Dynamics of Rising Bubbles and Their Impact with Viscoelastic Fluid Interfaces
Abstract
:1. Introduction
2. Methods
2.1. Experimental Setup
2.2. Experimental Materials
2.3. Experimental Methods
3. Experimental Results and Discussion
3.1. Bubble Flotation
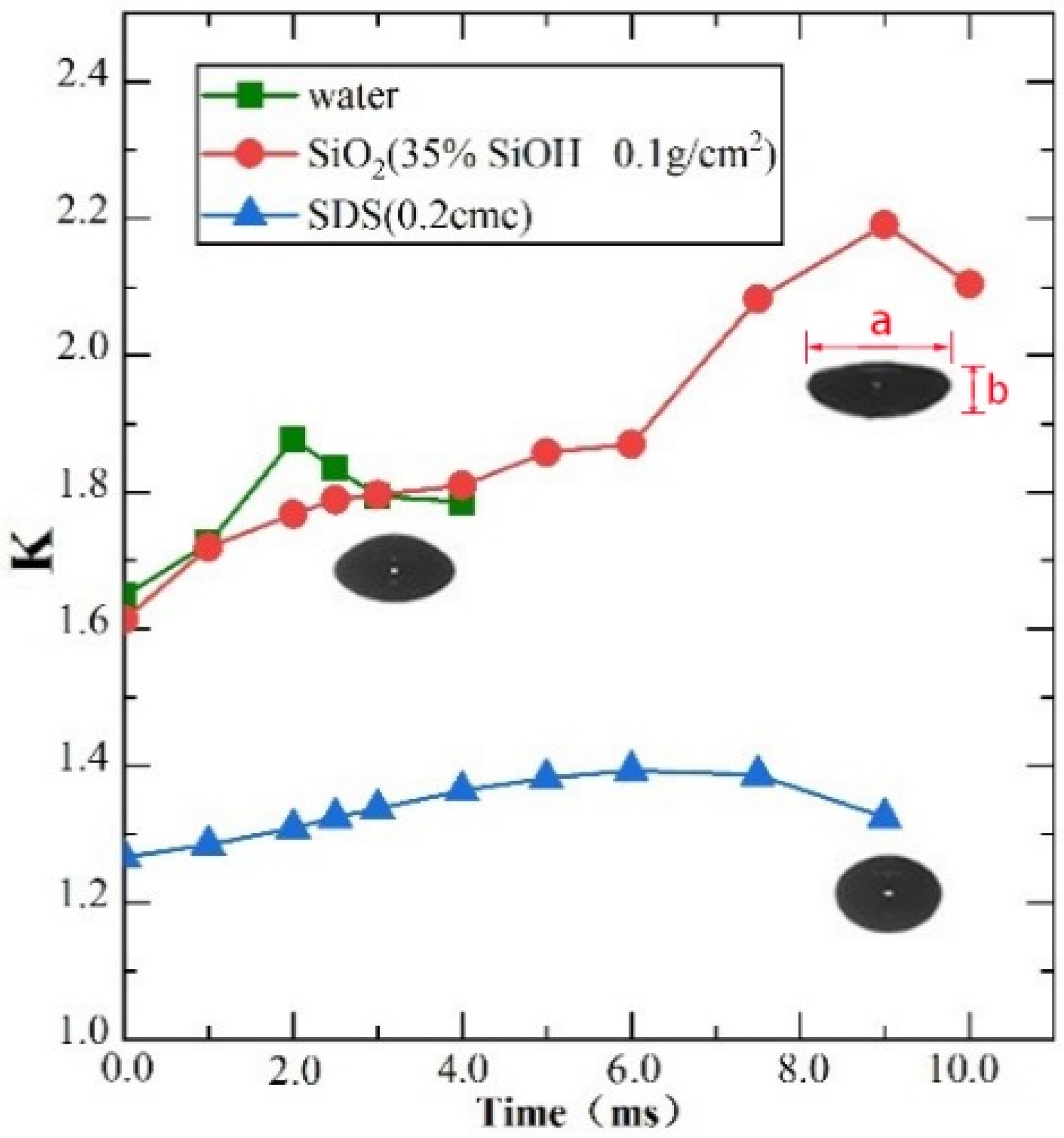
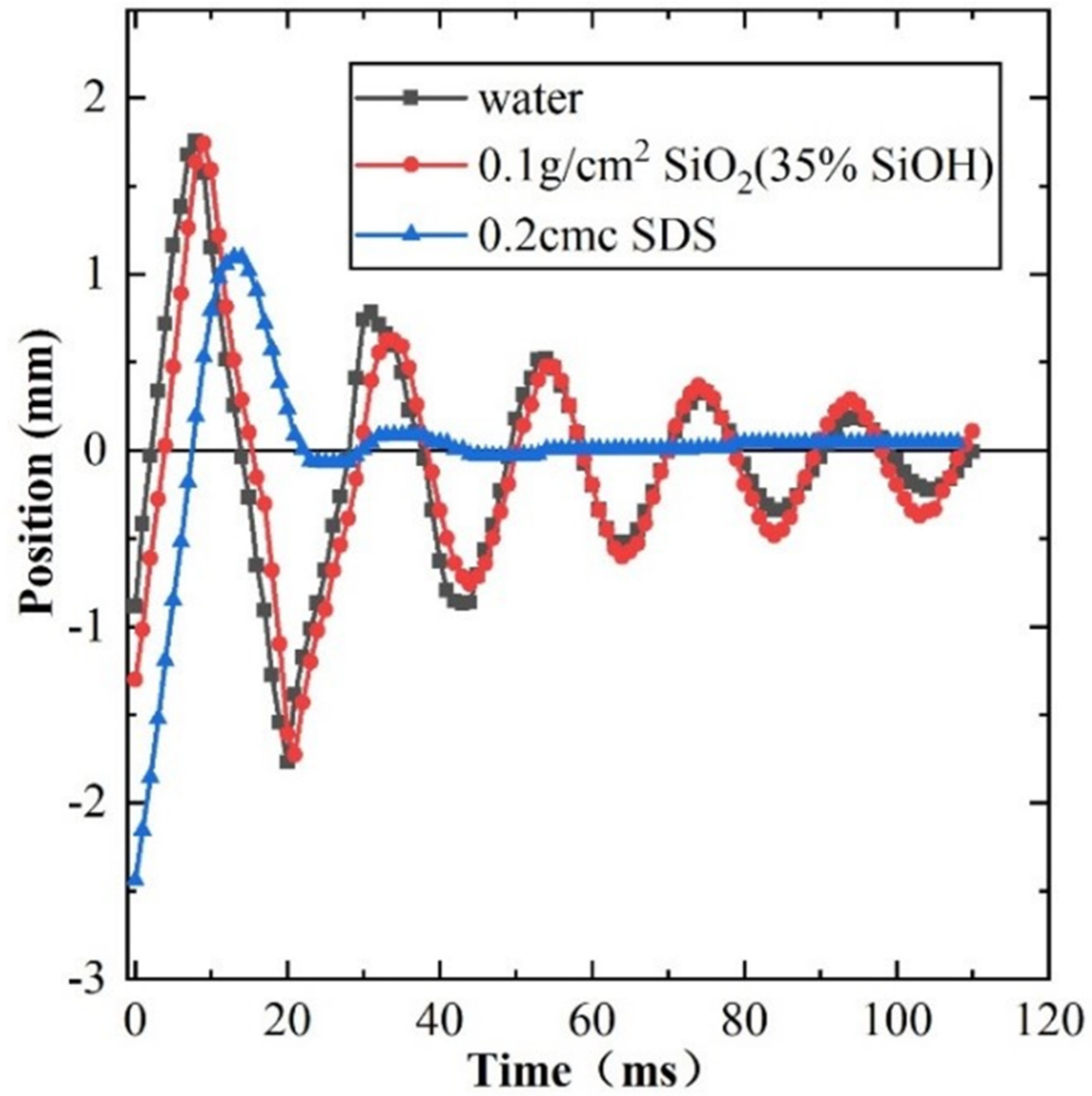
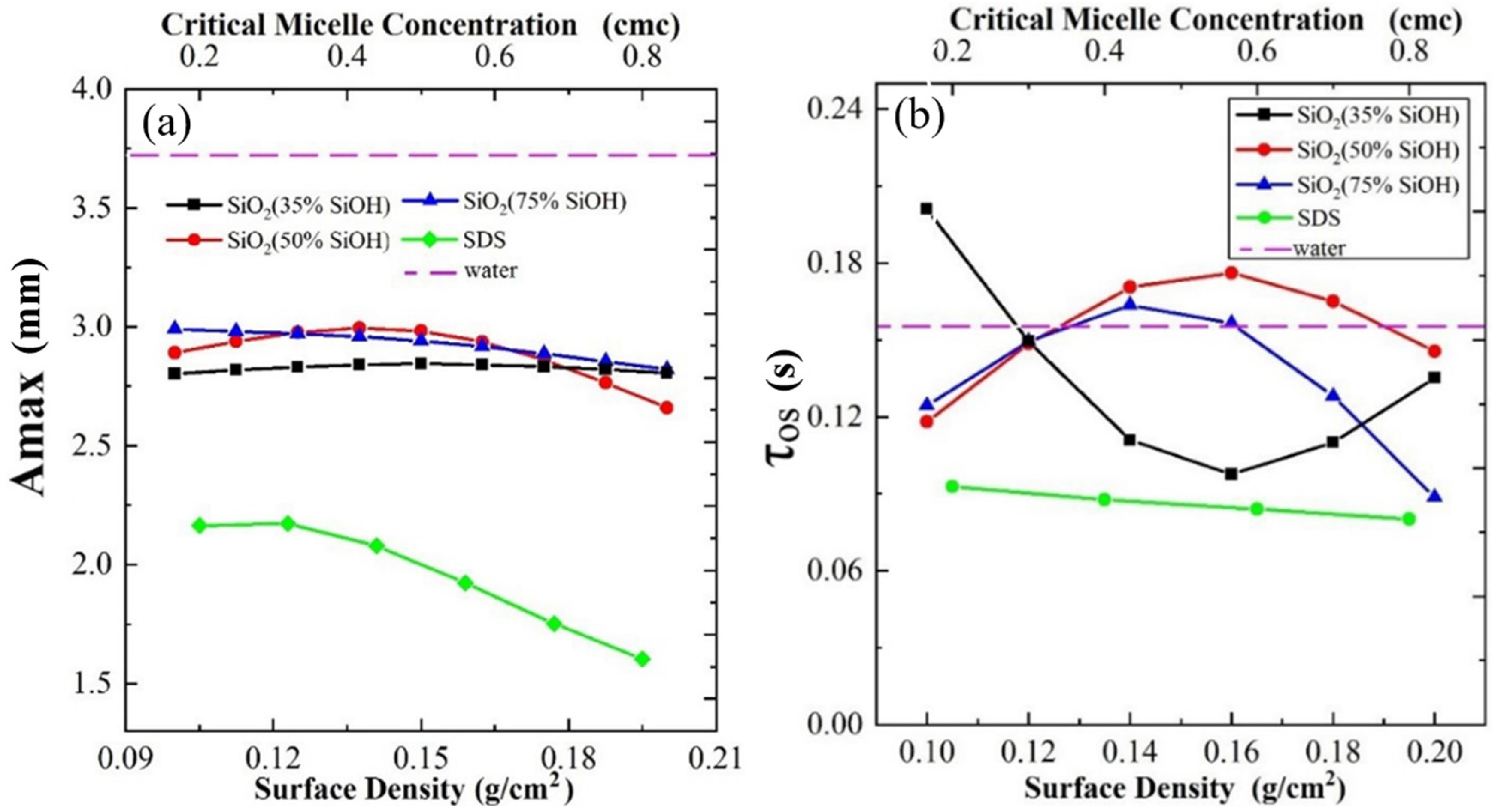
3.2. Impact between the Bubble and Gas-Liquid Interface
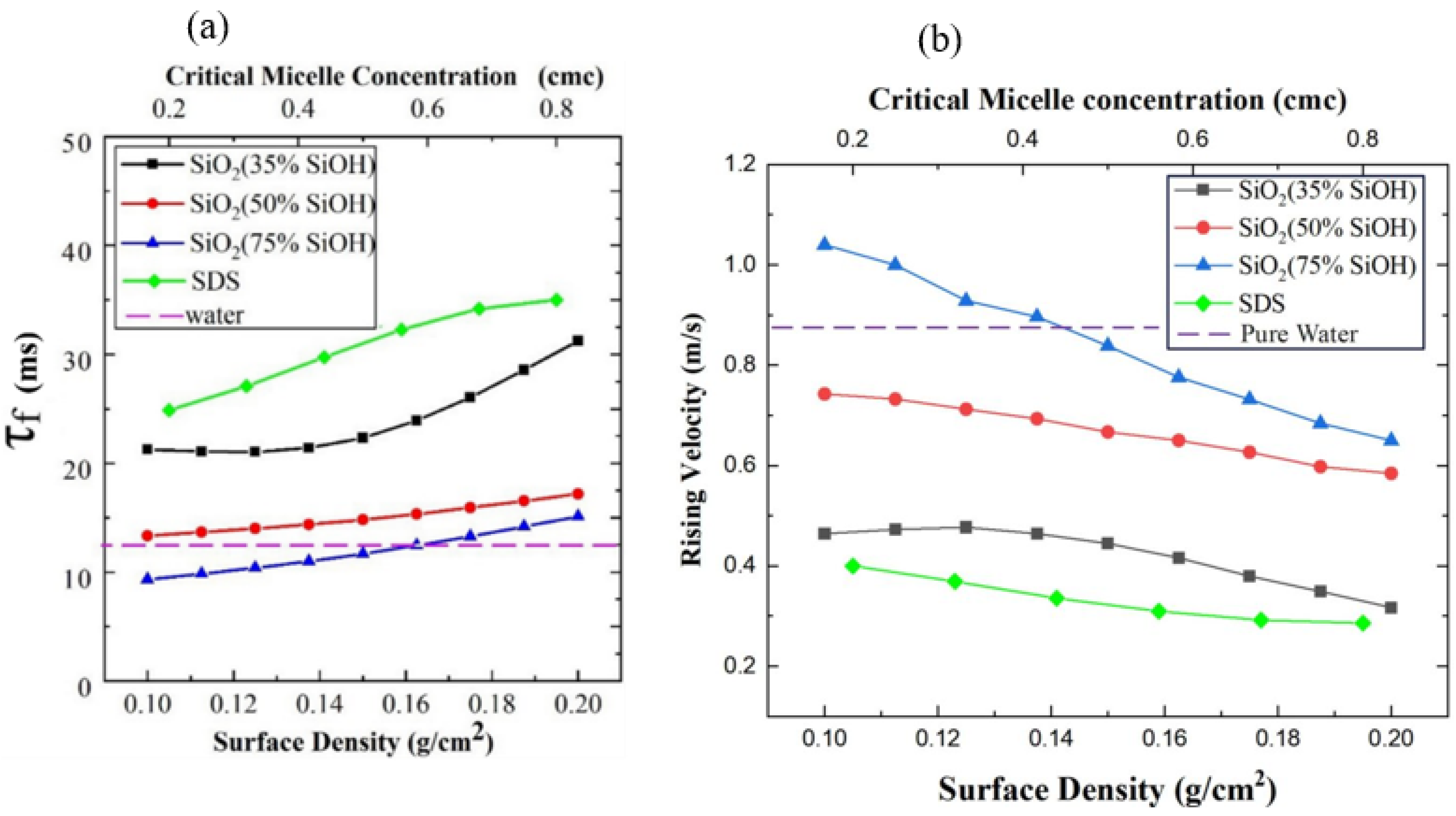
3.3. Liquid Drainage and Bubble Life of Liquid Film
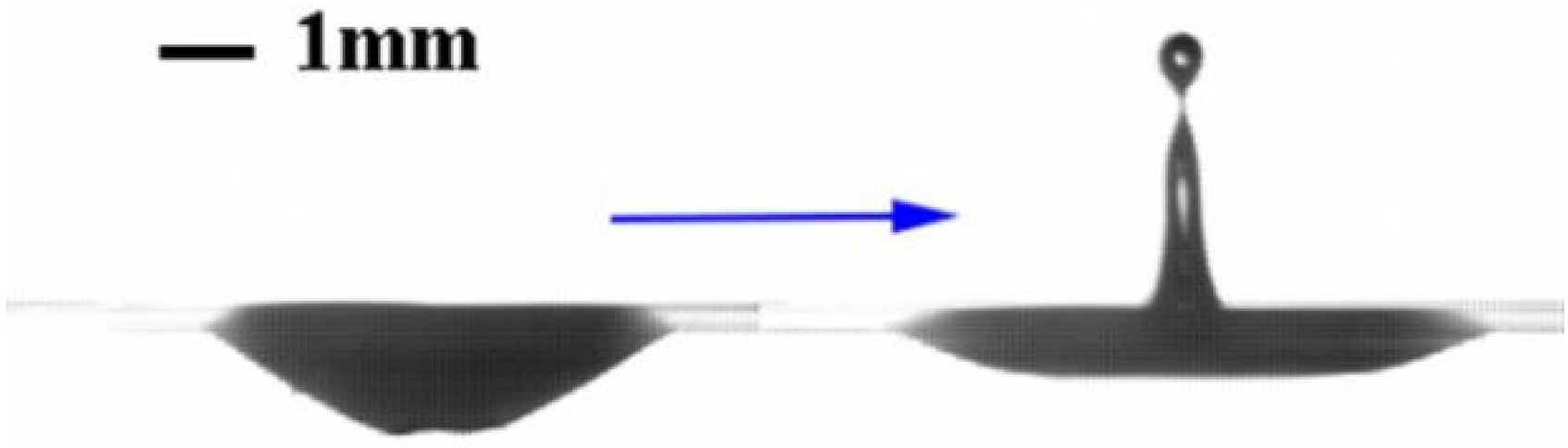
3.4. Bubble Rupture and Jet Flow
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grieves, R.B. Foam separations: A review. Chem. Eng. J. 1975, 9, 93–106. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, M.; Gui, X.; Cao, Y.; Rudolph, M.; Butt, H.-J.; Kappl, M. The role of surface forces in mineral flotation. Curr. Opin. Colloid Interface Sci. 2019, 44, 143–152. [Google Scholar] [CrossRef]
- Plesset, M.S.; Prosperetti, A. Bubble dynamics and cavitation. Annu. Rev. Fluid Mech. 1977, 9, 145–185. [Google Scholar] [CrossRef]
- Chen, L.; Garimella, S.V.; Reizes, J.A.; Leonardi, E. The development of a bubble rising in a viscous liquid. J. Fluid Mech. 1999, 387, 61–96. [Google Scholar] [CrossRef] [Green Version]
- Hua, J.; Lou, J. Numerical simulation of bubble rising in viscous liquid. J. Comput. Phys. 2007, 222, 769–795. [Google Scholar] [CrossRef]
- Somasundaran, P.; Chandar, P.; Chari, K. A study of the interactions between particles and bubbles in surfactant solutions. Colloids Surf. 1983, 8, 121–136. [Google Scholar] [CrossRef]
- Borkowski, M.; Kosior, D.; Zawala, J. Effect of initial adsorption coverage and dynamic adsorption layer formation at bubble surface in stability of single foam films. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124446. [Google Scholar] [CrossRef]
- Miguet, J.; Pasquet, M.; Rouyer, F.; Fang, Y.; Rio, E. Stability of big surface bubbles: Impact of evaporation and bubble size. Soft Matter 2020, 16, 1082–1090. [Google Scholar] [CrossRef] [Green Version]
- Krzan, M.; Malysa, K. Profiles of local velocities of bubbles in n-butanol, n-hexanol and n-nonanol solutions. Colloids Surf. A Physicochem. Eng. Asp. 2002, 207, 279–291. [Google Scholar] [CrossRef]
- Krzan, M.; Zawala, J.; Malysa, K. Development of steady state adsorption distribution over interface of a bubble rising in solutions of n-alkanols (C5, C8) and n-alkyltrimethylammonium bromides (C8, C12, C16). Colloids Surf. A Physicochem. Eng. Asp. 2007, 298, 42–51. [Google Scholar] [CrossRef]
- Kosior, D.; Zawala, J.; Todorov, R.; Exerowa, D.; Malysa, K. Bubble bouncing and stability of liquid films formed under dynamic and static conditions from n-octanol solutions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 391–400. [Google Scholar] [CrossRef]
- Boinovich, L. DLVO forces in thin liquid films beyond the conventional DLVO theory. Curr. Opin. Colloid Interface Sci. 2010, 15, 297–302. [Google Scholar] [CrossRef]
- Zhang, A.; Guo, Z.; Wang, Q.; Xiong, S. Three-dimensional numerical simulation of bubble rising in viscous liquids: A conservative phase-field lattice-Boltzmann study. Phys. Fluids 2019, 31, 063106. [Google Scholar] [CrossRef]
- Sanada, T.; Sugihara, K.; Shirota, M.; Watanabe, M. Motion and drag of a single bubble in super-purified water. Fluid Dyn. Res. 2008, 40, 534–545. [Google Scholar] [CrossRef]
- Sato, A.; Shirota, M.; Sanada, T.; Watanabe, M. Modeling of bouncing of a single clean bubble on a free surface. Phys. Fluids 2011, 23, 013307. [Google Scholar] [CrossRef]
- Feng, J.; Muradoglu, M.; Kim, H.; Ault, J.T.; Stone, H.A. Dynamics of a bubble bouncing at a liquid/liquid/gas interface. J. Fluid Mech. 2016, 807, 324–352. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhou, X.; Wang, Y. Comprehensive heat transfer performance analysis of nanofluid mixed forced and thermocapillary convection around a gas bubble in minichannel. Int. Commun. Heat Mass Transf. 2020, 110, 104386. [Google Scholar] [CrossRef]
- Manica, R.; Klaseboer, E.; Chan, D.Y.C. Force Balance Model for Bubble Rise, Impact, and Bounce from Solid Surfaces. Langmuir 2015, 31, 6763–6772. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, P.; Ming, F. An SPH modeling of bubble rising and coalescing in three dimensions. Comput. Methods Appl. Mech. Eng. 2015, 294, 189–209. [Google Scholar] [CrossRef]
- Tammaro, D.; Pasquino, R.; Villone, M.M.; D’Avino, G.; Ferraro, V.; Di Maio, E.; Langella, A.; Grizzuti, N.; Maffettone, P.L. Elasticity in Bubble Rupture. Langmuir 2018, 34, 5646–5654. [Google Scholar] [CrossRef]
- Sun, Q.-L.; Sun, L.; Wang, X.-W.; Fang, J.-L.; Kong, H.-Y.; He, J.-H.; Gu, H. Jet speed in bubble rupture. Therm. Sci. 2018, 22, 47–50. [Google Scholar] [CrossRef]
- Guo, W.; Li, H.; Wang, J.; Wang, Y.; Huang, C. Research progress of interaction between single cavitation and free surface. Chin. J. Theor. Appl. Mech. 2019, 51, 1682–1698. (In Chinese) [Google Scholar]
- Zhang, L.; Yin, Q. Theoretical and numerical studies on the bubblecollapse in water. J. Hydrodyn. 2012, 27, 68–73. (In Chinese) [Google Scholar]
- Zhang, A.; Wang, C.; Zhang, S. Experimental study of interaction between bubble and free surface. Acta Phys. Sin. 2012, 61, 300–312. (In Chinese) [Google Scholar] [CrossRef]
- Jan, Z.; Kazimierz, M. Influence of the Impact Velocity and Size of the Film Formed on Bubble Coalescence Time at Water Surface. Langmuir 2011, 27, 2250–2257. [Google Scholar]
- Kazimierz, M.; Krasowska, M.; Krzan, M. Influence of surface active substances on bubble motion and collision with various interfaces. Adv. Colloid Interface Sci. 2005, 114–115, 205–225. [Google Scholar]
- Rek, Z. Using a Dynamic and Constant Mesh in Numerical Simulation of the Free-Rising Bubble. Fluids 2019, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Gumulya, M.; Joshi, J.; Utikar, R.; Evans, G.; Pareek, V. Characteristics of energy production and dissipation around a bubble rising in water. Chem. Eng. Sci. 2018, 193, 38–52. [Google Scholar] [CrossRef]
- Kong, G.; Mirsandi, H.; Buist, K.A.; Peters, E.A.J.F.; Baltussen, M.W.; Kuipers, J.A.M. Oscillation dynamics of a bubble rising in viscous liquid. Exp. Fluids 2019, 60, 130. [Google Scholar] [CrossRef] [Green Version]
- Exerowa, D.; Platikanov, D. Thin liquid films from aqueous solutions of non-ionic polymeric surfactants. Adv. Colloid Interface Sci. 2009, 147–148, 74–87. [Google Scholar] [CrossRef]
- Vakarelski, I.U.; Manica, R.; Tang, X.; O’Shea, S.J.; Stevens, G.W.; Grieser, F.; Dagastine, R.R.; Chan, D.Y.C. Dynamic interactions between microbubbles in water. Proc. Natl. Acad. Sci. USA 2010, 107, 11177–11182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albadawi, A.; Donoghue, D.; Robinson, A.; Murray, D.; Delauré, Y. On the assessment of a VOF based compressive interface capturing scheme for the analysis of bubble impact on and bounce from a flat horizontal surface. Int. J. Multiph. Flow 2014, 65, 82–97. [Google Scholar] [CrossRef]
- Li, S.; Schwarz, M.P.; Yang, W.; Feng, Y.; Witt, P.; Sun, C. Experimental observations of bubble–particle collisional interaction relevant to froth flotation, and calculation of the associated forces. Miner. Eng. 2020, 151, 106335. [Google Scholar] [CrossRef]
- Zawala, J.; Dorbolo, S.; Terwagne, D.; Vandewalle, N.; Malysa, K. Bouncing bubble on a liquid/gas interface resting or vibrating. Soft Matter 2011, 7, 6719–6726. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; You, Y. Bouncing behaviors of a buoyancy-driven bubble on a horizontal solid wall. Chin. J. Theor. Appl. Mech. 2019, 51, 1285–1295. (In Chinese) [Google Scholar]
- Mohanram, R.; Jagtap, C.; Kumar, P. Isolation, screening, and characterization of surface-active agent-producing, oil-degrading marine bacteria of Mumbai Harbor. Mar. Pollut. Bull. 2016, 105, 131–138. [Google Scholar] [CrossRef]
- Gedde, U.W.; Helmholtz, F.E. Essential Classical Thermodynamics; Springer: Cham, Switzerland, 2020; pp. 21–24. [Google Scholar]
- Zang, D.Y.; Rio, E.; Langevin, D.; Binks, B.P. Viscoelastic properties of silica nanoparticle monolayers at the air-water interface. Eur. Phys. J. E 2010, 31, 125–134. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, C.; Tang, X.; Dong, X.; He, T.; Wang, H.; Zang, D. Dynamics of Rising Bubbles and Their Impact with Viscoelastic Fluid Interfaces. Polymers 2022, 14, 2948. https://doi.org/10.3390/polym14142948
Zhang Y, Liu C, Tang X, Dong X, He T, Wang H, Zang D. Dynamics of Rising Bubbles and Their Impact with Viscoelastic Fluid Interfaces. Polymers. 2022; 14(14):2948. https://doi.org/10.3390/polym14142948
Chicago/Turabian StyleZhang, Yongjian, Chenlong Liu, Xiuxing Tang, Xin Dong, Tan He, Heyi Wang, and Duyang Zang. 2022. "Dynamics of Rising Bubbles and Their Impact with Viscoelastic Fluid Interfaces" Polymers 14, no. 14: 2948. https://doi.org/10.3390/polym14142948
APA StyleZhang, Y., Liu, C., Tang, X., Dong, X., He, T., Wang, H., & Zang, D. (2022). Dynamics of Rising Bubbles and Their Impact with Viscoelastic Fluid Interfaces. Polymers, 14(14), 2948. https://doi.org/10.3390/polym14142948






