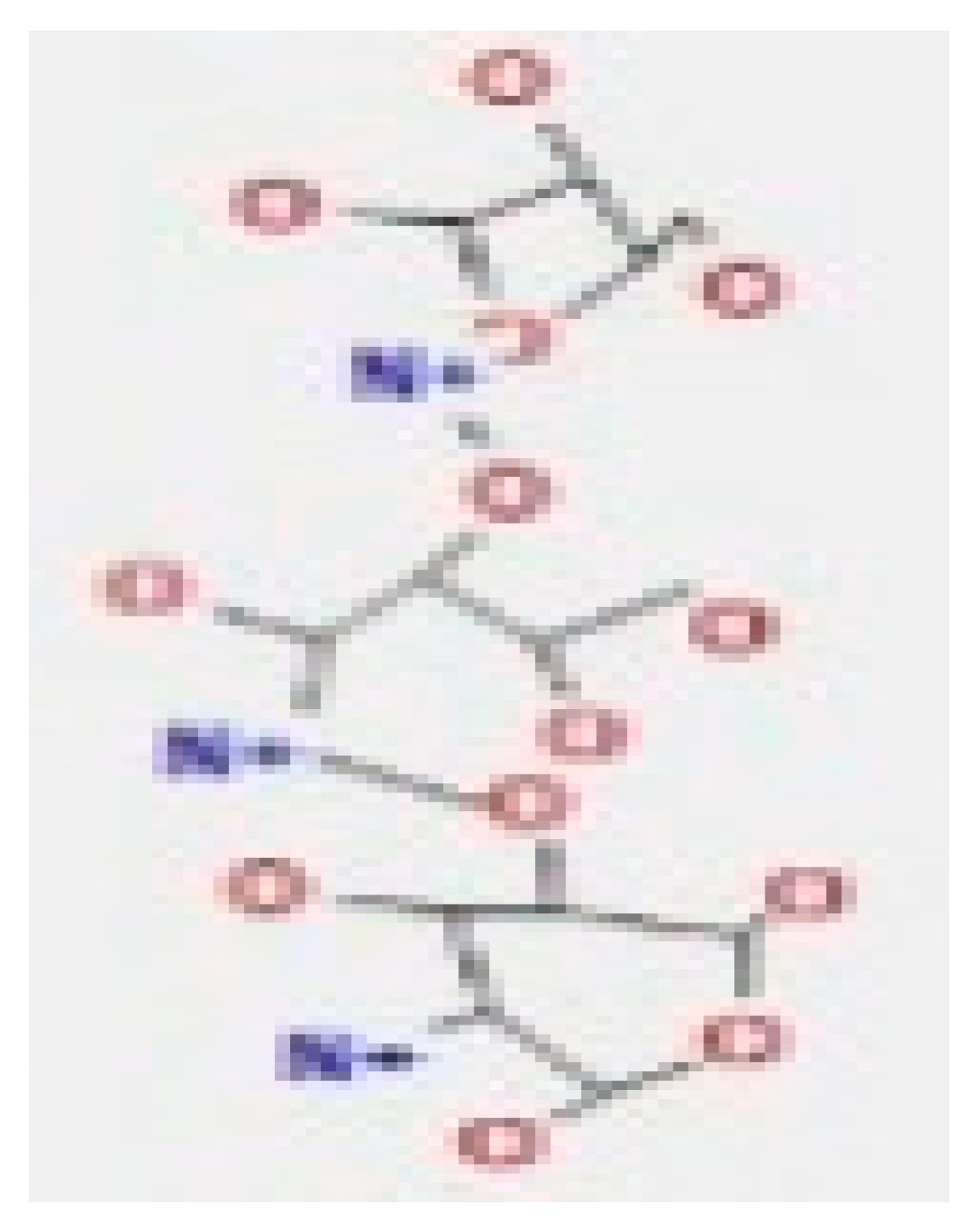
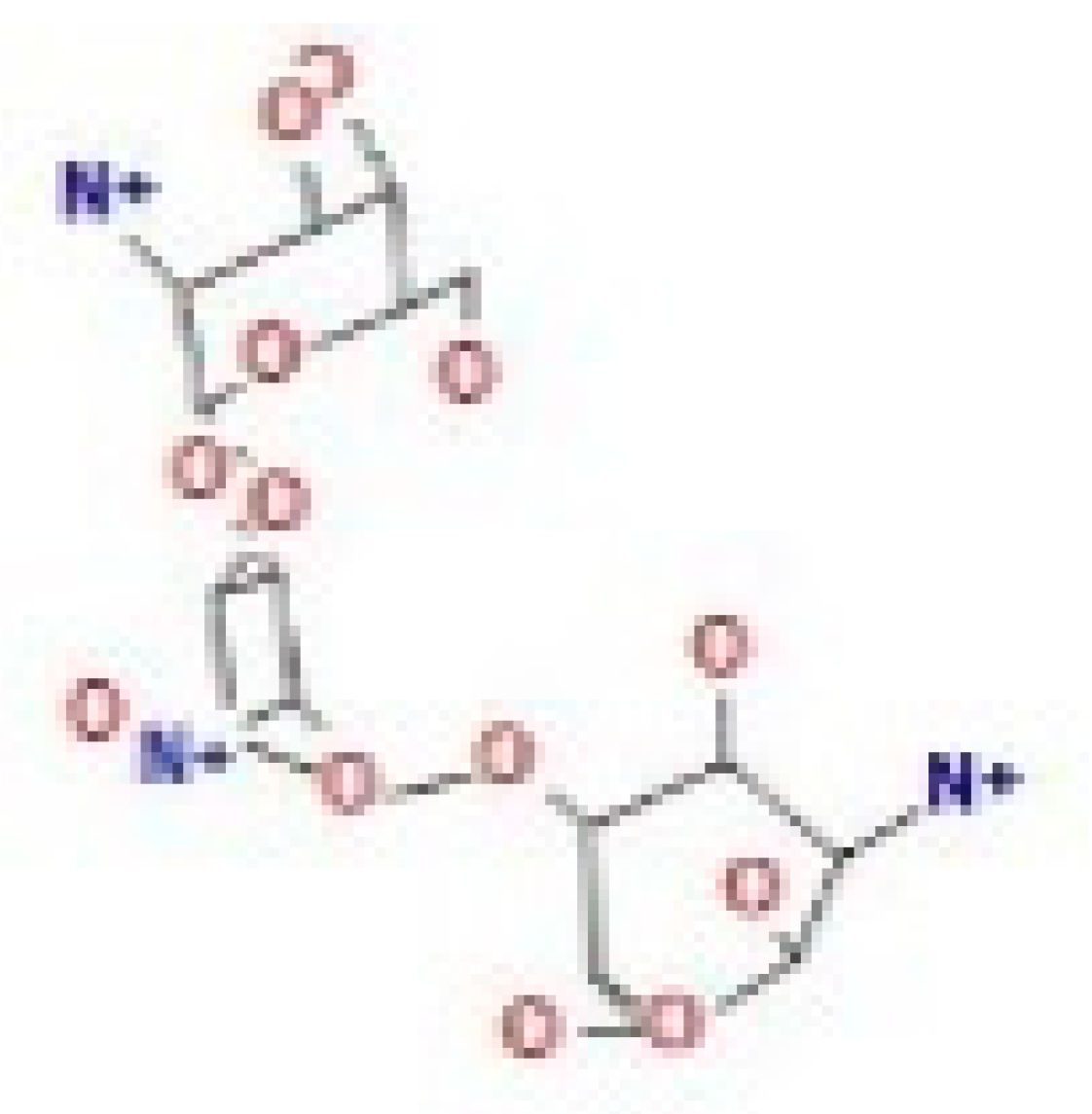
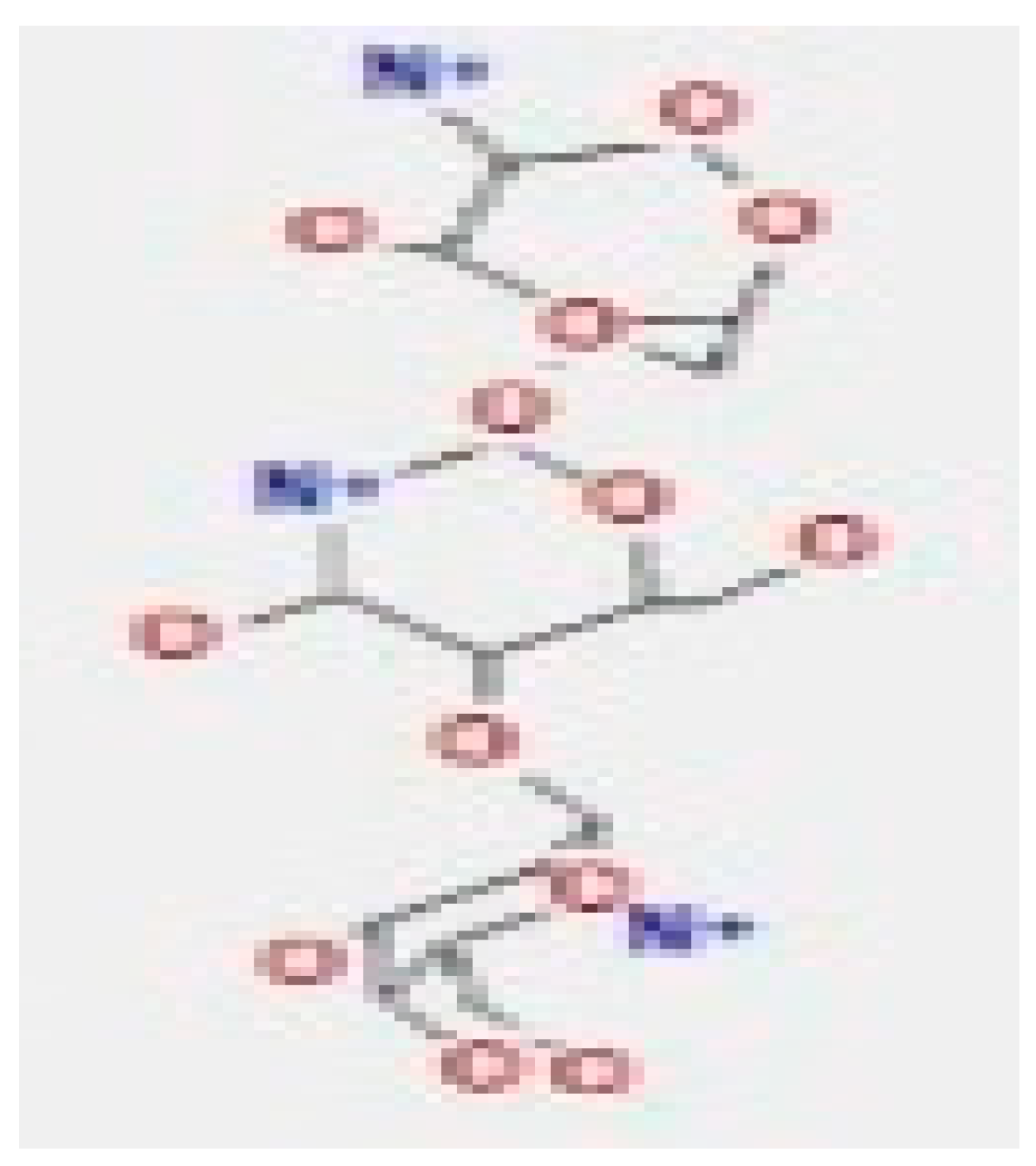
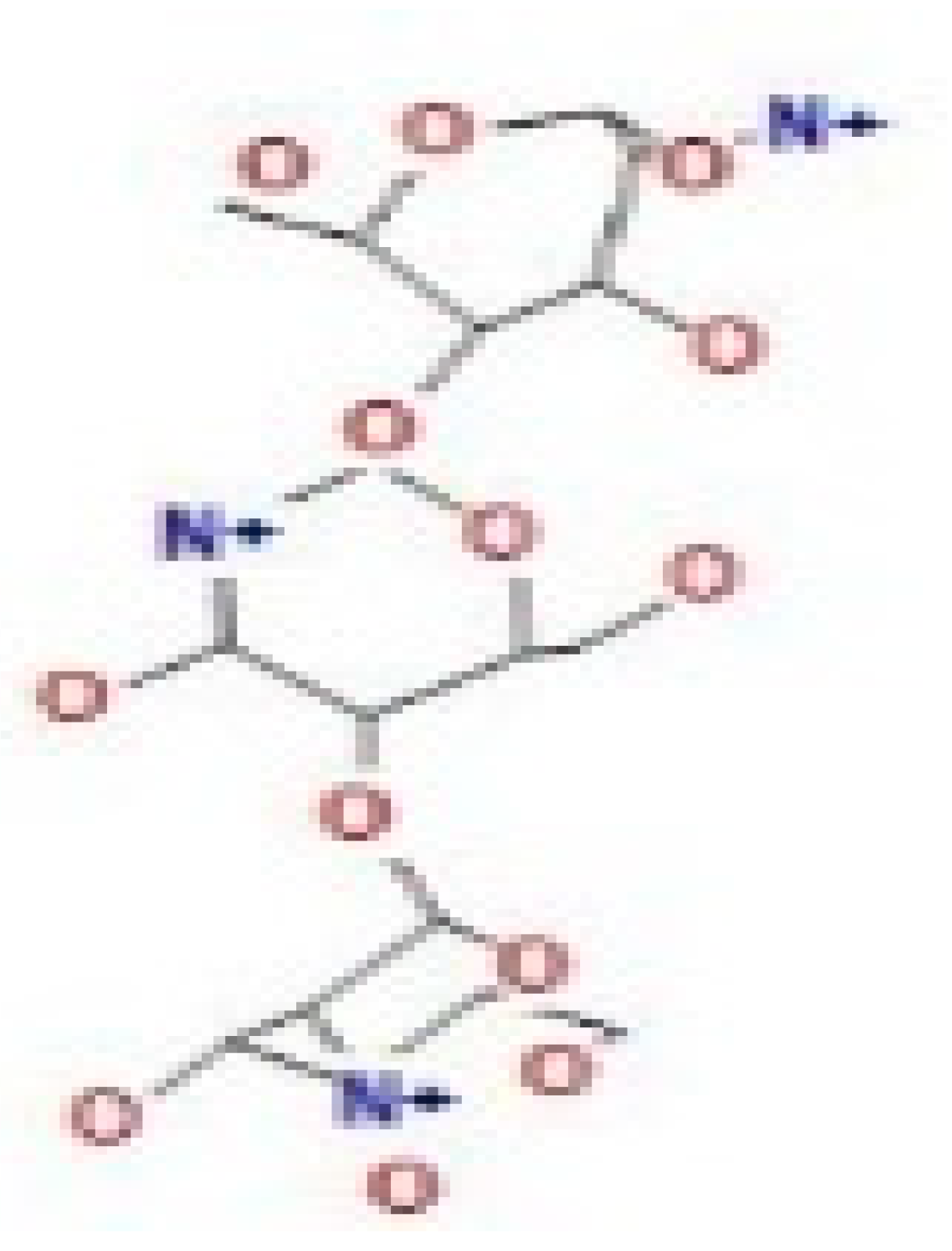
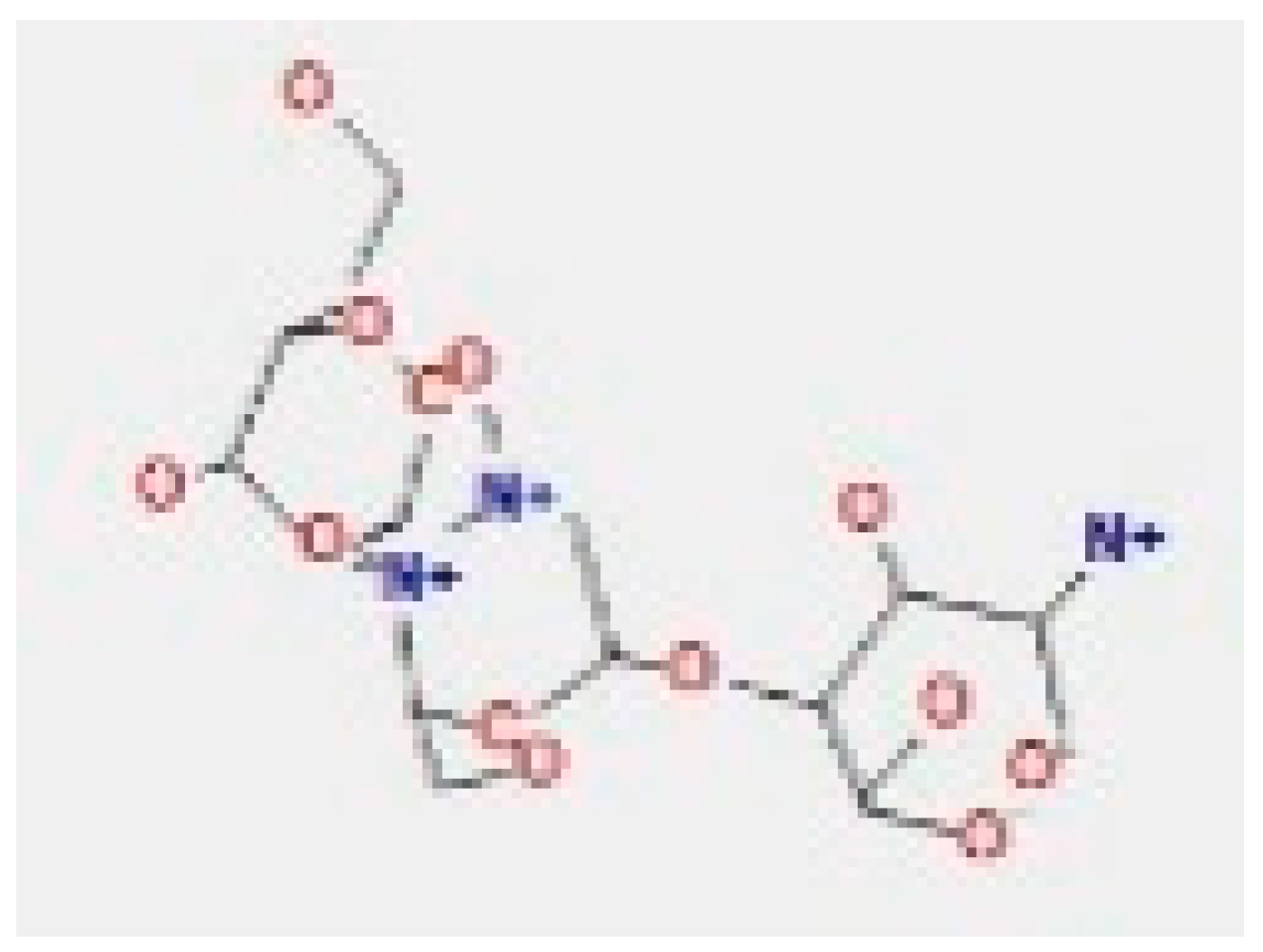
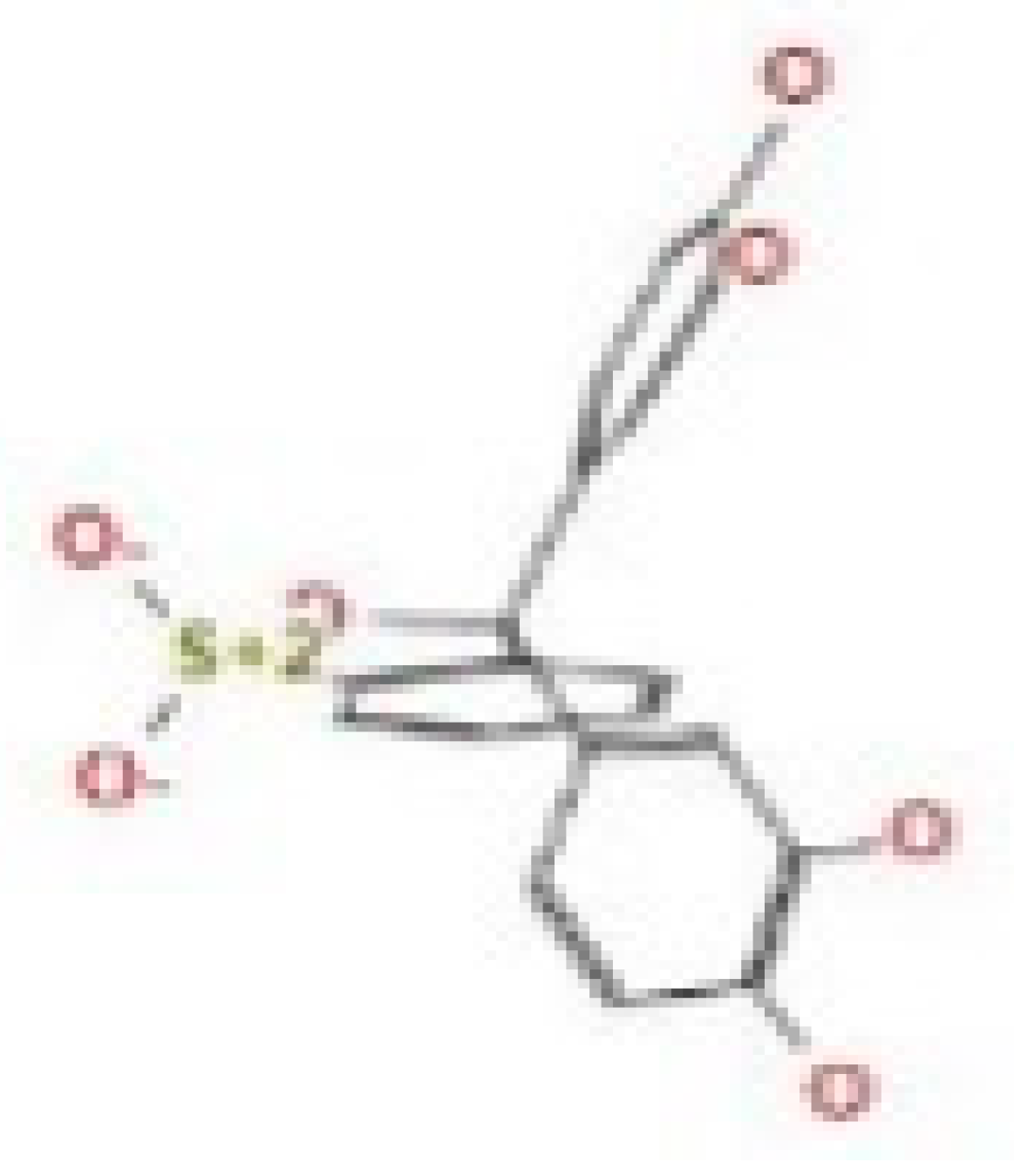
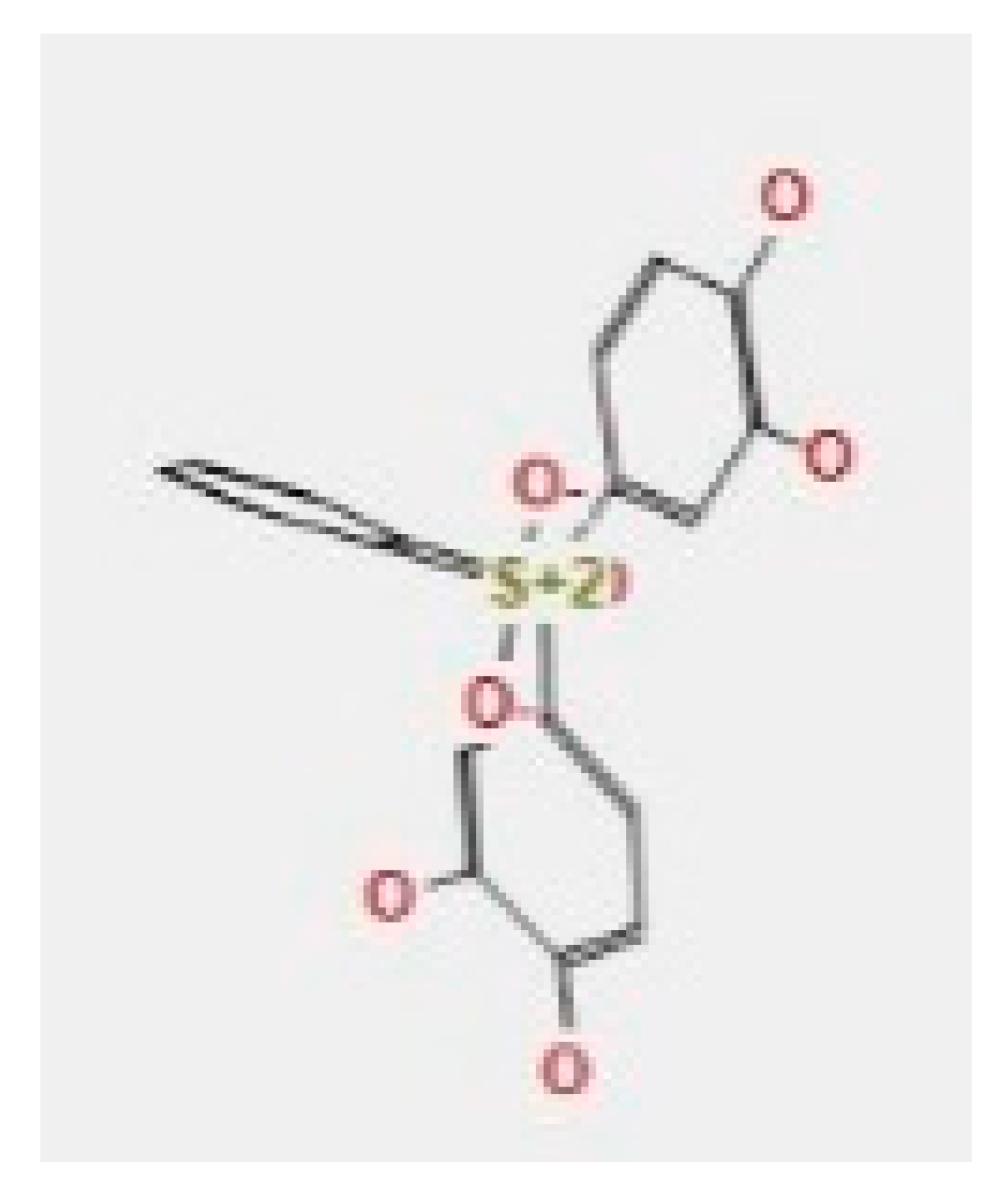
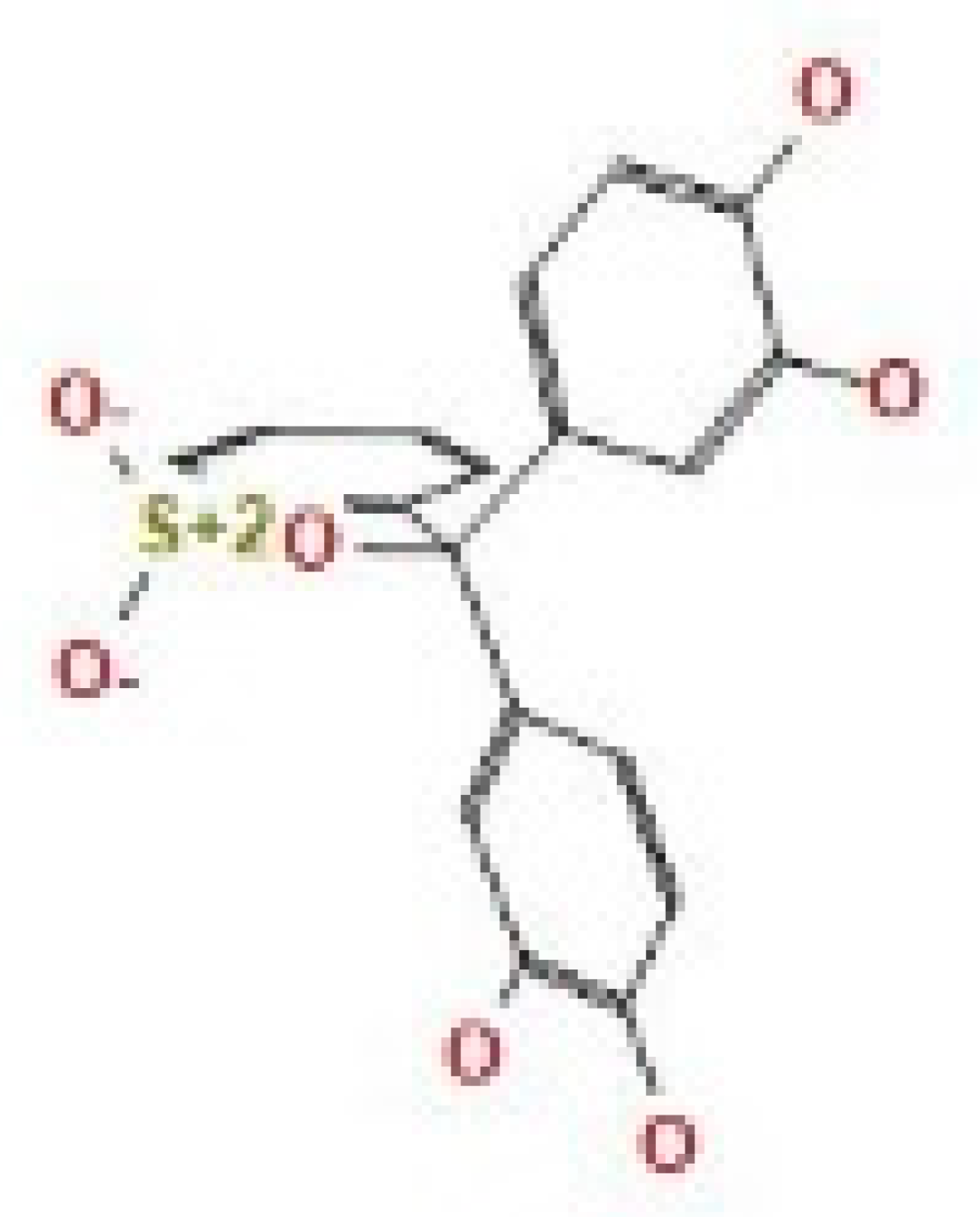
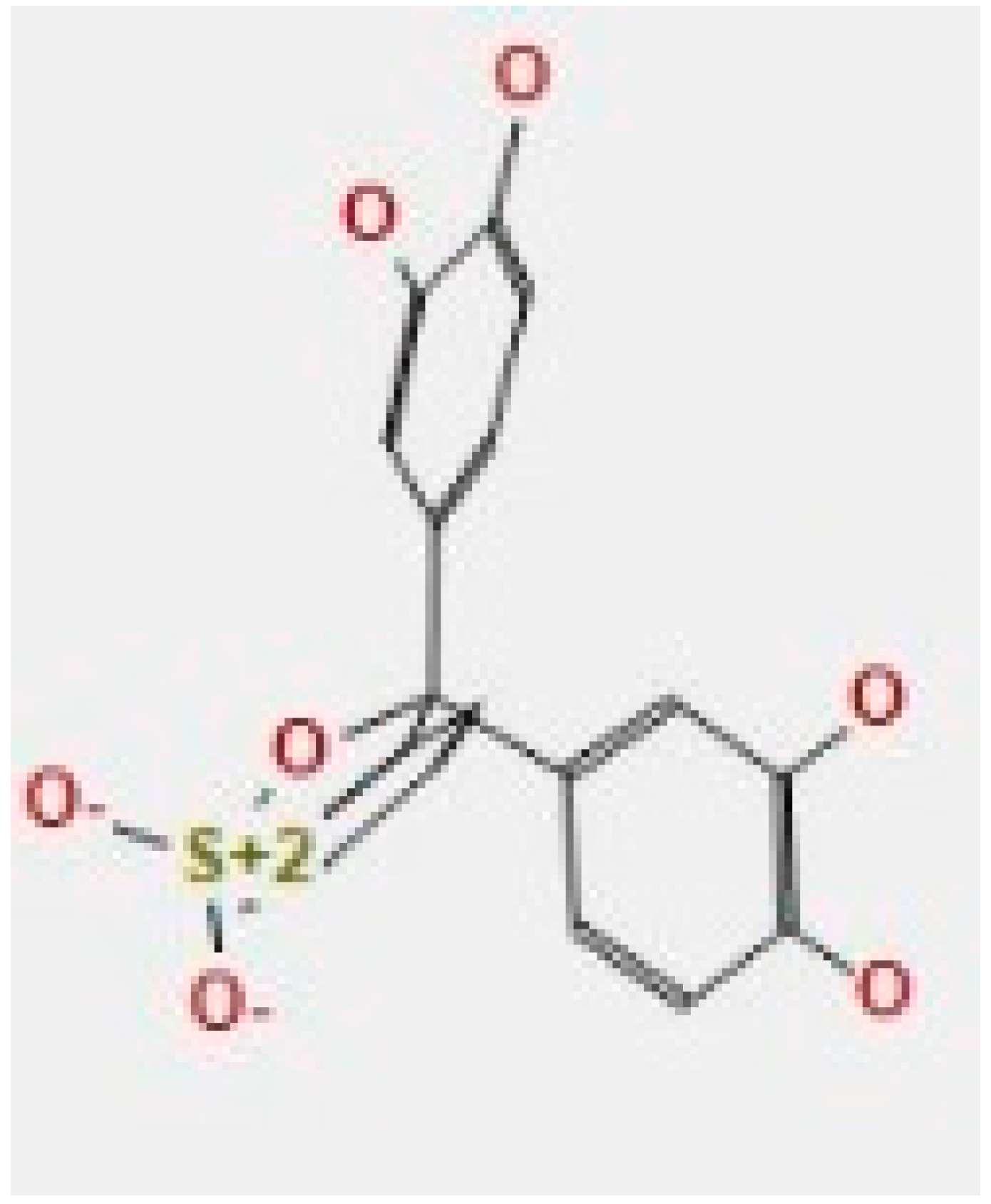
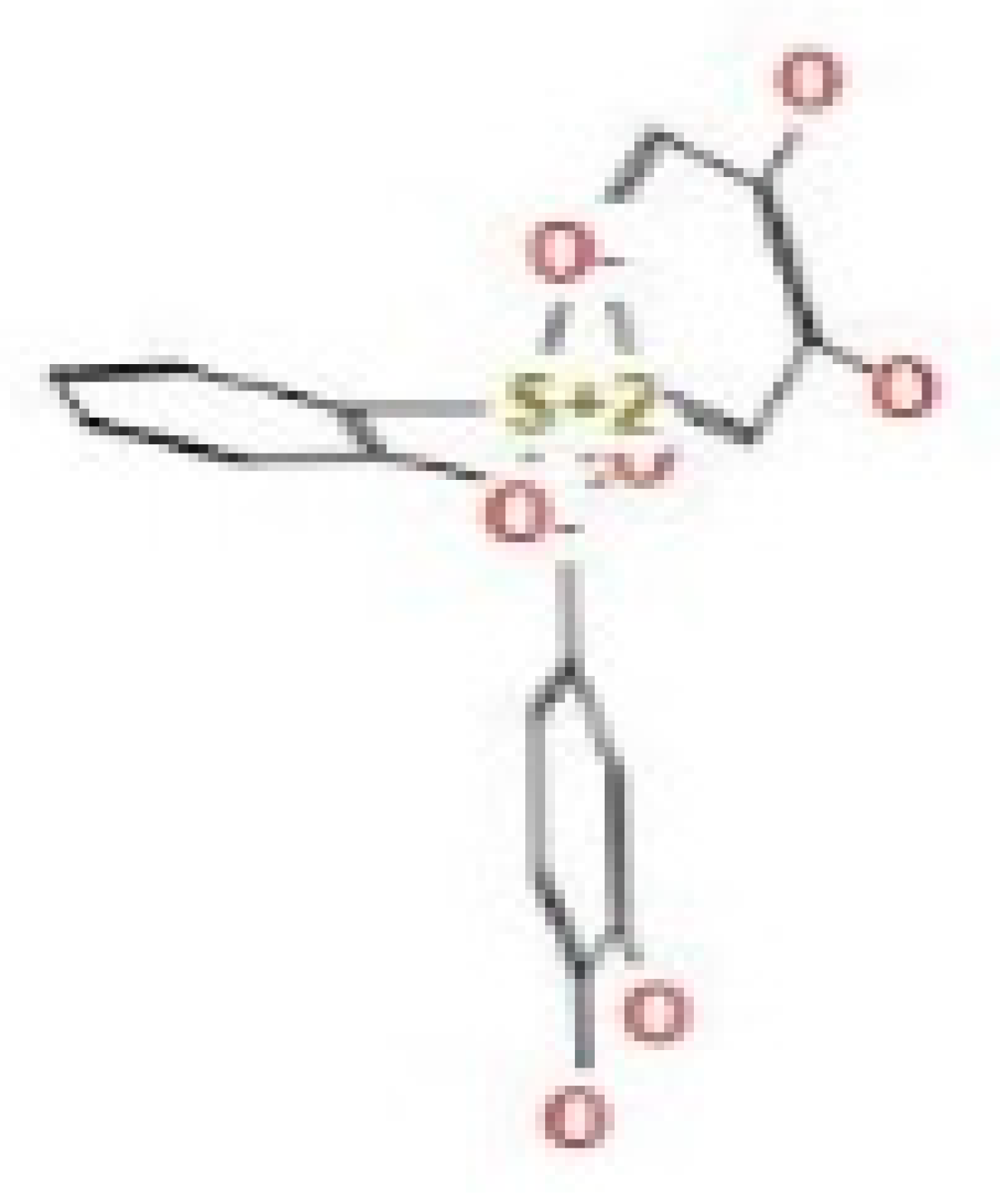
Here, we study the interaction between chlorogenic acid, chitosan, and pyrocatechol with the crystal structure of the H. pylori (4HI0) protein. The interaction among protein and compounds was visualized (Figure 11).
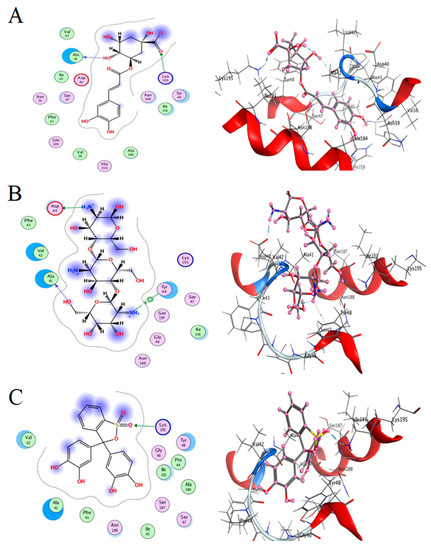
Figure 11.
Docking interactions of certain compounds of A. vera gel extract.2D and 3D diagrams show the interaction between chlorogenic acid and active sites of 4HI0 protein (A); 2D and 3D diagrams show the interaction between chitosan and active sites of 4HI0 protein (B); 2D and 3D diagrams show the interaction between pyrocatechol and active sites of 4HI0 protein (C).
The representative key for the types of interaction between chitosan, chlorogenic acid and pyrocatechol with 4HI0 protein of H. pylori was illustrated in Figure 12.
Figure 12.
The representative key for the types of interaction between chitosan and pyrocatechol.
The best fitted poses adopted by the compounds, binding energy scores, and energies are presented in Table 5. Chlorogenic acid exhibited a more potent binding energy than chitosan and pyrocatechol, which was observed as −6.4876 kcal/mol. In a recent study, chlorogenic acid had a strong interaction with the active site bound to chain (A) of the E.coli 7C7N protein, and its energy value was −6.0422 kcal mol- [49]. In addition, a molecular docking report indicated the appearance of a superior negative score of free binding energy as a result of the application of chlorogenic acid on Proteus vulgaris and Human coronavirus (HCoV 229E), validating its application for inhibiting the bacterial and viral propagation [50]. A list of hydrogen bonds between all compounds with the examined protein is presented in Table 6.

Table 5.
The best possible conformations of compounds inside the protein central activity.

Table 6.
Interaction of chlorogenic acid, pyrocatechol, and chitosan with 4HI0 Protein.
- Chlorogenic acid interacted via the (4HI0) protein with O 17 and O 23 by donating or accepting their H atoms through O ALA 41 and NZ LYS 195 of receptors.
- The interaction between chitosan and the active site bond of (4HI0) revealed the presence of a hydrogen donor atom between N 15 and N 67 in the ligand and the O ALA 41 and OD1 ASP 40 amino acid residue, in addition to the noncovalent molecular interaction (Cation-Pi) between the N 21 atom in the ligand and 6-ring TYR 48 amino acid receptor.
- The docked pyrocatechol with receptor active sites of (4HI0) indicated the presence of a hydrogen acceptor atom between O 41 of the ligand and the NZ LYS 195 amino acid residue with a distance of 2.85 oA.
The docking pose and types of interaction agreed with the experimental results of the antibacterial activity of the main constituents of A. vera gel and CSNPs against H. pylori.
4. Conclusions
From HPLC analysis, A. vera gel is rich with various powerful antimicrobial and antioxidant constituents comprising flavonoids and phenolic contents. CSNPs displayed a relevant enhancement of the bacteriostatic activity of A. vera gel against H. pylori, as well as antioxidant and hemolysis inhibition. The experimental findings of the efficacy of A. vera gel and A. vera gel incorporated with CSNPs against H. pylori were documented. A docking study on the interaction of chlorogenic acid and pyrocatechol as the major constituent of A. vera gel, as well as CSNPs with a 4HI0 protein of H. pylori, confirmed the anti- H. pylori activity.
Author Contributions
R.Y.: designed and performed some experiments, writing—review and editing; A.M.H.A.-R.: carried out some of the experiments, formal analysis, and editing of the manuscript; S.Z.A.: designed some experiments and wrote the introduction; M.A.A.A.: designed some experiments, writing—review and editing of the first draft of the manuscript; M.S.A.: designed some experiments and wrote the discussion; S.K.A.J.: designed some experiments, English editing of the manuscript; S.S.: designed some experiments, writing—review and editing of final draft; K.S.I.: development and design of methodology, writing—original draft preparation; T.M.A.: carried out some experiments, designed the docking studies, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data that support the findings of this study are available within the article.
Acknowledgments
To Princess Nourah bint Abdulrahman University for their grant through Researchers Supporting Project number PNURSP2022R217, Princess Nourah bint Abdulrahman University, Riyadh, Sau-di Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bajer, D.; Janczak, K.; Bajer, K. Novel Starch/Chitosan/Aloe vera Composites as Promising Biopackaging Materials. J. Polym. Environ. 2020, 28, 1021–1039. [Google Scholar] [CrossRef] [Green Version]
- Doddanna, S.J.; Patel, S.; Sundarrao, M.A.; Veerabhadrappa, R.S. Antimicrobial activity of plant extracts on Candida albicans: An in vitro study. Indian J. Dent. Res. 2013, 24, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants—A Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjbar, R.; Yousefi, A. Effects of Aloe vera and Chitosan Nanoparticle Thin-Film Membranes on Wound Healing in Full Thickness Infected Wounds with Methicillin Resistant. Staphylococcus aureus. Bull. Emerg. Trauma 2018, 6, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Tayal, E.; Sardana, D.; InduShekar, K.R.; Saraf, B.G.; Sheoran, N. Current perspectives on use of Aloe vera in dentistry. Eur. J. Med. Plants 2014, 4, 1408–1419. [Google Scholar] [CrossRef]
- Shilpa, M.; Bhat, V.; Shetty, A.V.; Reddy, M.S.; Punde, P. Antifungal Activity of Aloe vera Leaf and Gel Extracts Against Candida albicans: An In Vitro Study. World J. Dent. 2020, 11, 36–40. [Google Scholar] [CrossRef]
- Bhandari, A.; Crowe, S.E. Helicobacter pylori in gastric malignancies. Curr. Gastroenterol. Rep. 2012, 14, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Cellini, L.; Di Bartolomeo, S.; Di Campli, E.; Genovese, S.; Locatelli, M.; Di Giulio, M. In vitro activity of Aloe vera inner gel against Helicobacter pylori strains. Lett. Appl. Microbiol. 2014, 59, 43–48. [Google Scholar] [CrossRef]
- Nostro, A.; Cellini, L.; Di Bartolomeo, S.; Di Campli, E.; Grande, R.; Cannatelli, M.A.; Marzio, L.; Alonzo, V. Antibacterial effect of plant extracts against Helicobacter pylori. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005, 19, 198–202. [Google Scholar] [CrossRef]
- Babaee, N.; Zabihi, E.; Mohseni, S.; Moghadamnia, A.A. Evaluation of the therapeutic effects of Aloe vera gel on minor recurrent aphthous stomatitis. Dent. Res. J. 2012, 9, 381–385. [Google Scholar]
- Salem, S.S.; Hammad, E.N.; Mohamed, A.A.; El-Dougdoug, W. A Comprehensive Review of Nanomaterials: Types, Synthesis, Characterization, and Applications. Biointerface Res. Appl. Chem. 2022, 13, 2023. [Google Scholar]
- Hashem, A.H.; Shehabeldine, A.M.; Ali, O.M.; Salem, S.S. Synthesis of Chitosan-Based Gold Nanoparticles: Antimicrobial and Wound-Healing Activities. Polymers 2022, 14, 2293. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional Silver Nanoparticles Based on Chitosan: Antibacterial, Antibiofilm, Antifungal, Antioxidant, and Wound-Healing Activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Salem, S.S. Green and ecofriendly biosynthesis of selenium nanoparticles using Urtica dioica (stinging nettle) leaf extract: Antimicrobial and anticancer activity. Biotechnol. J. 2021, 17, 2100432. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, T.M. Stachybotrys chartarum: A novel biological agent for the extracellular synthesis of silver nanoparticles and their antimicrobial activity. Indones. J. Biotechnol. 2013, 18, 75–82. [Google Scholar]
- Abdelghany, T.M.; Al-Rajhi, A.M.; Al Abboud, M.A.; Alawlaqi, M.M.; Ganash Magdah, A.; Helmy, E.A.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. BioNanoScience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Ganash, M.; Abdel Ghany, T.M.; Omar, A.M. Morphological and biomolecules dynamics of Phytopathogenic Fungi under stress of silver nanoparticles. BioNanoScience 2018, 8, 566–573. [Google Scholar] [CrossRef]
- Salem, S.S. Bio-fabrication of Selenium Nanoparticles Using Baker’s Yeast Extract and Its Antimicrobial Efficacy on Food Borne Pathogens. Appl. Biochem. Biotechnol. 2022, 194, 1898–1910. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.; Yahya, R.; Bakri, M.M.; Yahya, R.; Abdelghany, T.M. In situ green synthesis of Cu-doped ZnO based polymers nanocomposite with studying antimicrobial, antioxidant and anti-inflammatory activities. Appl. Biol. Chem. 2022, 65, 35. [Google Scholar] [CrossRef]
- Salem, S.S.; Badawy, M.S.E.M.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green Biosynthesis of Selenium Nanoparticles Using Orange Peel Waste: Characterization, Antibacterial and Antibiofilm Activities against Multidrug-R sistant Bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Salem, S.S.; Alharbi, A.A.; Abdelghany, T.M. Ecofriendly synthesis of silver nanoparticles using Kei-apple (Dovyalis caffra) fruit and their efficacy against cancer cells and clinical pathogenic microorganisms. Arab. J. Chem. 2022, 15, 103927. [Google Scholar] [CrossRef]
- Salem, S.S.; Ali, O.M.; Reyad, A.M.; Abd-Elsalam, K.A.; Hashem, A.H. Pseudomonas indica-Mediated Silver Nanoparticles: Antifungal and Antioxidant Biogenic Tool for Suppressing Mucormycosis Fungi. J. Fungi 2022, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Calina, D. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-based (Nano) materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, R.; Osman, H.; Hassan, I. Preparation of biocompatible chitosan nanoparticles loaded with Aloe vera extract for use as a novel drug delivery mechanism to improve the antibacterial characteristics of cellulose-based fabrics. Egypt. J. Chem. 2022, 65, 589–604. [Google Scholar] [CrossRef]
- Amiri, A.; Ramezanian, A.; Mortazavi, S.M.H.; Hosseini, S.M.H.; Yahia, E. Shelf-life extension of pomegranate arils using chitosan nanoparticles loaded with Satureja hortensis essential oil. J. Sci. Food Agric. 2021, 101, 3778–3786. [Google Scholar] [CrossRef]
- Kesharwani, S.; Gupta, D.K.; Gupta, M.K. In-Vitro Evaluation of Drug Release and Antioxidant Activity of Aloe Loaded Chitosan Nanoparticles. J. Drug Deliv. Ther. 2019, 9, 43–52. [Google Scholar] [CrossRef]
- Barrera-Necha, L.L.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Hernández-López, M.; Jiménez, J.E.M.; Mejía, A.F.M. Synthesis and Characterization of Chitosan Nanoparticles Loaded Botanical Extracts with Antifungal Activity on Colletotrichum gloeosporioides and Alternaria species. Adv. Microbiol. 2018, 8, 286–296. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Yahya, R.; Bakri, M.M.; Ganash, M.; Amin, B.H.; Qanash, H. Effect of Thevetia peruviana Seeds Extract for Microbial Pathogens and Cancer Control. Int. J. Pharmacol. 2021, 17, 643–655. [Google Scholar] [CrossRef]
- Torlak, E.; Sert, D. Antibacterial effectiveness of chitosan–propolis coated-polypropylene films against foodborne pathogens. International. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Juarez, I.; Rivero-Cruz, F.; Celis, H.; Romero, I. Anti-Helicobacter pylori activity of anacardic acids from Amphipterygium adstringens. J. Ethnopharmacol. 2007, 114, 72–77. [Google Scholar] [CrossRef] [PubMed]
- French, G.L. Bactericidal agents in the treatment of MRSA infections–the potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107. [Google Scholar] [CrossRef] [Green Version]
- López, A.; De Tangil, M.S.; Vega-Orellana, O.; Ramírez, A.S.; Rico, M. Phenolic Constituents, Antioxidant and Preliminary Antimycoplasmic Activities of Leaf Skin and Flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules 2013, 18, 4942–4954. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Mei, N. Aloe vera: A review of toxicity and adverse clinical effects. J. Environ. Sci. Health. Part C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 77–96. [Google Scholar] [CrossRef]
- Hassan, H.S.; EL-Hefny, M.; Ghoneim, I.M.; El-Lahot, M.S.R.A.; Akrami, M.; Al-Huqail, A.A.; Ali, H.M.; Abd-Elkader, D.Y. Assessing the Use of Aloe vera Gel Alone and in Combination with Lemongrass Essential Oil as a Coating Material for Strawberry Fruits: HPLC and EDX Analyses. Coatings 2022, 12, 489. [Google Scholar] [CrossRef]
- Numan, I.N. Identification of Flavonoids and Phenolic Compound in Aloe vera gel by HPLC. Tikrit J. Pure Sci. 2018, 23, 91–94. [Google Scholar]
- Thamilarasan, V.; Sethuraman, V.; Gopinath, K.; Balalakshmi, C.; Govindarajan, M.; Mothana, R.A.; Benelli, G. Single Step Fabrication of Chitosan Nanocrystals Using Penaeus semisulcatus: Potential as New Insecticides, Antimicrobials and Plant Growth Promoters. J. Clust. Sci. 2018, 29, 375–384. [Google Scholar] [CrossRef]
- Vaezifar, S.; Razavi, S.; Golozar, M.A.; Karbasi, S.; Neaz, M.M.; Kamali, M. Effects of Some Parameters on Particle Size Distribution of Chitosan Nanoparticles Prepared by Ionic Gelation Method. J. Clust. Sci. 2013, 24, 891–903. [Google Scholar] [CrossRef]
- Ishkeh, S.R.; Shirzad, H.; Asghari, M.; Alirezalu, A.; Pateiro, M.; Lorenzo, J.M. Effect of Chitosan Nanoemulsion on Enhancing the Phytochemical Contents, Health Promoting Components, and Shelf Life of Raspberry (Rubus sanctus Schreber). Appl. Sci. 2021, 11, 2224. [Google Scholar] [CrossRef]
- Branca, C.; Angelo, G.; Crupi, C.; Khouzami, K.; Rifci, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay flms. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Nejatzadeh-Barandozi, F.; Enferadi, S.T. FT-IR study of the polysaccharides isolated from the skin juice, gel juice, and flower of Aloe vera tissues affected by fertilizer treatment. Org. Med. Chem. Lett. 2012, 2, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Giner, S.; Wilkanowicz, S.; Melendez-Rodriguez, B.; Lagaron, J.M. Nanoencapsulation of Aloe vera in Synthetic and Naturally Occurring Polymers by Electrohydrodynamic Processing of Interest in Food Technology and Bioactive Packaging. J. Agric. Food Chem. 2017, 65, 4439–4448. [Google Scholar] [CrossRef]
- Prabjone, R.; Thong-Ngam, D.; Wisedopas, N.; Chatsuwan, T.; Patumraj, S. Anti-inflammatory effects of Aloe vera on leukocyte-endothelium interaction in the gastric microcirculation of Helicobacter pylori-infected rats. Clin. Hemorheol. Microcirc. 2006, 35, 359–366. [Google Scholar]
- Gorsi, F.I.; Kausar, T.; Murtaza, M.A. Evaluation of antibacterial and antioxidant activity of Aloe vera (Aloe barbadensis Miller) gel powder using different solvents. Pure Appl. Biol. 2019, 8, 1265–1270. [Google Scholar] [CrossRef]
- Pandey, R.; Mishra, A. Antibacterial activities of crude extract of Aloe barbadensis to clinically isolated bacterial pathogens. Appl. Biochem. Biotechnol. 2010, 160, 1356–1361. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Ganash, M.; Alawlaqi, M.M.; Al-Rajhi, A.M.H. Antioxidant, antitumor, antimicrobial activities evaluation of Musa paradisiaca L. pseudostem exudate cultivated in Saudi Arabia. BioNanoScience 2019, 9, 172–178. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Yahya, R.; Abdelghany, T.M.; Fareid, M.A.; Mohamed, A.M.; Amin, B.H.; Masrahi, A.S. Anticancer, Anticoagulant, Antioxidant and Antimicrobial Activities of Thevetia peruviana Latex with Molecular Docking of Antimicrobial and Anticancer Activities. Molecules 2022, 27, 3165. [Google Scholar] [CrossRef]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; TM, A. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).