Microbial Poly-γ-Glutamic Acid (γ-PGA) as an Effective Tooth Enamel Protectant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosynthesis of γ-PGA
2.1.1. Microorganism
2.1.2. Fermentation Media
2.1.3. Cultivation Parameters
2.1.4. Statistical Analysis
2.1.5. Isolation and Purification of γ-PGA
2.1.6. Characterisation of γ-PGA
2.1.7. Formulation of γ-PGA
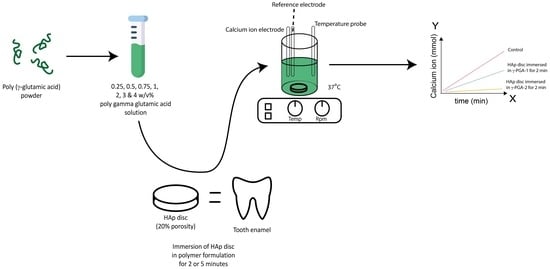
2.1.8. Artificial Demineralisation Monitored Using ISE
2.1.9. ISE or Real-Time Monitoring of Artificial Caries
3. Results
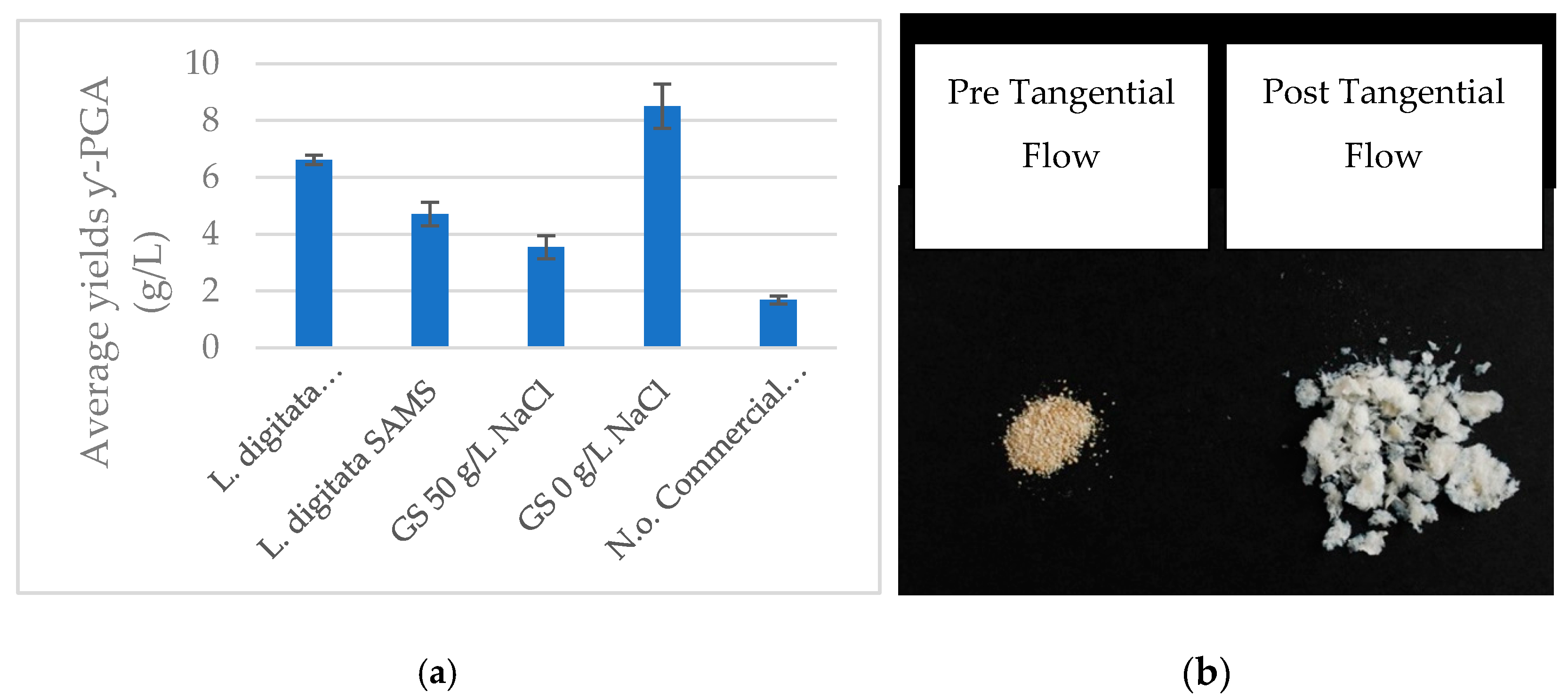
3.1. Biosynthesis of γ-PGA from Macro-Algae and Micro-Algae Waste Fraction
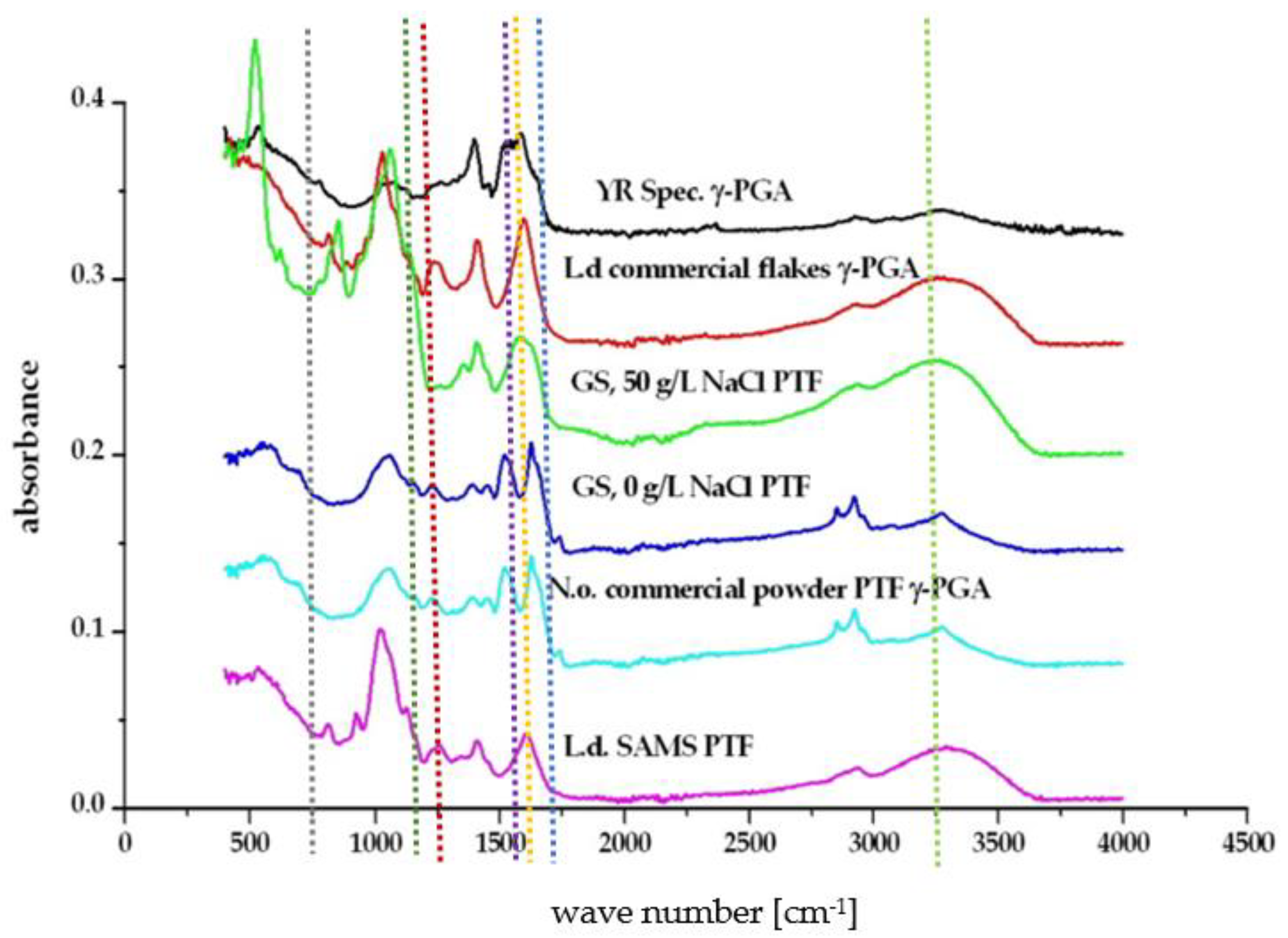
3.2. Physico-Chemical Characteristics of γ-PGA Synthesised from Macro-Algae and Micro-Algae Waste Fraction
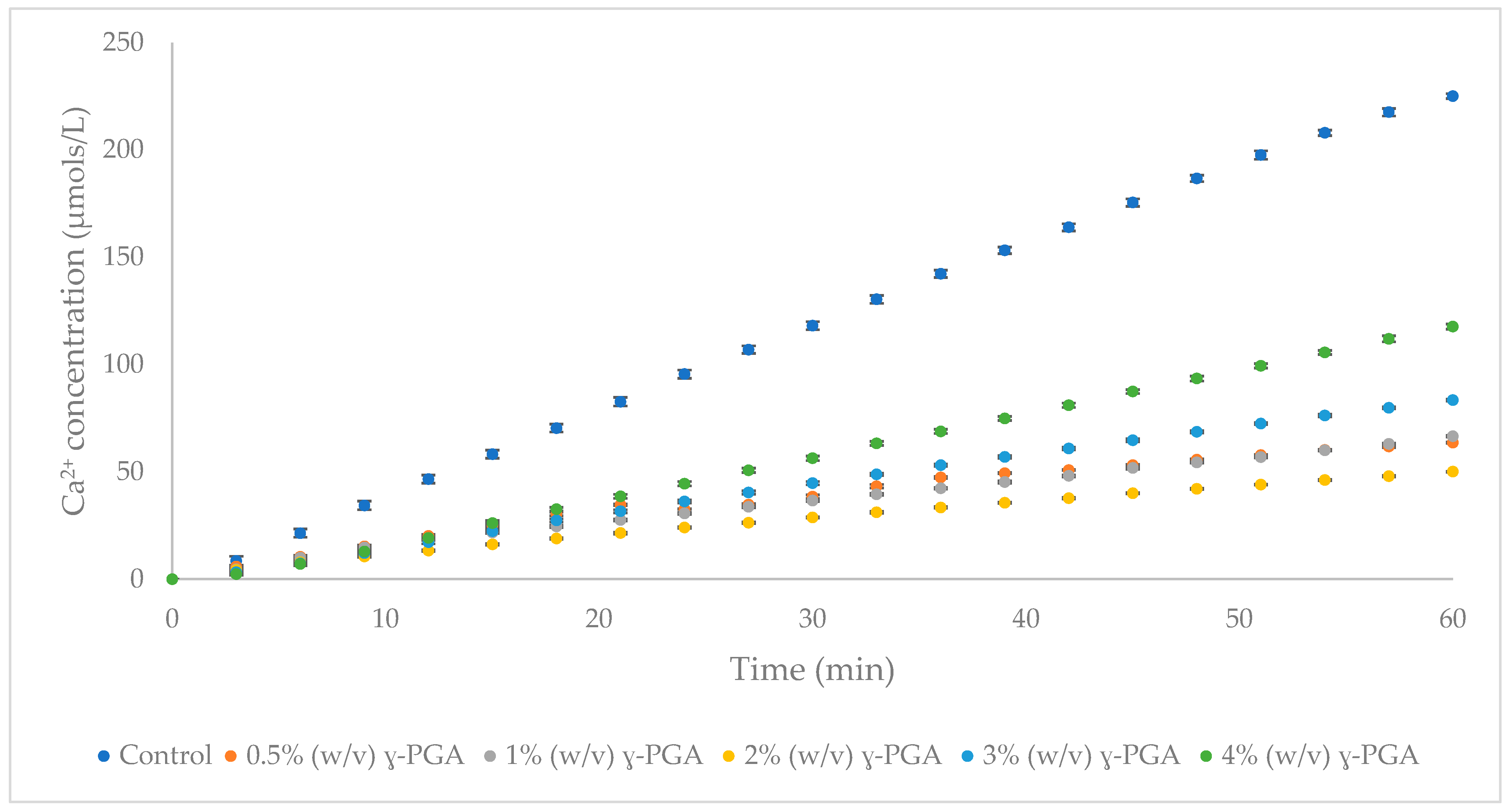
3.3. The Impact of γ-PGA Concentrations on HAp Demineralisation
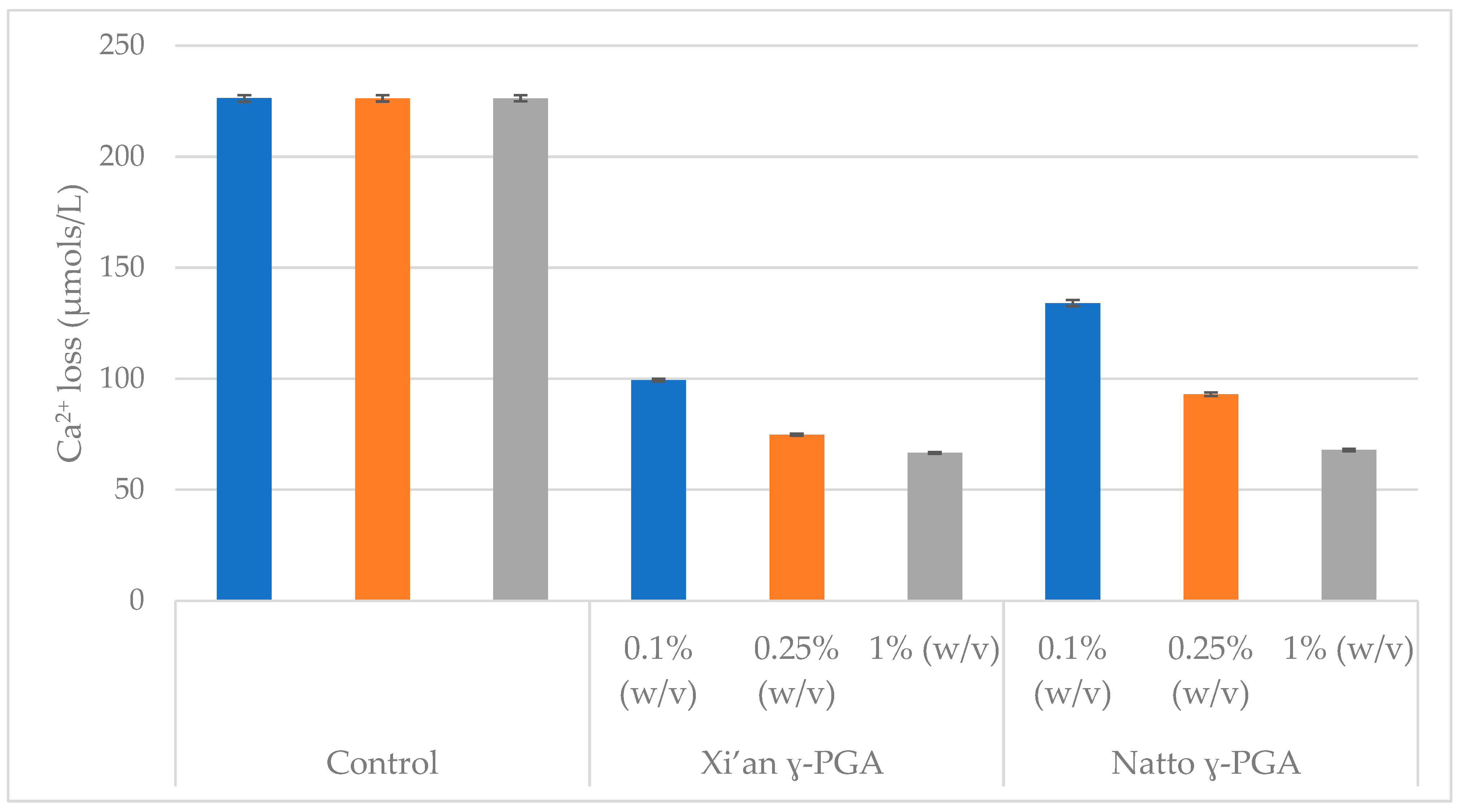
3.4. Comparison of γ-PGA Average Molar Mass on HAp Protection
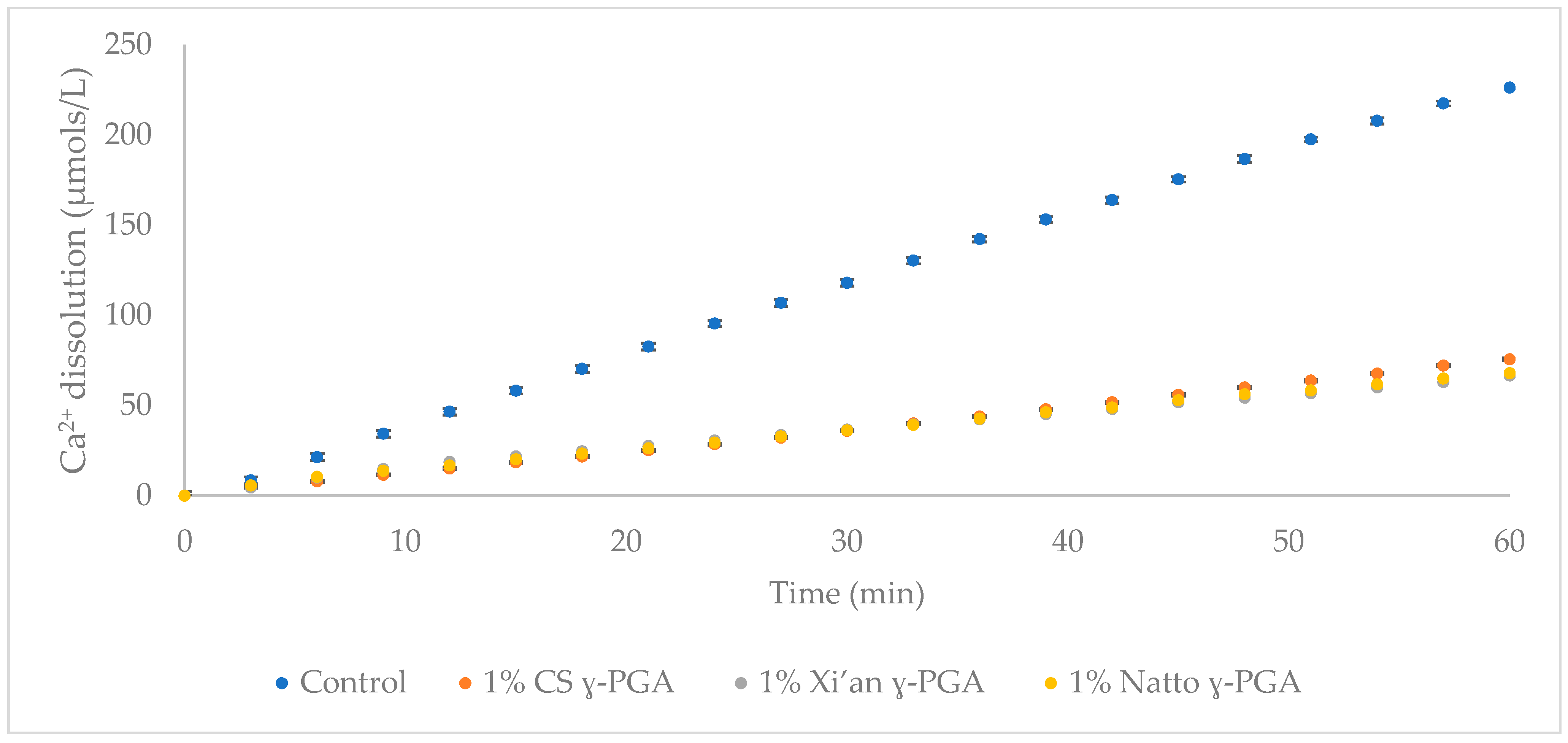
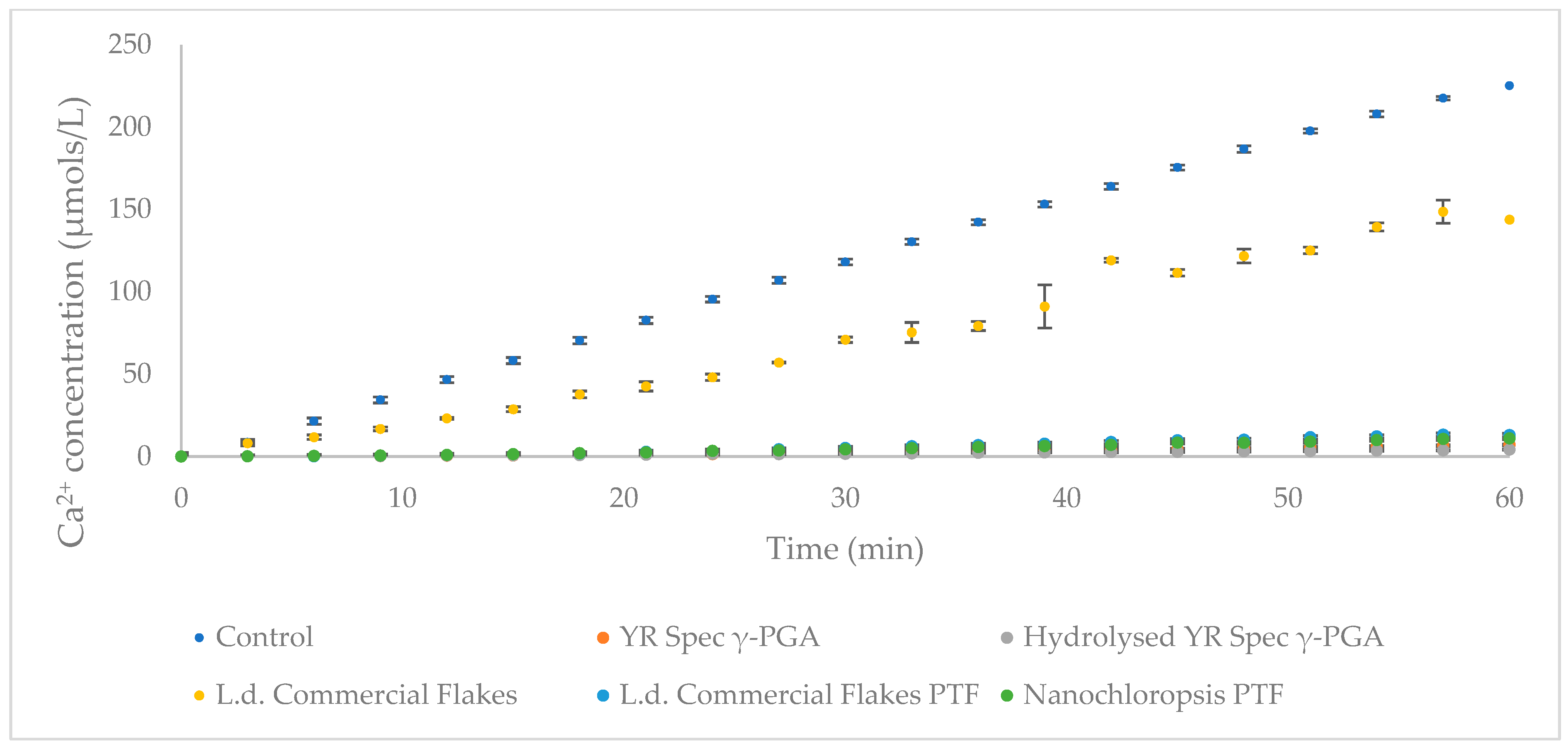
3.5. Enamel Protection from Micro- and Macro-Algal Produced γ-PGA
4. Discussion
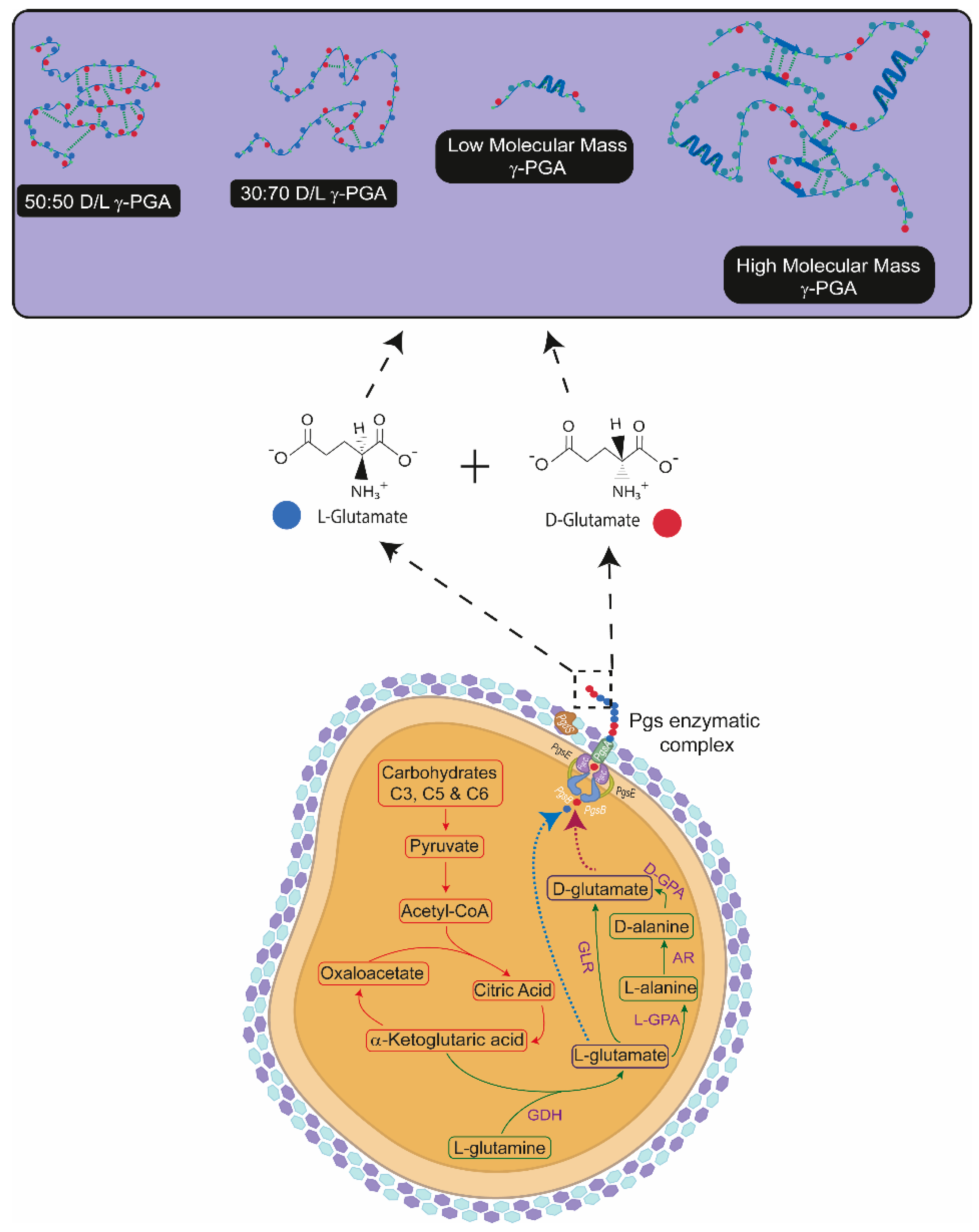
4.1. Variation in γ-PGA Yields and Physico-Chemical Properties with Micro- and Macro-Algal Substrate
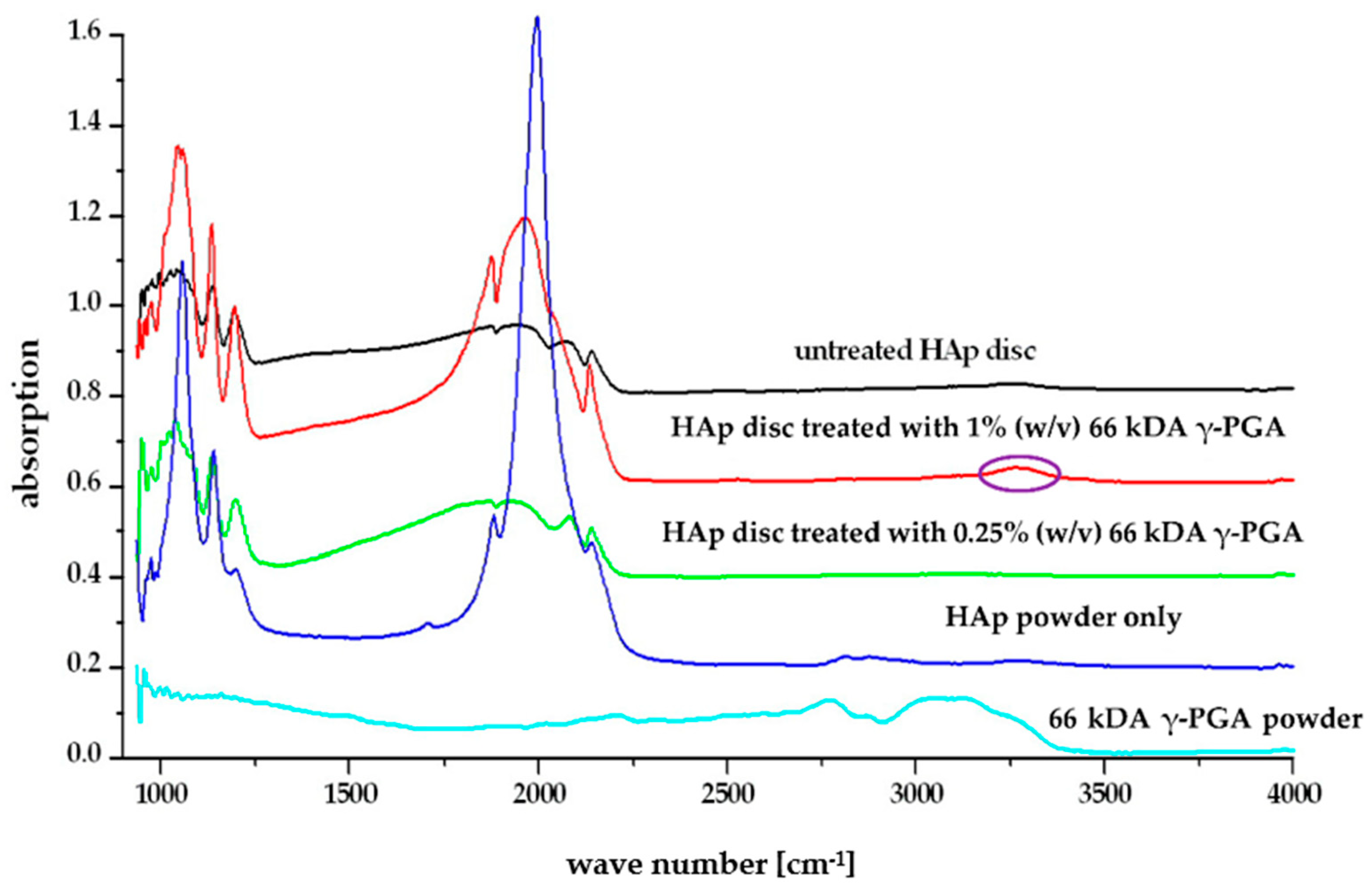
4.2. Affinity and Protection of γ-PGA towards HAp
4.3. HAp Protection through Micro- and Macro-Algal Produced γ-PGA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Villa, A.; Connell, C.; Abati, S. Diagnosis and Management of Xerostomia and Hyposalivation. Ther. Clin. Risk Manag. 2014, 11, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary Secretion in Health and Disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Wolff, A.; Narayana, N.; Dawes, C.; Aframian, D.; Lynge Pedersen, A.; Vissink, A.; Aliko, A.; Sia, Y.; Joshi, R.; et al. World Workshop on Oral Medicine VI: A Systematic Review of Medication-Induced Salivary Gland Dysfunction. Oral Dis. 2016, 22, 365–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neville, B.W.; Damm, D.D.; Allen, C.M.; Chi, A.C. Physical and Chemical Injuries. In Color Atlas of Oral and Maxillofacial Diseases; Elsevier: New York, NY, USA, 2019. [Google Scholar]
- Reed, R.; Xu, C.; Liu, Y.; Gorski, J.P.; Wang, Y.; Walker, M.P. Radiotherapy Effect on Nano-Mechanical Properties and Chemical Composition of Enamel and Dentine. Arch. Oral Biol. 2015, 60, 690–697. [Google Scholar] [CrossRef] [Green Version]
- Anderson, P.; Hector, M.P.; Rampersad, M.A. Critical PH in Resting and Stimulated Whole Saliva in Groups of Children and Adults. Int. J. Paediatr. Dent. 2008, 11, 266–273. [Google Scholar] [CrossRef]
- Söderling, E.; Le Bell, A.; Kirstilä, V.; Tenovuo, J. Betaine-Containing Toothpaste Relieves Subjective Symptoms of Dry Mouth. Acta Odontol. Scand. 1998, 56, 65–69. [Google Scholar] [CrossRef]
- Fehrenbach, M.J.; Popowics, T. Illustrated Dental Embryology, Histology, and Anatomy; Missouri Elsevier: St. Louis, MO, USA, 2020; ISBN 9780323611084. [Google Scholar]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The Functions of Human Saliva: A Review Sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- van der Reijden, W.A.; Veerman, E.C.I.; Nieuw Amerongen, A.V. Rheological Properties of Commercially Available Polysaccharides with Potential Use in Saliva Substitutes. Biorheology 1994, 31, 631–642. [Google Scholar] [CrossRef]
- Chawhuaveang, D.D.; Yu, O.Y.; Yin, I.X.; Lam, W.Y.-H.; Mei, M.L.; Chu, C.-H. Acquired Salivary Pellicle and Oral Diseases: A Literature Review. J. Dent. Sci. 2021, 16, 523–529. [Google Scholar] [CrossRef]
- Qamar, Z.; Haji, Z.B.; Rahim, A.; Chew, H.P.; Fatima, T. Poly-γ-Glutamic Acid a Substitute of Salivary Protein Statherin? J. Chem. Soc. Pak. 2016, 38, 730–736. [Google Scholar]
- Goobes, G.; Goobes, R.; Shaw, W.J.; Gibson, J.M.; Long, J.R.; Raghunathan, V.; Schueler-Furman, O.; Popham, J.M.; Baker, D.; Campbell, C.T.; et al. The Structure, Dynamics, and Energetics of Protein Adsorption—Lessons Learned from Adsorption of Statherin to Hydroxyapatite. Magn. Reson. Chem. 2007, 45, S32–S47. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Schlueter, N.; Rakhmatullina, E.; Ganss, C. Dental Erosion—An Overview with Emphasis on Chemical and Histopathological Aspects. Caries Res. 2011, 45, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Agostini, B.A.; Cericato, G.O.; da Silveira, E.R.; Nascimento, G.G.; Costa, F.d.S.; Thomson, W.M.; Demarco, F.F. How Common Is Dry Mouth? Systematic Review and Meta-Regression Analysis of Prevalence Estimates. Braz. Dent. J. 2018, 29, 606–618. [Google Scholar] [CrossRef] [Green Version]
- Namita; Rai, R. Adolescent Rampant Caries. Contemp. Clin. Dent. 2012, 3, 122. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A. Bacterial Poly-Gamma-Glutamic Acid (γ-pga)—A Promising Biosorbent of Heavy Metals; University of Wolverhampton: Wolverhampton, UK, 2015. [Google Scholar]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial Synthesis of Poly-γ-Glutamic Acid: Current Progress, Challenges, and Future Perspectives. Biotechnol. Biofuels 2016, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashiuchi, M.; Misono, H. Biochemistry and Molecular Genetics of Poly-γ-Glutamate Synthesis. Appl. Microbiol. Biotechnol. 2002, 59, 9–14. [Google Scholar] [CrossRef]
- Qamar, Z.; Haji Abdul Rahim, Z.B.; Neon, G.S.; Chew, H.P.; Zeeshan, T. Effectiveness of Poly-γ-Glutamic Acid in Maintaining Enamel Integrity. Arch. Oral Biol. 2019, 106, 104482. [Google Scholar] [CrossRef]
- Buescher, J.M.; Margaritis, A. Microbial Biosynthesis of Polyglutamic Acid Biopolymer and Applications in the Biopharmaceutical, Biomedical and Food Industries. Crit. Rev. Biotechnol. 2007, 27, 1–19. [Google Scholar] [CrossRef]
- Ho, G.-H.; Ho, T.-I.; Hsieh, K.-H.; Su, Y.-C.; Lin, P.-Y.; Yang, J.; Yang, K.-H.; Yang, S.-C. γ-Polyglutamic Acid Produced ByBacillus Subtilis(Natto): Structural Characteristics, Chemical Properties and Biological Functionalities. J. Chin. Chem. Soc. 2006, 53, 1363–1384. [Google Scholar] [CrossRef]
- Tanimoto, H.; Fox, T.; Eagles, J.; Satoh, H.; Nozawa, H.; Okiyama, A.; Morinaga, Y.; Fairweather-Tait, S.J. Acute Effect of Poly-γ-Glutamic Acid on Calcium Absorption in Post-Menopausal Women. J. Am. Coll. Nutr. 2007, 26, 645–649. [Google Scholar] [CrossRef]
- Tanimoto, H.; Mori, M.; Motoki, M.; Torii, K.; Kadowaki, M.; Noguchi, T. Natto Mucilage Containing Poly-γ-Glutamic Acid Increases Soluble Calcium in the Rat Small Intestine. Biosci. Biotechnol. Biochem. 2001, 65, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K. Characterization of Bacillus Subtilis Gamma-Glutamyltransferase and Its Involvement in the Degradation of Capsule Poly-Gamma-Glutamate. Microbiology 2004, 150, 4115–4123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradali, M.F.; Rehm, B.H.A. Bacterial Biopolymers: From Pathogenesis to Advanced Materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Hezayen, F.F.; Rehm, B.H.; Tindall, B.J.; Steinbüchel, A. Transfer of Natrialba Asiatica B1T to Natrialba Taiwanensis Sp. Nov. And Description of Natrialba Aegyptiaca Sp. Nov., a Novel Extremely Halophilic, Aerobic, Non-Pigmented Member of the Archaea from Egypt That Produces Extracellular Poly(Glutamic Acid). Int. J. Syst. Evol. Microbiol. 2001, 51, 1133–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, R.J.C.; Beauchemin, D.; Clapham, L.; Beveridge, T.J. Metal-Binding Characteristics of the Gamma-Glutamyl Capsular Polymer of Bacillus Licheniformis ATCC 9945. Appl. Environ. Microbiol. 1990, 56, 3671–3677. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, D. Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1998; ISBN 9783540635673. [Google Scholar]
- Bajaj, I.; Singhal, R. Poly (Glutamic Acid)—An Emerging Biopolymer of Commercial Interest. Bioresour. Technol. 2011, 102, 5551–5561. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, X.; Zhou, Z.; Zhang, Y.; Xu, H. Economical Production of Poly(γ-Glutamic Acid) Using Untreated Cane Molasses and Monosodium Glutamate Waste Liquor by Bacillus Subtilis NX-2. Bioresour. Technol. 2012, 114, 583–588. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.M.; Jang, W.J.; Park, H.D.; Kim, Y.; Kim, C.; Kong, I. Efficient Production of Poly γ-D-Glutamic Acid from the Bloom-Forming Green Macroalgae, Ulva Sp., by Bacillus Sp. SJ-10. Biotechnol. Bioeng. 2019, 116, 1594–1603. [Google Scholar] [CrossRef]
- Duarte, C.M.; Bruhn, A.; Krause-Jensen, D. A Seaweed Aquaculture Imperative to Meet Global Sustainability Targets. Nat. Sustain. 2021, 1–9. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Seaweeds as Renewable Sources for Biopolymers and Its Composites: A Review. Curr. Anal. Chem. 2018, 14, 249–267. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Lele, S.S.; Singhal, R.S. Enhanced Production of Poly (γ-Glutamic Acid) from Bacillus Licheniformis NCIM 2324 in Solid State Fermentation. J. Ind. Microbiol. Biotechnol. 2008, 35, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Rapid Determination of Bulk Microalgal Biochemical Composition by Fourier-Transform Infrared Spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The Estimation of the Bactericidal Power of the Blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.-T.; Shahid, S.; Anderson, P. Validation of a Real-Time ISE Methodology to Quantify the Influence of Inhibitors of Demineralization Kinetics in Vitro Using a Hydroxyapatite Model System. Caries Res. 2018, 52, 598–603. [Google Scholar] [CrossRef]
- Fang, J.; Huan, C.; Liu, Y.; Xu, L.; Yan, Z. Bioconversion of Agricultural Waste into Poly-γ-Glutamic Acid in Solid-State Bioreactors at Different Scales. Waste Manag. 2020, 102, 939–948. [Google Scholar] [CrossRef]
- Kedia, G.; Hill, D.; Hill, R.; Radecka, I. Production of Poly-γ-Glutamic Acid by Bacillus Subtilis and Bacillus Licheniformis with Different Growth Media. J. Nanosci. Nanotechnol. 2010, 10, 5926–5934. [Google Scholar] [CrossRef]
- Birrer, G.A.; Cromwick, A.-M.; Gross, R.A. γ-Poly(Glutamic Acid) Formation by Bacillus Licheniformis 9945a: Physiological and Biochemical Studies. Int. J. Biol. Macromol. 1994, 16, 265–275. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Lele, S.S.; Singhal, R.S. A Statistical Approach to Optimization of Fermentative Production of Poly(γ-Glutamic Acid) from Bacillus Licheniformis NCIM 2324. Bioresour. Technol. 2009, 100, 826–832. [Google Scholar] [CrossRef]
- Ashiuchi, M. Effective Synthesis of Bio-nylon Materials Using Bacillus Megaterium; Kochi University Repository: Kochi, Japan, 2007. [Google Scholar]
- Shimizu, K.; Nakamura, H.; Ashiuchi, M. Salt-Inducible Bionylon Polymer from Bacillus Megaterium. Appl. Environ. Microbiol. 2007, 73, 2378–2379. [Google Scholar] [CrossRef] [Green Version]
- Ashiuchi, M.; Shimizu, K. Process for Producing Poly-y-Glutamic Acid Having High Optical Purity. U.S. 20100233764A1, 16 September 2010. [Google Scholar]
- Ji, Y.; Song, W.; Xu, L.; Yu, D.-G.; Annie Bligh, S.W. A Review on Electrospun Poly(Amino Acid) Nanofibers and Their Applications of Hemostasis and Wound Healing. Biomolecules 2022, 12, 794. [Google Scholar] [CrossRef]
- Ashiuchi, M.; Kamei, T.; Baek, D.-H.; Shin, S.-Y.; Sung, M.-H.; Soda, K.; Yagi, T.; Misono, H. Isolation of Bacillus Subtilis (Chungkookjang), a Poly-Gamma-Glutamate Producer with High Genetic Competence. Appl. Microbiol. Biotechnol. 2001, 57, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.-L.; Van, Y.-T. The Production of Poly-(γ-Glutamic Acid) from Microorganisms and Its Various Applications. Bioresour. Technol. 2001, 79, 207–225. [Google Scholar] [CrossRef]
- Yamashiro, D.; Yoshioka, M.; Ashiuchi, M. Bacillus Subtilis PgsE (Formerly YwtC) Stimulates Poly-γ-Glutamate Production in the Presence of Zinc. Biotechnol. Bioeng. 2010, 108, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.R.; Irorere, V.U.; Bartlett, T.; Hill, D.; Kedia, G.; Morris, M.R.; Charalampopoulos, D.; Radecka, I. Bacillus Subtilis Natto: A Non-Toxic Source of Poly-γ-Glutamic Acid That Could Be Used as a Cryoprotectant for Probiotic Bacteria. AMB Express 2013, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Jens, B.H.-N.; Ehiaze, A.E. Biomass Supply Chains for Bioenergy and Biorefining; Woodhead Publishing: Oxford, UK, 2016; pp. 319–332. ISBN 9781782423669. [Google Scholar]
- Kamarudin, A.; Anderson, P.; Hill, R. The Effect of Different Fluoride Varnishes on the Release of Calcium Ions from Hydroxyapatite Discs: An Ion-Selective Electrodes Study. Padjadjaran J. Dent. 2020, 32, 82–90. [Google Scholar] [CrossRef]
- Abou Neel, E.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.; Bozec, L.; Mudera, V. Demineralization–Remineralization Dynamics in Teeth and Bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Gross, R.A. Bacterial Y-Poly(Glutamic Acid). In Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 1998; pp. 195–219. [Google Scholar]
- Park, S.-B.; Hasegawa, U.; van der Vlies, A.J.; Sung, M.-H.; Uyama, H. Preparation of Poly(γ-Glutamic Acid)/Hydroxyapatite Monolith via Biomineralization for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2014, 25, 1875–1890. [Google Scholar] [CrossRef]
| Code | Description | Substrate |
|---|---|---|
| Xi’an γ-PGA | Commercial γ-PGA, hydrolysed to 66 kDa | Not specified |
| Natto γ-PGA | 166 kDa commercial γ-PGA | Not specified |
| CS γ-PGA | 520 kDa commercial γ-PGA | Not specified |
| YR Spec γ-PGA | 440 kDa commercial γ-PGA | Not specified |
| Hydrolysed YR spec γ-PGA | 102 kDa commercial hydrolysed γ-PGA | Not specified |
| GS, 0 g/L NaCl PTF | Modified commercial substrate | 20 g/L L-glut, 50 g/L sucrose, 2.7 g/L KH2PO4, 4.2 g/L Na2HPO4, 0 g/L NaCl, 5 g/L MgSO4 ·7H2O, 1 mL/L of Murashige-Skoog vitamin solution |
| GS, 50 g/L NaCl PTF | Commercial substrate | 20 g/L L-glut, 50 g/L sucrose, 2.7 g/L KH2PO4, 4.2 g/L Na2HPO4, 50 g/L NaCl, 5 g/L MgSO4 ·7H2O, 1 mL/L of Murashige-Skoog vitamin solution |
| L.d. Commercial flakes | Flakes from Cornish Seaweed | High shear mixing 3000 rpm for 3 min, 10 g/L L-glut, 25 g/L sucrose |
| L.d. Commercial flakes PTF | ||
| L.d. SAMS PTF | Powder from Scottish Association for Marine Science ltd. | |
| N.o. Commercial Powder PTF | Powder from Algaecytes Ltd. | Ethanol extraction for 24 h, 10 g/L L-glut, 25 g/L sucrose |
| Production Method | Mn [g/mol] | Mw [g/mol] | Mw/Mn | XRD |
|---|---|---|---|---|
| Xi’an γ-PGA | N.S. | 66,000 | Not Specified | Amorphous |
| Natto γ-PGA | 47,800 | 166,000 | 3.5 | Crystalline |
| CS γ-PGA | N.S. | 520,000 | Not Specified | Amorphous |
| Commercial γ-PGA | 250,000 | 440,000 | 1.8 | Amorphous |
| Hydrolysed commercial γ-PGA | 33,200 | 102,000 | 3.1 | Amorphous |
| GS, 0 g/L NaCl PTF | 3,320,000 | 3,700,000 | 1.1 | Amorphous |
| GS, 50 g/L NaCl PTF | 1,810,000 | 2,700,000 | 1.5 | Crystalline |
| L.d. Commercial flakes | 10,900 | 145,000 | 13.3 | Amorphous |
| L.d. Commercial flakes PTF | 59,000 | 183,000 | 3.1 | Amorphous |
| L.d. SAMS PTF | 1,760,000 | 2,700,000 | 1.5 | Amorphous |
| N.o. Commercial Powder PTF | 1500 | 4600 | 3.1 | Amorphous |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parati, M.; Clarke, L.; Anderson, P.; Hill, R.; Khalil, I.; Tchuenbou-Magaia, F.; Stanley, M.S.; McGee, D.; Mendrek, B.; Kowalczuk, M.; et al. Microbial Poly-γ-Glutamic Acid (γ-PGA) as an Effective Tooth Enamel Protectant. Polymers 2022, 14, 2937. https://doi.org/10.3390/polym14142937
Parati M, Clarke L, Anderson P, Hill R, Khalil I, Tchuenbou-Magaia F, Stanley MS, McGee D, Mendrek B, Kowalczuk M, et al. Microbial Poly-γ-Glutamic Acid (γ-PGA) as an Effective Tooth Enamel Protectant. Polymers. 2022; 14(14):2937. https://doi.org/10.3390/polym14142937
Chicago/Turabian StyleParati, Mattia, Louisa Clarke, Paul Anderson, Robert Hill, Ibrahim Khalil, Fideline Tchuenbou-Magaia, Michele S. Stanley, Donal McGee, Barbara Mendrek, Marek Kowalczuk, and et al. 2022. "Microbial Poly-γ-Glutamic Acid (γ-PGA) as an Effective Tooth Enamel Protectant" Polymers 14, no. 14: 2937. https://doi.org/10.3390/polym14142937
APA StyleParati, M., Clarke, L., Anderson, P., Hill, R., Khalil, I., Tchuenbou-Magaia, F., Stanley, M. S., McGee, D., Mendrek, B., Kowalczuk, M., & Radecka, I. (2022). Microbial Poly-γ-Glutamic Acid (γ-PGA) as an Effective Tooth Enamel Protectant. Polymers, 14(14), 2937. https://doi.org/10.3390/polym14142937