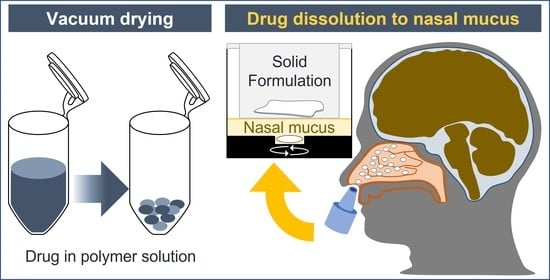
Formulation and In Vitro Characterization of a Vacuum-Dried Drug–Polymer Thin Film for Intranasal Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Vacuum Drying
2.3. Physical Analysis of Film Formulations
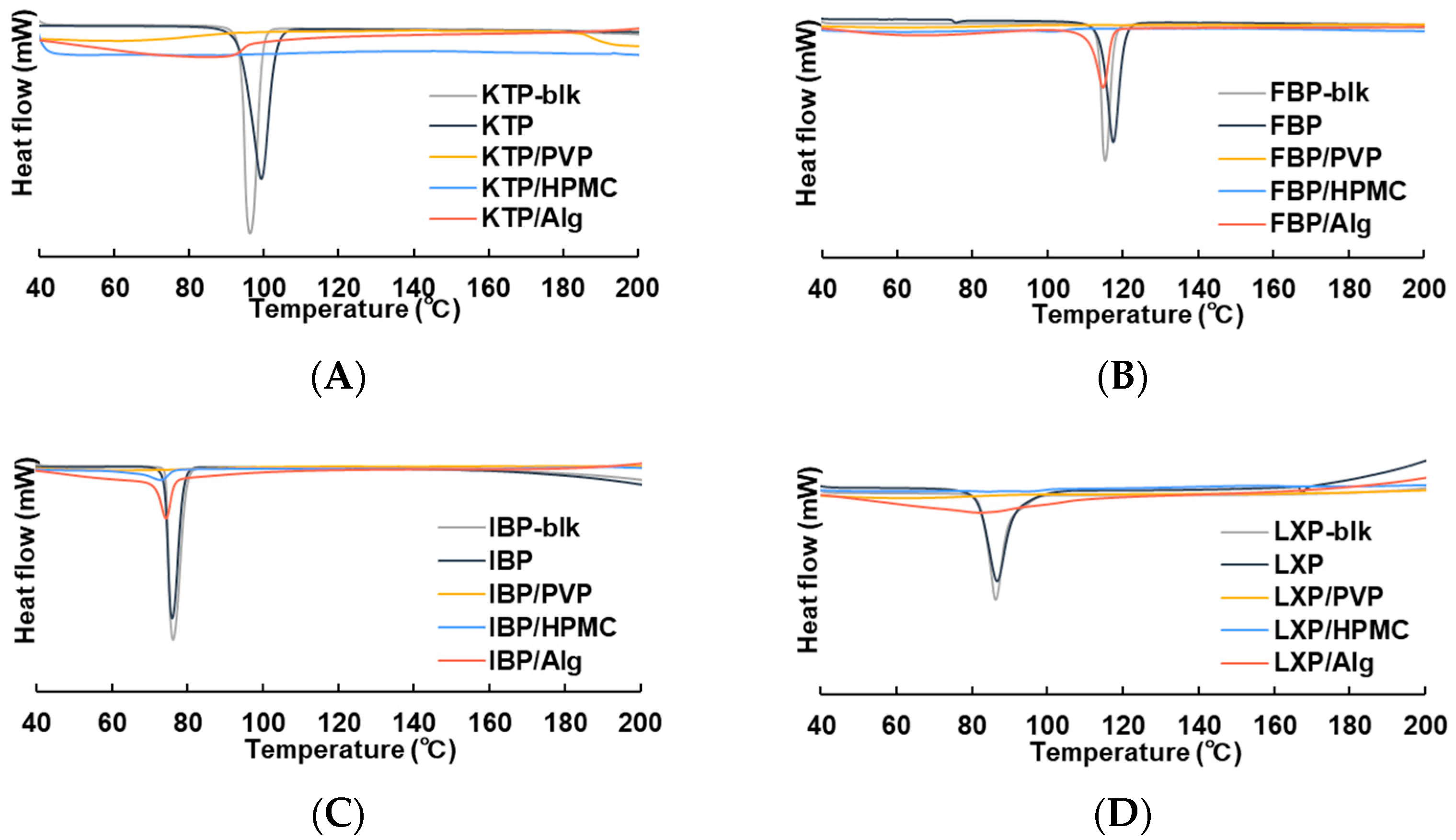
2.3.1. Differential Scanning Calorimetry (DSC)
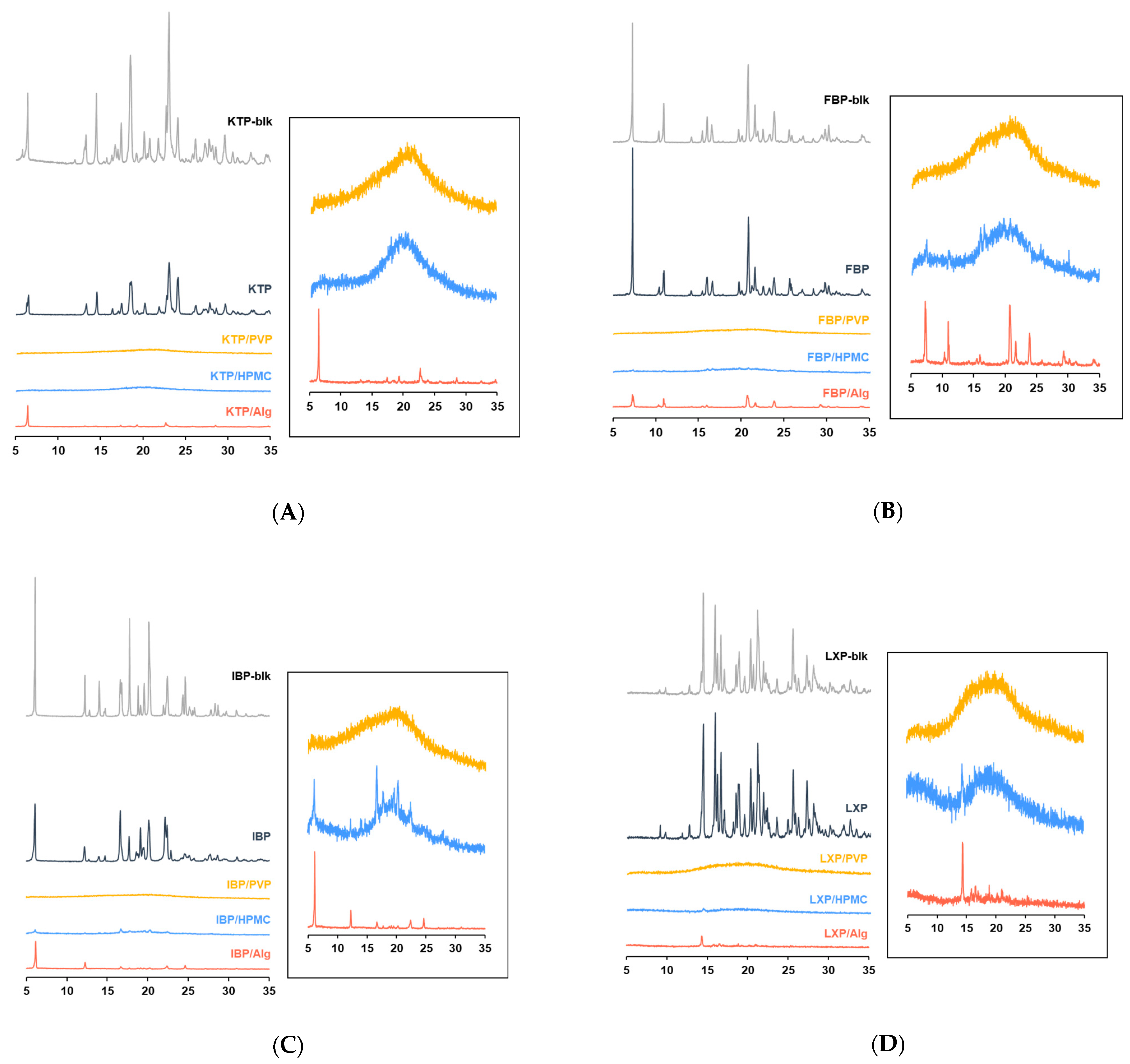
2.3.2. Powder X-ray Diffraction Analysis (PXRD)
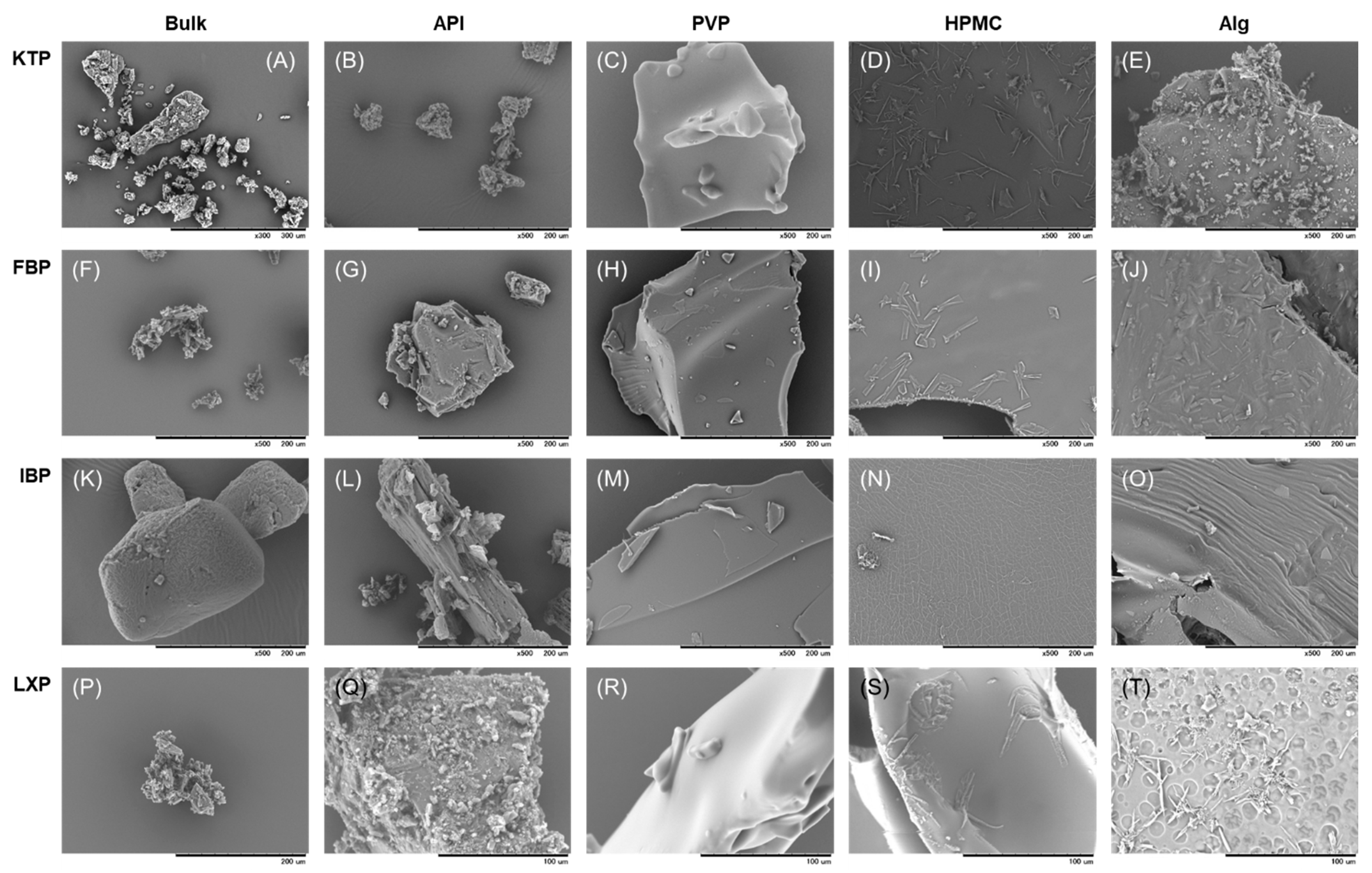
2.3.3. Scanning Electron Microscopy (SEM)
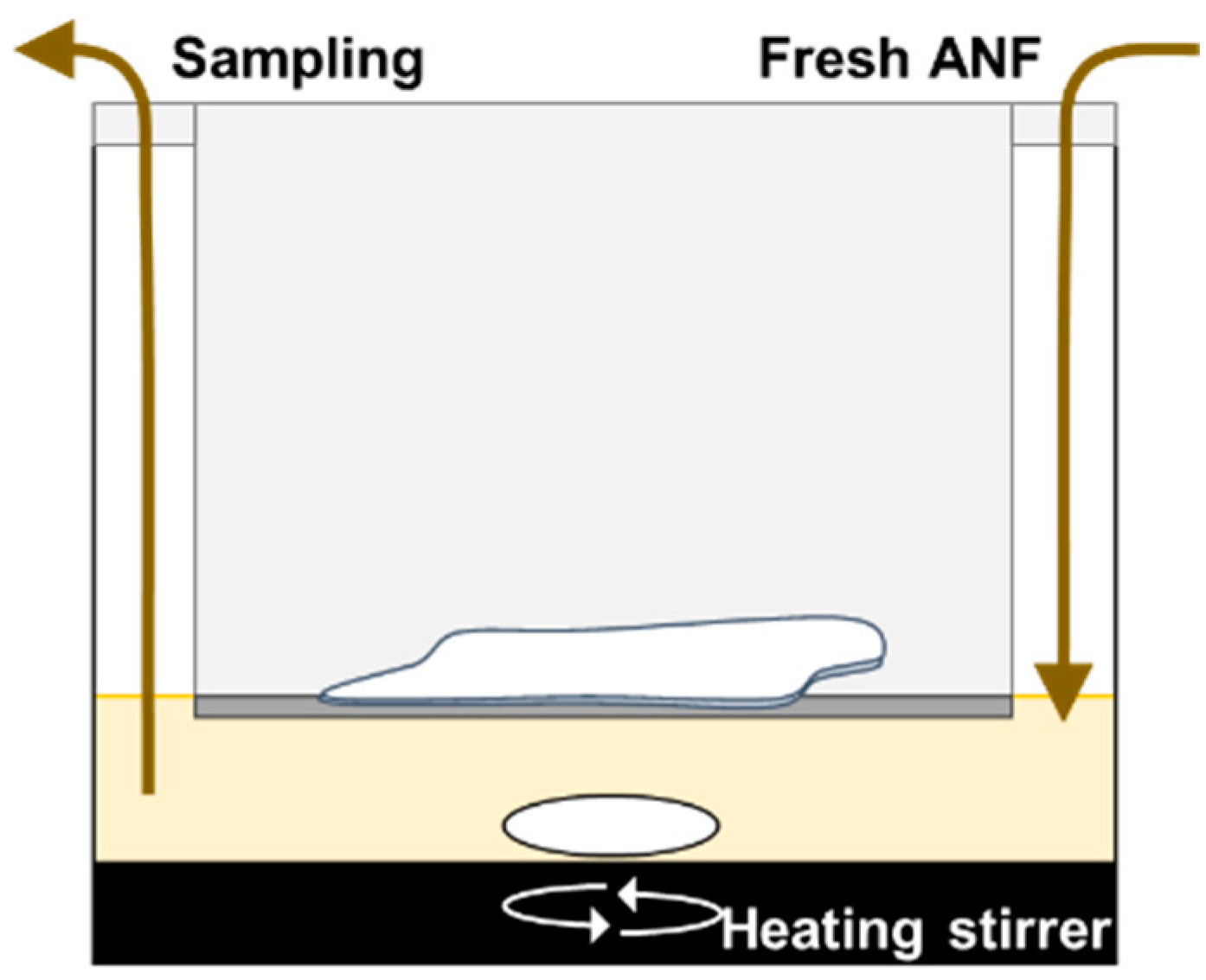
2.4. Preparation of Artificial Nasal Fluid (ANF)
2.5. In Vitro Evaluation for Drug Dissolution in Nasal Mucus
2.5.1. In Vitro Evaluation System for Intranasal Dissolution of Solid Formulation
2.5.2. Sample Treatment
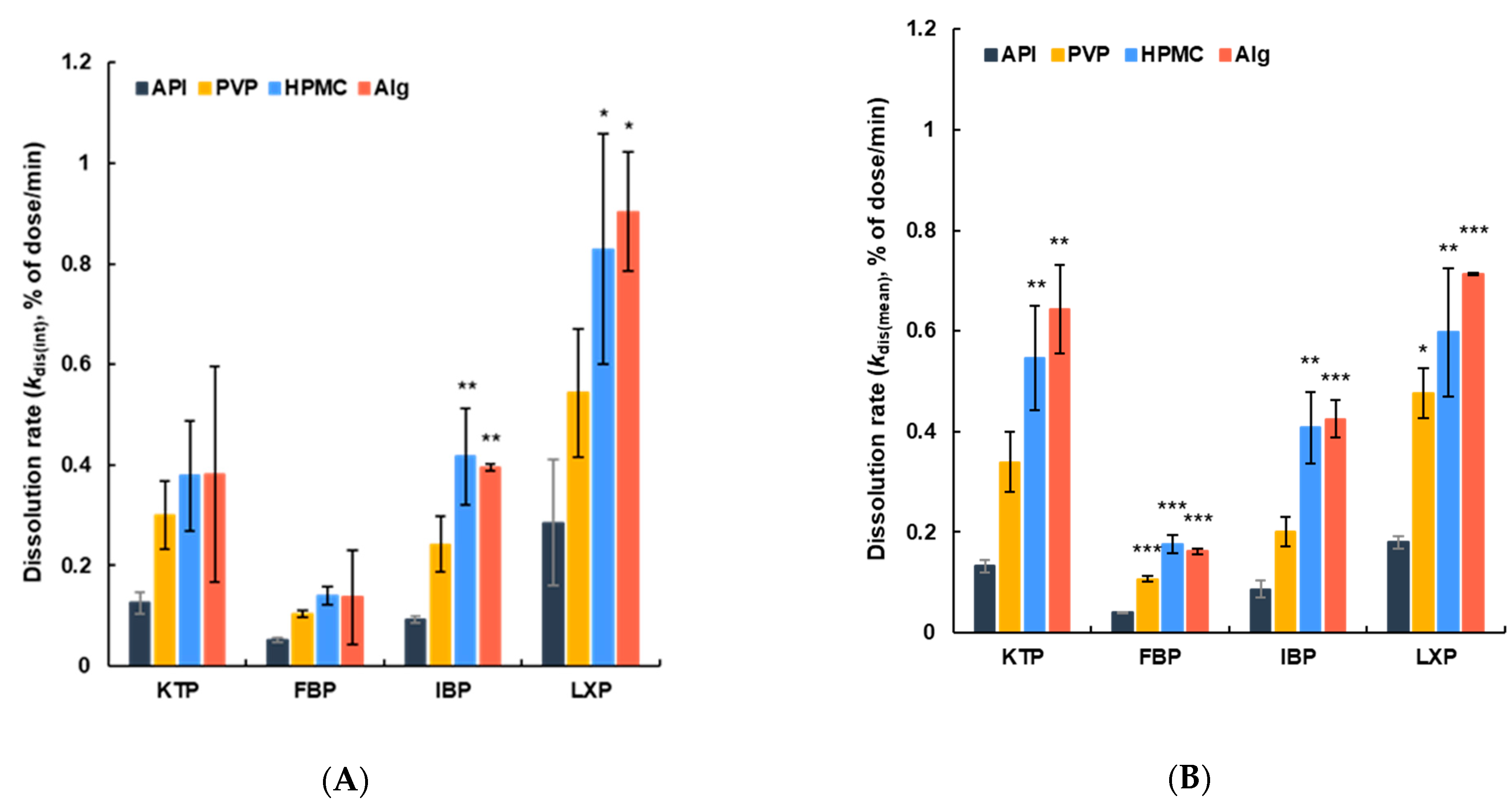
2.5.3. Dissolution Rate
2.6. Statistical Analysis
3. Results
3.1. Characterization of Vacuum Dried Thin Films
3.1.1. DSC
3.1.2. PXRD
3.1.3. SEM
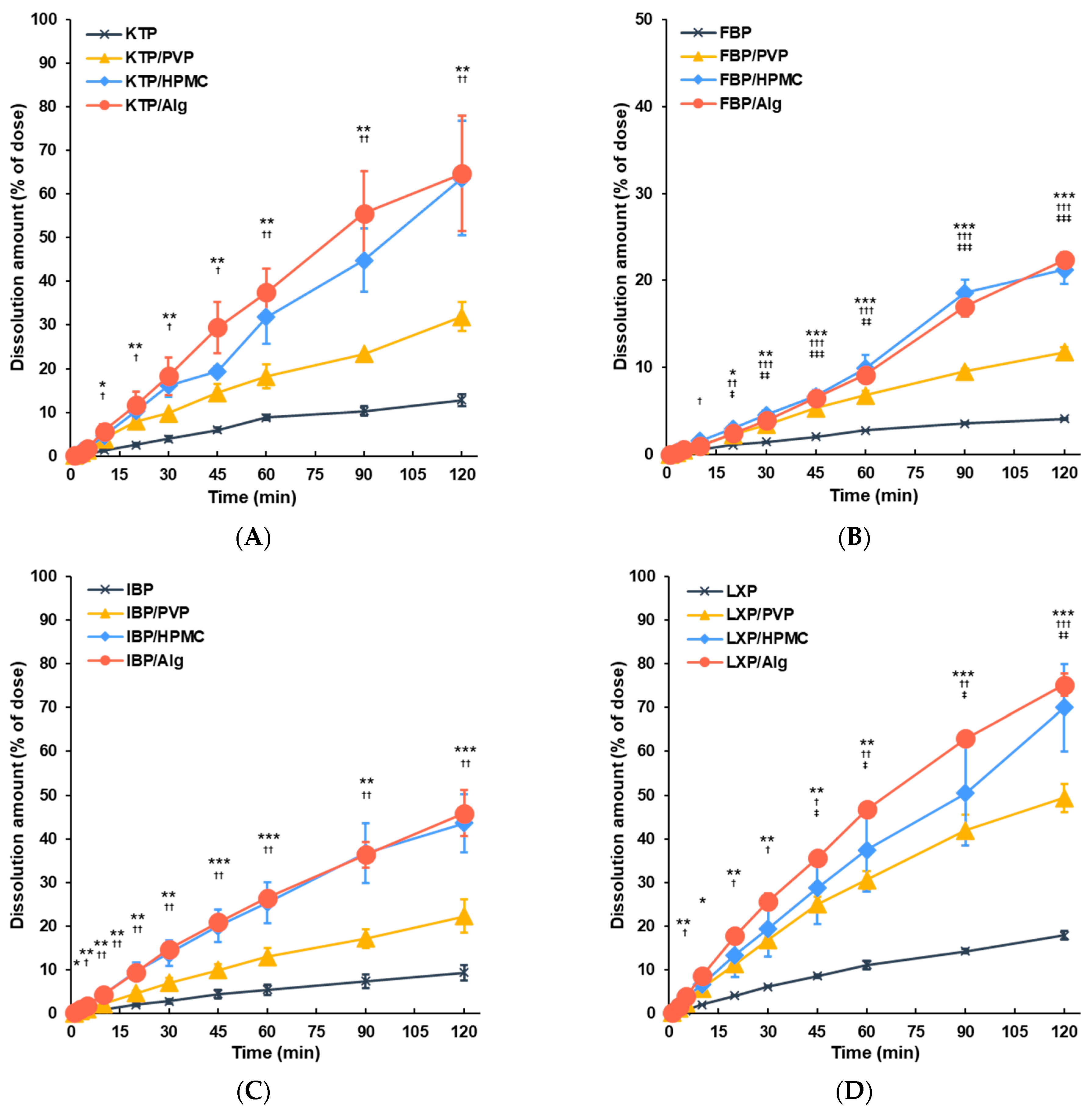
3.2. Dissolution of APIs from Vacuum Dried Film in ANF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, A.A.; Hirai, S.; Bawarshi, R. Nasal absorption of propranolol in rats. J. Pharm. Sci. 1979, 68, 1196. [Google Scholar] [CrossRef] [PubMed]
- Shinichiro, H.; Takatsuka, Y.; Tai, M.; Hiroyuki, M. Absorption of drugs from the nasal mucosa of rat. Int. J. Pharm. 1981, 7, 317–325. [Google Scholar] [CrossRef]
- de la Torre, B.G.; Albericio, F. The pharmaceutical industry in 2021. An analysis of FDA drug approvals from the perspective of molecules. Molecules 2022, 27, 1075. [Google Scholar] [CrossRef] [PubMed]
- Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007, 337, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Furubayashi, T.; Ogawara, K.; Kimura, T.; Higaki, K.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Higashi, Y. In vitro evaluation of nasal mucociliary clearance using excised rat nasal septum. Biol. Pharm. Bull. 2012, 35, 889–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, T.; Shiraki, M.; Hagino, H.; Iinuma, H.; Nakamura, T. Daily nasal spray of HPTH(1-34) for 3 months increases bone mass in osteoporotic subjects: A pilot study. Osteoporos. Int. 2006, 17, 1532–1538. [Google Scholar] [CrossRef]
- Deurloo, M.J.M.; Hermens, W.A.J.J.; Romeyn, S.G.; Verhoef, J.C.; Merkus, F.W.H.M. Absorption enhancement of intranasally administered insulin by sodium taurodihydrofusidate (STDHF) in rabbits and rats. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1989, 6, 853–856. [Google Scholar]
- Zhang, Y.; Zhang, Q.; Sun, Y.; Sun, J.; Wang, X.; Chen, M. Nasal recombinant hirudin-2 delivery: Absorption and its mechanism in vivo and in vitro studies. Biol. Pharm. Bull. 2005, 28, 2263–2267. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings 1PII of original article: S0169-409X(96)00423-1. The article was originally published in advanced drug delivery reviews 23 (1997). Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery–the potential of nanotechnology. Bioorg. Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Hallschmid, M.; Schmitz, K.; Schultes, B.; Ratter, F.; Fehm, H.L.; Born, J.; Kern, W. Intranasal insulin improves memory in humans: Superiority of insulin aspart. Neuropsychopharmacology 2007, 32, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Guastella, A.J.; Gray, K.M.; Rinehart, N.J.; Alvares, G.A.; Tonge, B.J.; Hickie, I.B.; Keating, C.M.; Cacciotti-Saija, C.; Einfeld, S.L. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: A randomized controlled trial. J. Child Psychol. Psychiatry Allied Discip. 2015, 56, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.; Jack, A.; Pretzsch, C.M.; Vander Wyk, B.; Leckman, J.F.; Feldman, R.; Pelphrey, K.A. Intranasal oxytocin enhances connectivity in the neural circuitry supporting social motivation and social perception in children with autism. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallschmid, M.; Benedict, C.; Born, J.; Fehm, H.L.; Kern, W. Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol. Behav. 2004, 83, 55–64. [Google Scholar] [CrossRef]
- Baier, P.C.; Weinhold, S.L.; Huth, V.; Gottwald, B.; Ferstl, R.; Hinze-Selch, D. Olfactory dysfunction in patients with narcolepsy with cataplexy is restored by intranasal orexin A (hypocretin-1). Brain 2008, 131, 2734–2741. [Google Scholar] [CrossRef] [Green Version]
- Marttin, E.; Schipper, N.G.M.; Coos Verhoef, J.; Merkus, F.W.H.M. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Schipper, N.G.; Verhoef, J.; Merkus, F.W. The nasal mucociliary clearance: Relevance to nasal drug delivery. Pharm. Res. 1991, 8, 807–814. [Google Scholar] [CrossRef]
- Donovan, M.D.; Zhou, M. Drug effects on in vivo nasal clearance in rats. Int. J. Pharm. 1995, 116, 77–86. [Google Scholar] [CrossRef]
- Zhou, M.; Donovan, M.D. Intranasal mucociliary clearance of putative bioadhesive polymer gels. Int. J. Pharm. 1996, 135, 115–125. [Google Scholar] [CrossRef]
- Inoue, D.; Tanaka, A.; Kimura, S.; Kiriyama, A.; Katsumi, H.; Yamamoto, A.; Ogawara, K.I.; Kimura, T.; Higaki, K.; Yutani, R.; et al. The relationship between in vivo nasal drug clearance and in vitro nasal mucociliary clearance: Application to the prediction of nasal drug absorption. Eur. J. Pharm. Sci. 2018, 117, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Kimura, S.; Kiriyama, A.; Katsumi, H.; Yamamoto, A.; Ogawara, K.I.; Higaki, K.; Tanaka, A.; Yutani, R.; Sakane, T.; et al. Quantitative estimation of the effect of nasal mucociliary function on in vivo absorption of norfloxacin after intranasal administration to rats. Mol. Pharm. 2018, 15, 4462–4469. [Google Scholar] [CrossRef]
- Racaniello, G.F.; Laquintana, V.; Summonte, S.; Lopedota, A.; Cutrignelli, A.; Lopalco, A.; Franco, M.; Bernkop-Schnürch, A.; Denora, N. Spray-dried mucoadhesive microparticles based on s-protected thiolated hydroxypropyl-β-cyclodextrin for budesonide nasal delivery. Int. J. Pharm. 2021, 603, 120728. [Google Scholar] [CrossRef] [PubMed]
- Trenkel, M.; Scherließ, R. Nasal powder formulations: In-vitro characterisation of the impact of powders on nasal residence time and sensory effects. Pharmaceutics 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xie, J.; Shuai, S.; Wei, S.; Chen, Y.; Guan, Z.; Zheng, Q.; Yue, P.; Wang, C. Nose-to-brain delivery of drug nanocrystals by using Ca2+ responsive deacetylated gellan gum based in situ-nanogel. Int. J. Pharm. 2021, 594, 120182. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.; Alves, G.; Oliveira, P.; Fortuna, A.; Falcão, A. Intranasal delivery of ciprofloxacin to rats: A topical approach using a thermoreversible in situ gel. Eur. J. Pharm. Sci. 2017, 97, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Gotoda, Y.; Hirota, D.; Hidaka, F.; Sato, T.; Matsuura, T.; Imanaka, H.; Ishida, N.; Imamura, K. Surfactant-free solid dispersions of hydrophobic drugs in an amorphous sugar matrix dried from an organic solvent. Mol. Pharm. 2017, 14, 791–798. [Google Scholar] [CrossRef]
- Inoue, D.; Furubayashi, T.; Tanaka, A.; Sakane, T.; Sugano, K. Quantitative estimation of drug permeation through nasal mucosa using in vitro membrane permeability across calu-3 cell layers for predicting in vivo bioavailability after intranasal administration to rats. Eur. J. Pharm. Biopharm. 2020, 149, 145–153. [Google Scholar] [CrossRef]
- Beule, A.G. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2010, 9, Doc07. [Google Scholar] [CrossRef]
- Gross, E.A.; Swenberg, J.A.; Fields, S.; Popp, J.A. Comparative morphometry of the nasal cavity in rats and mice. J. Anat. 1982, 135, 83–88. [Google Scholar] [PubMed]
- Widdicombe, J.G. Airway liquid: A barrier to drug diffusion? Eur. Respir. J. 1997, 10, 2194–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schriever, V.A.; Hummel, T.; Lundström, J.N.; Freiherr, J. Size of nostril opening as a measure of intranasal volume. Physiol. Behav. 2013, 110, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F. Nasal Adhesive Patches-Approach for Topical Application for Dry Nasal Syndrome. Int. J. Biol. Macromol. 2018, 111, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.A.; Badawi, A.M.; Mansour, H.F. Insulin fast-dissolving film for intranasal delivery via olfactory region, a promising approach for the treatment of anosmia in COVID-19 patients: Design, in-vitro characterization and clinical evaluation. Int. J. Pharm. 2021, 601, 120600. [Google Scholar] [CrossRef]
- Bartos, C.; Varga, P.; Szabó-Révész, P.; Ambrus, R. Physico-chemical and in vitro characterization of chitosan-based microspheres intended for nasal administration. Pharmaceutics 2021, 13, 608. [Google Scholar] [CrossRef]
- Tiozzo Fasiolo, L.; Manniello, M.D.; Bortolotti, F.; Buttini, F.; Rossi, A.; Sonvico, F.; Colombo, P.; Valsami, G.; Colombo, G.; Russo, P. Anti-inflammatory flurbiprofen nasal powders for nose-to-brain delivery in alzheimer’s disease. J. Drug Target. 2019, 27, 984–994. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Andersson, S.; Velaga, S.P. Preparation of zolmitriptan-chitosan microparticles by spray drying for nasal delivery. Eur. J. Pharm. Sci. 2009, 38, 206–214. [Google Scholar] [CrossRef]
- Truzzi, E.; Rustichelli, C.; de Oliveira Junior, E.R.; Ferraro, L.; Maretti, E.; Graziani, D.; Botti, G.; Beggiato, S.; Iannuccelli, V.; Lima, E.M.; et al. Nasal biocompatible powder of geraniol oil complexed with cyclodextrins for neurodegenerative diseases: Physicochemical characterization and in vivo evidences of nose to brain delivery. J. Control. Release 2021, 335, 191–202. [Google Scholar] [CrossRef]
- Rautiola, D.; Updyke, J.L.; Nelson, K.M.; Siegel, R.A. Diazepam prodrug stabilizes human aminopeptidase B during lyophilization. Mol. Pharm. 2020, 17, 453–460. [Google Scholar] [CrossRef]
- Thakkar, S.G.; Warnken, Z.N.; Alzhrani, R.F.; Valdes, S.A.; Aldayel, A.M.; Xu, H.; Williams, R.O.; Cui, Z. Intranasal immunization with aluminum salt-adjuvanted dry powder vaccine. J. Control. Release 2018, 292, 111–118. [Google Scholar] [CrossRef]
- Yusuf, H.; Ali, A.A.; Orr, N.; Tunney, M.M.; McCarthy, H.O.; Kett, V.L. Novel freeze-dried DDA and TPGS liposomes are suitable for nasal delivery of vaccine. Int. J. Pharm. 2017, 533, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luppi, B.; Bigucci, F.; Abruzzo, A.; Corace, G.; Cerchiara, T.; Zecchi, V. Freeze-dried chitosan/pectin nasal inserts for antipsychotic drug delivery. Eur. J. Pharm. Biopharm. 2010, 75, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Amponsah-Efah, K.K.; Mistry, P.; Eisenhart, R.; Suryanarayanan, R. The influence of the strength of drug-polymer interactions on the dissolution of amorphous solid dispersions. Mol. Pharm. 2021, 18, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Thilagavathi, R.; Chakraborti, A.K.; Bansal, A.K. Role of molecular interaction in stability of celecoxib-PVP amorphous systems. Mol. Pharm. 2005, 2, 384–391. [Google Scholar] [CrossRef]
- Xua, S.; Dai, W.G. Drug precipitation inhibitors in supersaturable formulations. Int. J. Pharm. 2013, 453, 36–43. [Google Scholar] [CrossRef]
- LaFountaine, J.S.; Prasad, L.K.; Brough, C.; Miller, D.A.; McGinity, J.W.; Williams, R.O. Thermal processing of PVP- and HPMC-based amorphous solid dispersions. AAPS PharmSciTech 2016, 17, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Simões, M.F.; Pinto, R.M.A.; Simões, S. Hot-Melt Extrusion: A Roadmap for Product Development. AAPS PharmSciTech 2021, 22, 1–20. [Google Scholar] [CrossRef]
- Giri, B.R.; Kwon, J.; Vo, A.Q.; Bhagurkar, A.M.; Bandari, S.; Kim, D.W. Hot-melt extruded amorphous solid dispersion for solubility, stability, and bioavailability enhancement of telmisartan. Pharmaceuticals 2021, 14, 73. [Google Scholar] [CrossRef]
- Siriwannakij, N.; Heimbach, T.; Serajuddin, A.T.M. Aqueous dissolution and dispersion behavior of polyvinylpyrrolidone vinyl acetate-based amorphous solid dispersion of ritonavir prepared by hot-melt extrusion with and without added surfactants. J. Pharm. Sci. 2021, 110, 1480–1494. [Google Scholar] [CrossRef]
- Bochmann, E.S.; Steffens, K.E.; Gryczke, A.; Wagner, K.G. Numerical simulation of hot-melt extrusion processes for amorphous solid dispersions using model-based melt viscosity. Eur. J. Pharm. Biopharm. 2018, 124, 34–42. [Google Scholar] [CrossRef]
- Lang, B.; McGinity, J.W.; Williams, R.O. Hot-melt extrusion–basic principles and pharmaceutical applications. Drug Dev. Ind. Pharm. 2014, 40, 1133–1155. [Google Scholar] [CrossRef]
- Dedroog, S.; Adriaensens, P.; Van den Mooter, G. Gaining insight into the role of the solvent during spray drying of amorphous solid dispersions by studying evaporation kinetics. Mol. Pharm. 2022, 19, 1604–1618. [Google Scholar] [CrossRef]
- Poudel, S.; Kim, D.W. Developing Ph-modulated spray dried amorphous solid dispersion of candesartan cilexetil with enhanced in vitro and in vivo performance. Pharmaceutics 2021, 13, 497. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Faucher, A.; O’Reilly, E.; Walker, G. Spray drying ternary amorphous solid dispersions of ibuprofen–an investigation into critical formulation and processing parameters. Eur. J. Pharm. Biopharm. 2017, 120, 43–51. [Google Scholar] [CrossRef]
- Duarte, Í.; Corvo, M.L.; Serôdio, P.; Vicente, J.; Pinto, J.F.; Temtem, M. Production of nano-solid dispersions using a novel solvent-controlled precipitation process—benchmarking their in vivo performance with an amorphous micro-sized solid dispersion produced by spray drying. Eur. J. Pharm. Sci. 2016, 93, 203–214. [Google Scholar] [CrossRef]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef]
- Schönfeld, B.; Westedt, U.; Wagner, K.G. Vacuum drum drying–a novel solvent-evaporation based technology to manufacture amorphous solid dispersions in comparison to spray drying and hot melt extrusion. Int. J. Pharm. 2021, 596, 120233. [Google Scholar] [CrossRef]
- Schönfeld, B.V.; Westedt, U.; Wagner, K.G. Compression of amorphous solid dispersions prepared by hot-melt extrusion, spray drying and vacuum drum drying. Int. J. Pharm. X 2021, 3, 100102. [Google Scholar] [CrossRef]
- Jadhav, N.R.; Gaikwad, V.L.; Nair, K.J.; Kadam, H.M. Glass transition temperature: Basics and application in pharmaceutical sector. Asian J. Pharm. 2009, 3, 82–89. [Google Scholar] [CrossRef]
- Paradkar, A.; Maheshwari, M.; Tyagi, A.K.; Chauhan, B.; Kadam, S.S. Preparation and characterization of flurbiprofen beads by melt solidification technique. AAPS PharmSciTech 2003, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Luebbert, C.; Huxoll, F.; Sadowski, G.; Van Den Mooter, G.; Grohganz, H. Amorphous-amorphous phase separation in API/polymer formulations. Molecules 2017, 22, 296. [Google Scholar] [CrossRef]
- Yang, D.; Fang, L.; Yang, C. Roles of molecular interaction and mobility on loading capacity and release rate of drug-ionic liquid in long-acting controlled release transdermal patch. J. Mol. Liq. 2022, 352, 118752. [Google Scholar] [CrossRef]
- Gao, P.; Shi, Y. Characterization of supersaturatable formulations for improved absorption of poorly soluble drugs. AAPS J. 2012, 14, 703–713. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.A.; DiNunzio, J.C.; Yang, W.; McGinity, J.W.; Williams, R.O. Enhanced in vivo absorption of itraconazole via stabilization of supersaturation following acidic-to-neutral PH transition. Drug Dev. Ind. Pharm. 2008, 34, 890–902. [Google Scholar] [CrossRef]
- França, M.T.; O’Reilly Beringhs, A.; Nicolay Pereira, R.; Martins Marcos, T.; Bazzo, G.C.; Stulzer, H.K. The role of sodium alginate on the supersaturation state of the poorly soluble drug chlorthalidone. Carbohydr. Polym. 2019, 209, 207–214. [Google Scholar] [CrossRef]
| APIs | API Concentration (w/v%) | Polymer Concentration (w/v%) | Solvent Concentration (v/v%) | ||||
|---|---|---|---|---|---|---|---|
| HPMC | PVP | Alg | Water | Methanol | |||
| Ketoprofen (KTP) | Control | 2 | - | - | - | - | 100 |
| HPMC | 1 | 1 | - | - | 10 | 90 | |
| PVP | 1 | - | 1 | - | - | 100 | |
| Alg | 1 | - | - | 1 | 60 | 40 | |
| Flurbiprofen (FBP) | Control | 2 | - | - | - | - | 100 |
| HPMC | 1 | 1 | - | - | 10 | 90 | |
| PVP | 1 | - | 1 | - | - | 100 | |
| Alg | 1 | - | - | 1 | 50 | 50 | |
| Ibuprofen (IBP) | Control | 2 | - | - | - | - | 100 |
| HPMC | 1 | 1 | - | - | 10 | 90 | |
| PVP | 1 | - | 1 | - | - | 100 | |
| Alg | 1 | - | - | 1 | 60 | 40 | |
| Loxoprofen (LXP) | Control | 2 | - | - | - | - | 100 |
| HPMC | 1 | 1 | - | - | 10 | 90 | |
| PVP | 1 | - | 1 | - | - | 100 | |
| Alg | 1 | - | - | 1 | 70 | 30 | |
| Species | Length (mm) | Volume (mm3) | Surface Area (mm2) | Mucus Thickness (μm) | ||||
|---|---|---|---|---|---|---|---|---|
| Squamous Epithelium | Respiratory Epithelium | Olfactory Epithelium | Total Surface Area | Periciliary Layer | Surface Layer | |||
| Rat | 91 ± 0.3 [31] | 256.7 ± 4.1 [31] | 44.2 ± 5.2 [31] | 623.1 ± 14.0 [31] | 675.2 ± 43.0 [31] | 1343.5 ± 55.0 [31] | 5–10 [32] | 1–10 [32] |
| Human | 100–140 [33] | 20,000 [33] | - | - | 200–400 [33] | 16,000 [33] | 5 [30] | 10–15 [30] |
| APIs | Mobile Phase (Acetonitrile/0.1% TFA) | UV Wavelength (nm) | Injection Volume (μL) | Retention Time (min) |
|---|---|---|---|---|
| KTP | 50/50 | 254 | 10 | 1.35 |
| FBP | 60/40 | 220 | 20 | 1.01 |
| IBP | 60/40 | 244 | 20 | 0.82 |
| LXP | 35/65 | 223 | 30 | 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, D.; Yamashita, A.; To, H. Formulation and In Vitro Characterization of a Vacuum-Dried Drug–Polymer Thin Film for Intranasal Application. Polymers 2022, 14, 2954. https://doi.org/10.3390/polym14142954
Inoue D, Yamashita A, To H. Formulation and In Vitro Characterization of a Vacuum-Dried Drug–Polymer Thin Film for Intranasal Application. Polymers. 2022; 14(14):2954. https://doi.org/10.3390/polym14142954
Chicago/Turabian StyleInoue, Daisuke, Ayari Yamashita, and Hideto To. 2022. "Formulation and In Vitro Characterization of a Vacuum-Dried Drug–Polymer Thin Film for Intranasal Application" Polymers 14, no. 14: 2954. https://doi.org/10.3390/polym14142954
APA StyleInoue, D., Yamashita, A., & To, H. (2022). Formulation and In Vitro Characterization of a Vacuum-Dried Drug–Polymer Thin Film for Intranasal Application. Polymers, 14(14), 2954. https://doi.org/10.3390/polym14142954