Synthesis and Characterization of New Polycarbonate-Based Poly(thiourethane-urethane)s
Abstract
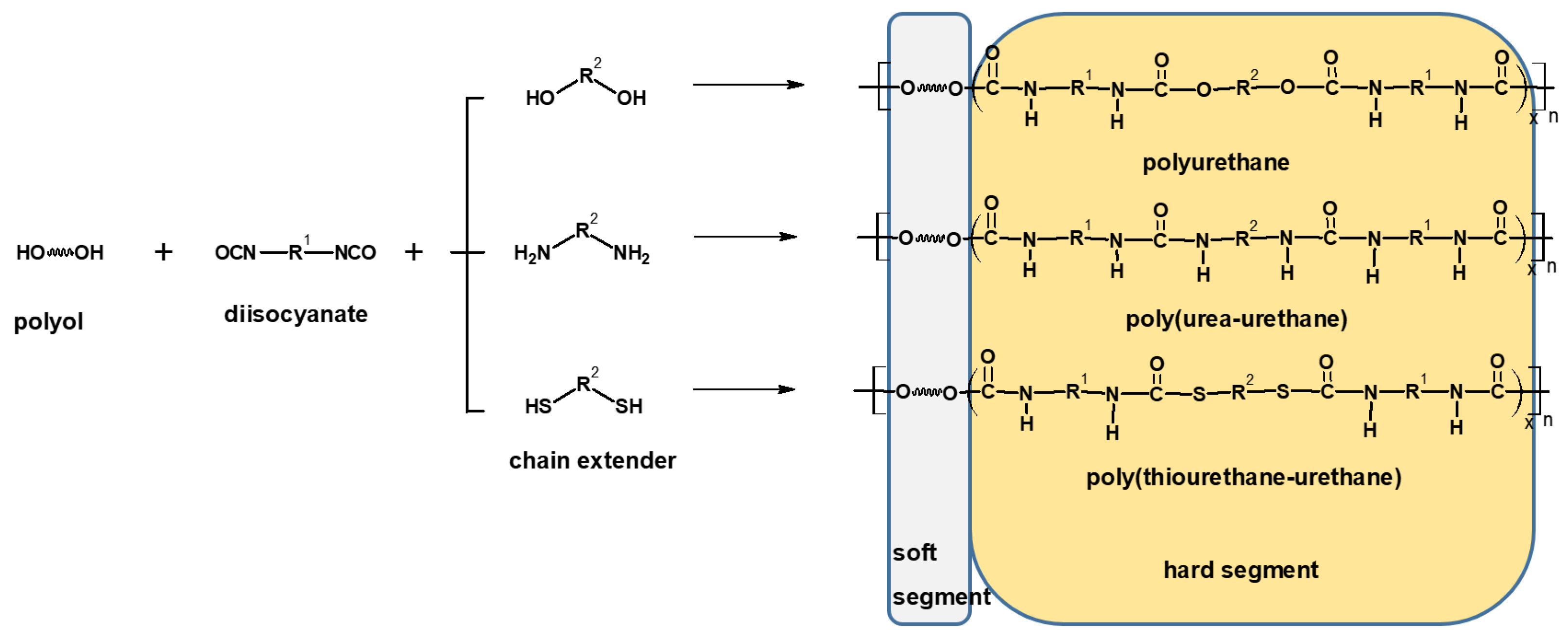
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements Methods
2.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.2. Physicochemical Characterization
- Gel permeation chromatography (GPC)
- 2.
- Reduced viscosities
- 3.
- Contact angles (CAs) and Surface Free Energy (SFE)
- 4.
- Hydrolytic resistance
2.2.3. Thermal and Thermomechanical Properties
- Differential Scanning Calorimetry (DSC)
- 2.
- Thermogravimetric Analysis (TGA)
- 3.
- Dynamic Mechanical Thermal Analysis (DMTA)
2.2.4. Mechanical Properties
2.2.5. Optical Properties
- Refractive index (RI)
- 2.
- Transmittance
- 3.
- Color
2.3. Polymer Synthesis
3. Results and Discussion
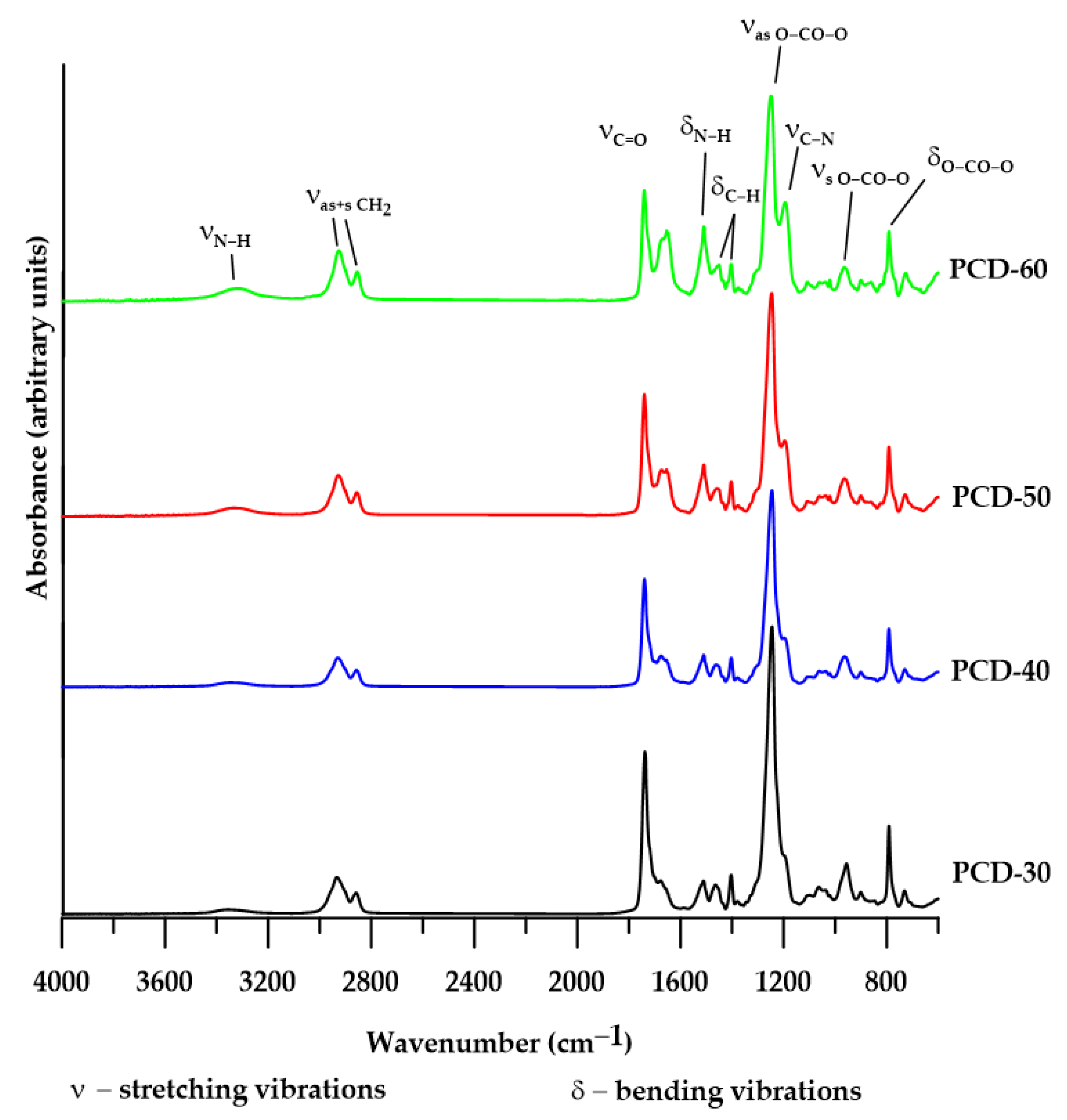
3.1. FTIR
3.2. Physicochemical Characterization
3.2.1. Reduced Viscosities and GPC
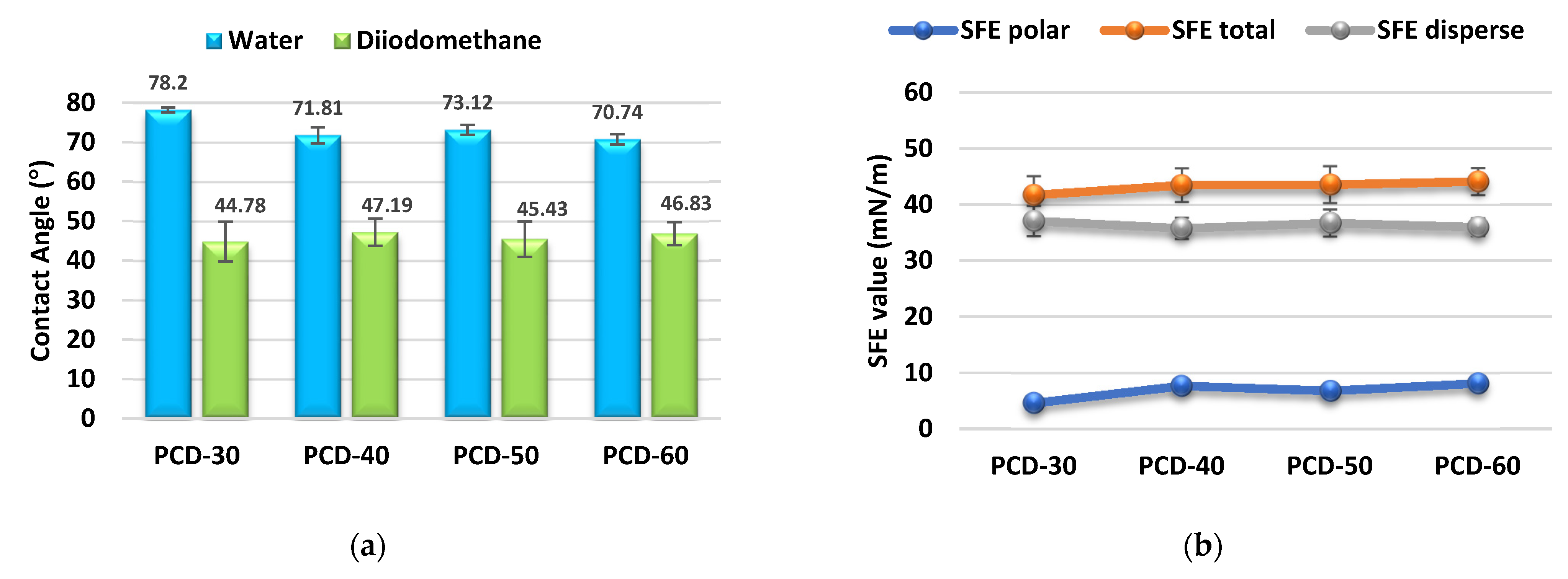
3.2.2. CAs and SFE
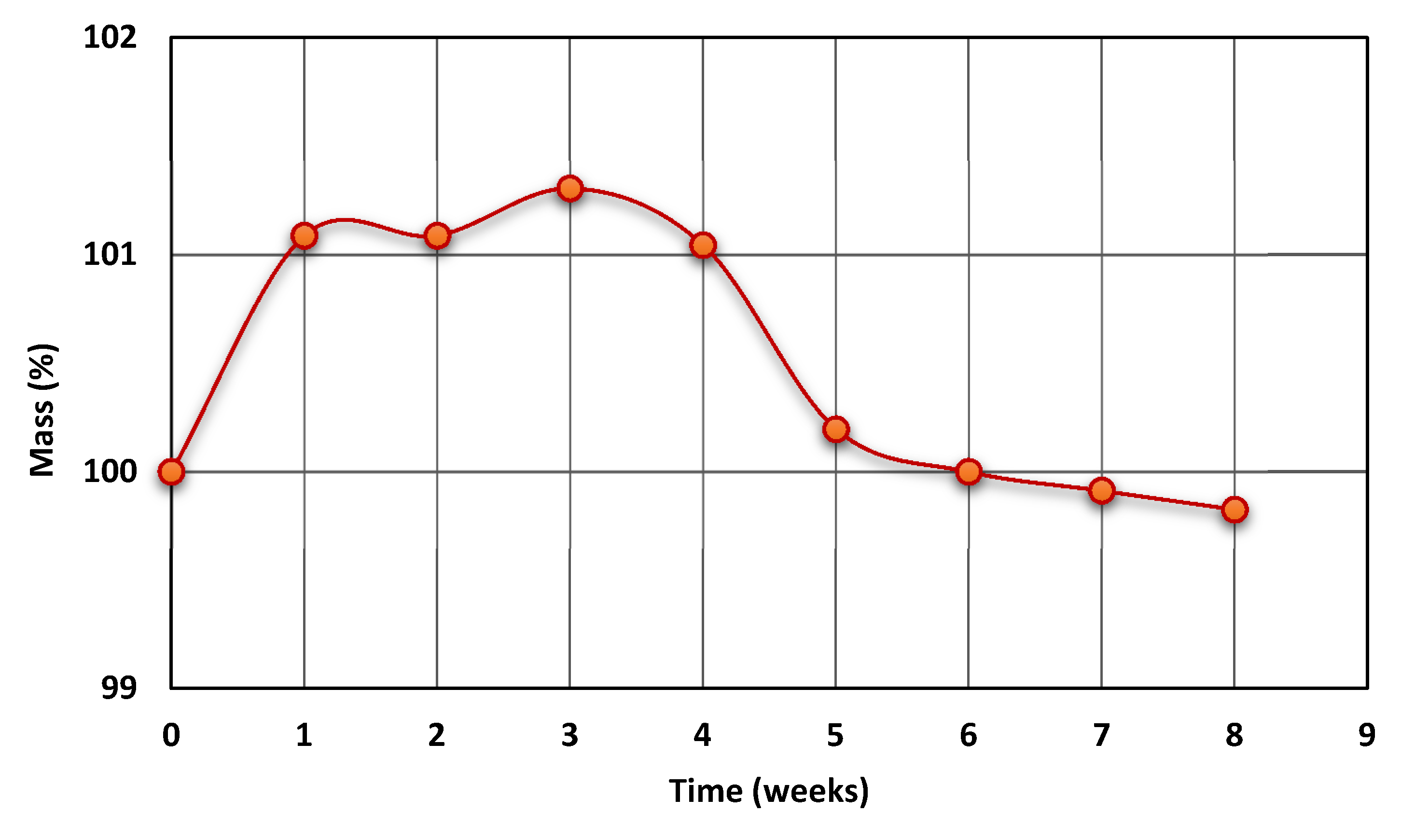
3.2.3. Hydrolytic Resistance
3.3. Thermal and Thermomechanical Properties
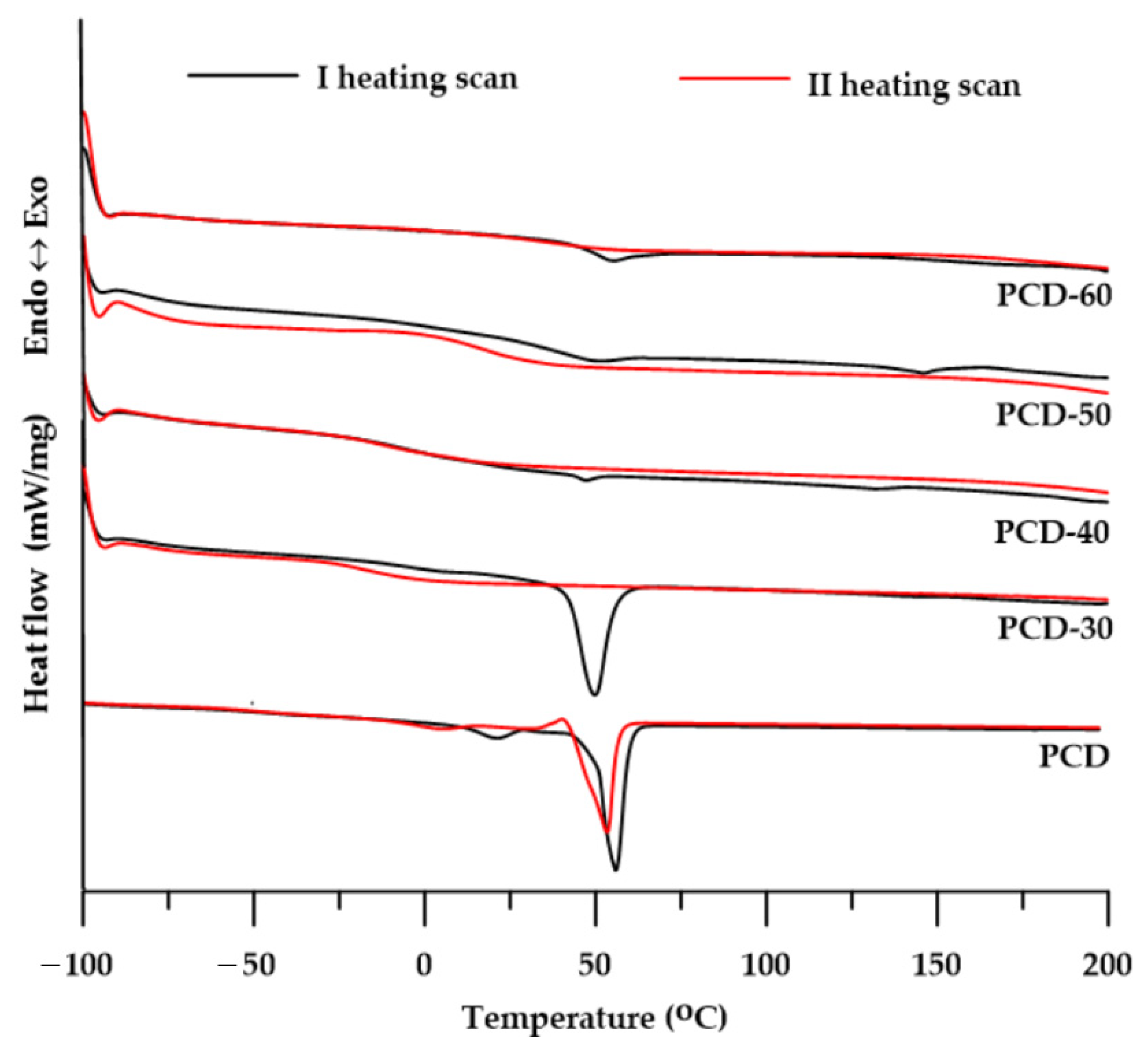
3.3.1. DSC
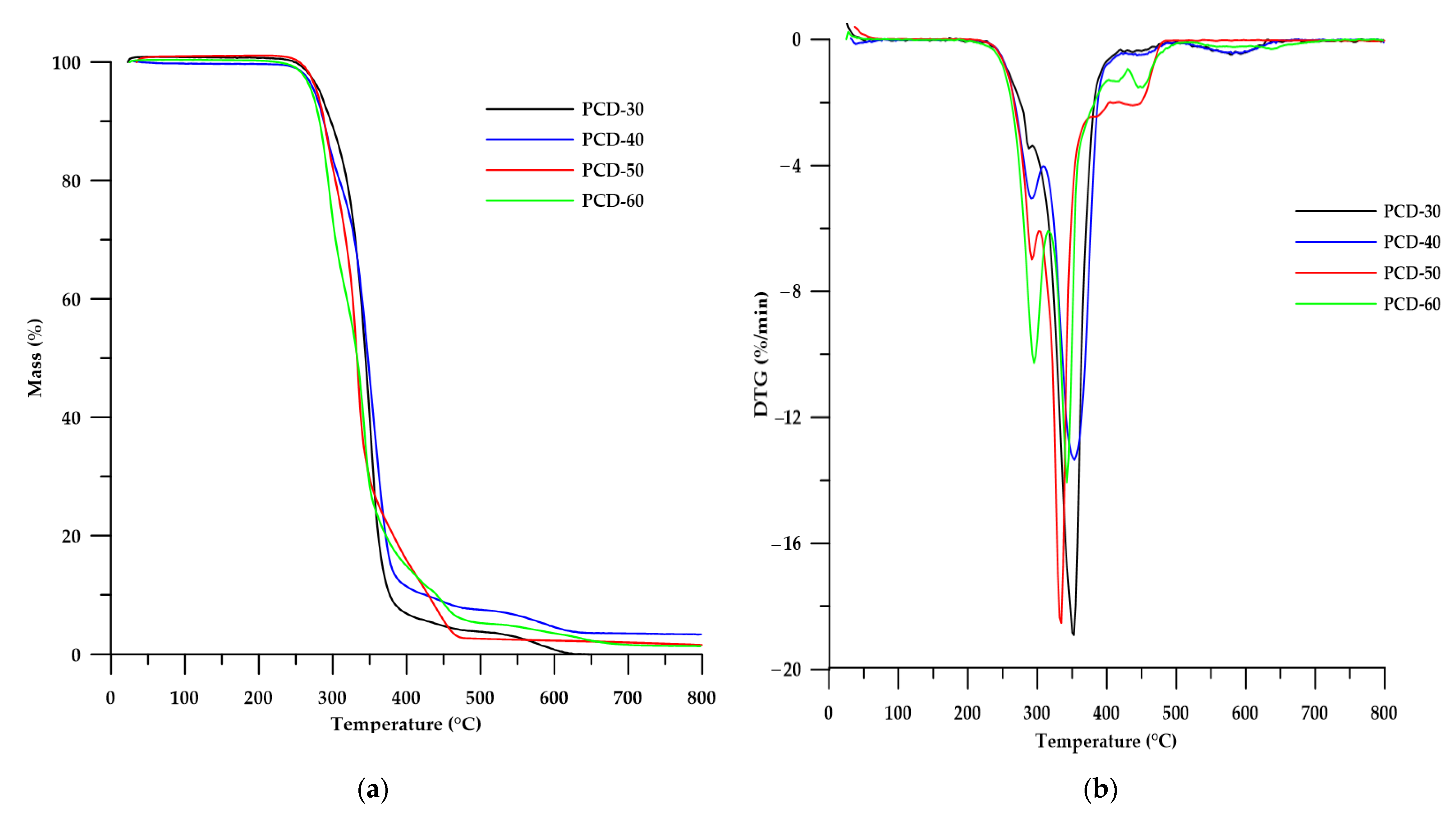
3.3.2. TGA
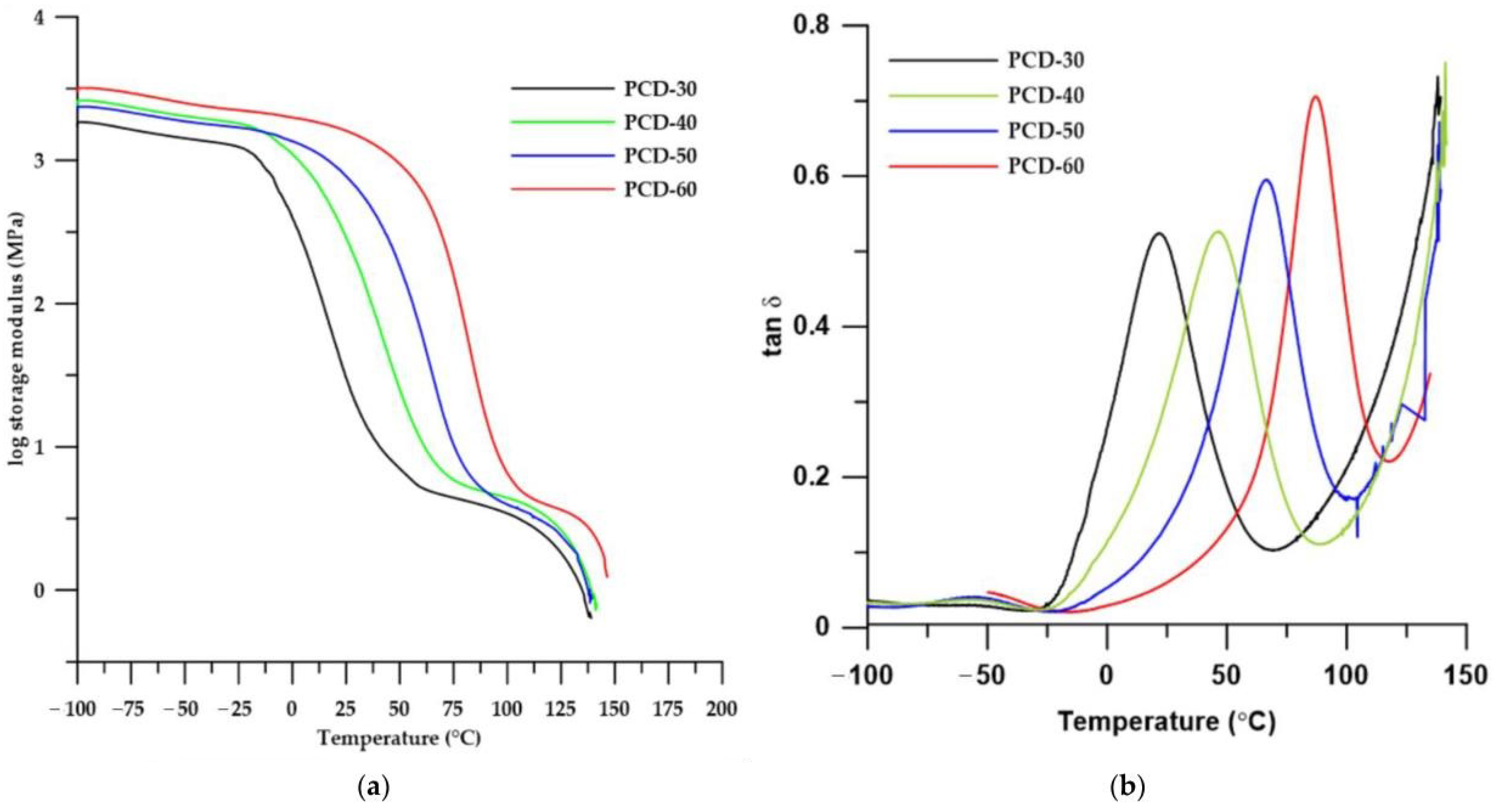
3.3.3. DMTA
3.4. Mechanical Properties
3.5. Optical Properties
3.5.1. Refractive Index and Transparency
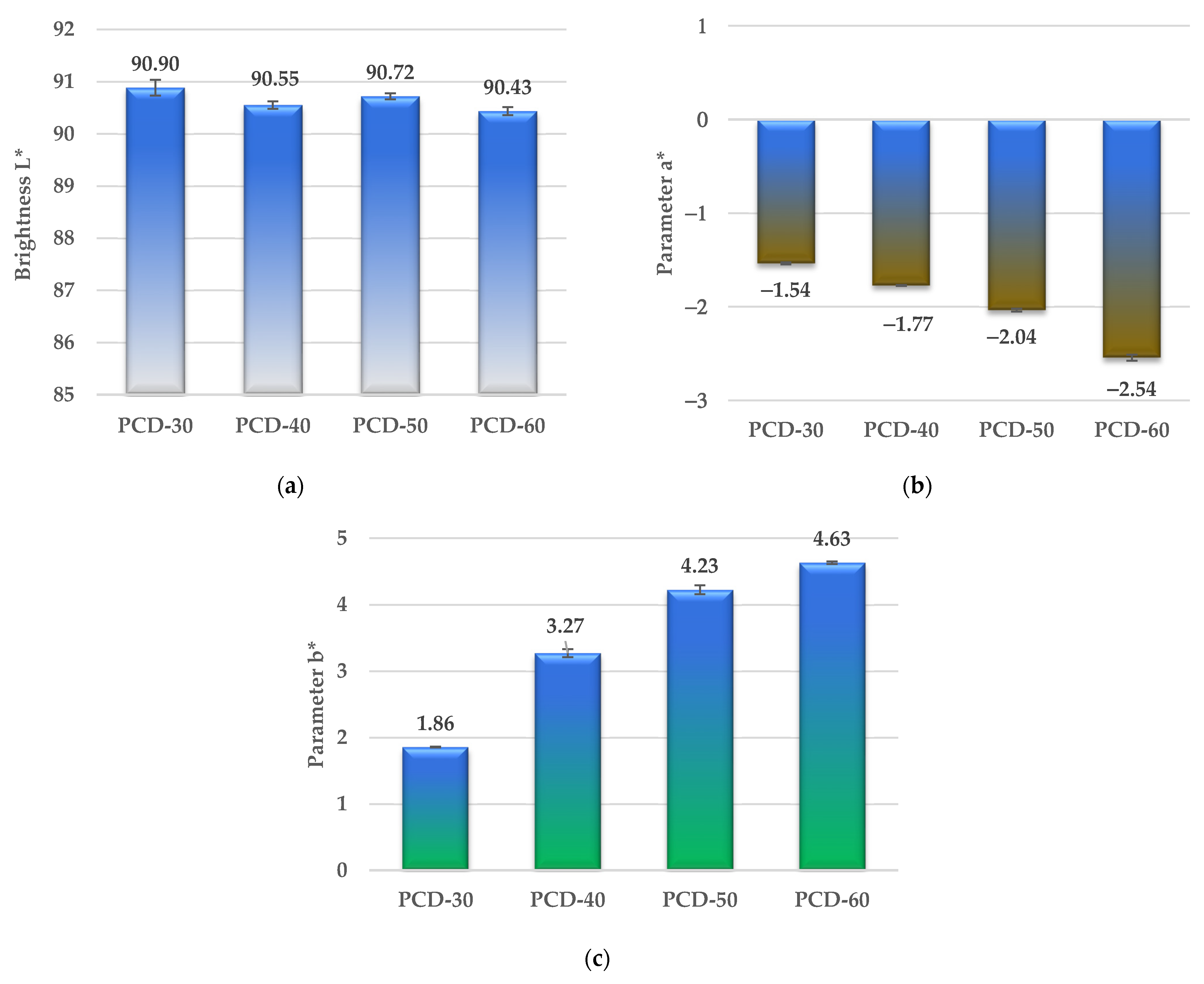
3.5.2. Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janik, H.; Sienkiewicz, M.; Kucinska-Lipka, J. 9-Polyurethanes. In Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; William Andrew Publishing: Norwich, NY, USA, 2014; pp. 253–295. [Google Scholar] [CrossRef]
- Wirpsza, Z. Polyurethanes—Chemistry, Technology, Application. Warszawa: WNT; Ellis Horwood Ltd.: Chichester, UK, 1991. [Google Scholar]
- Drobny, J.G. 9-Thermoplastic Polyurethane Elastomers. In Plastics Design Library, Handbook of Thermoplastic Elastomers, 2nd ed.; Drobny, J.G., Ed.; William Andrew Publishing: Norwich, NY, USA, 2014; pp. 233–253. [Google Scholar] [CrossRef]
- Fink, J.K. Chapter 2-Poly(urethane)s. In Plastics Design Library, Reactive Polymers Fundamentals and Applications, 2nd ed.; Fink, J.K., Ed.; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 49–93. [Google Scholar] [CrossRef]
- Datta, J.; Kasprzyk, P.; Błażek, K.; Włoch, M. Synthesis, structure and properties of poly(ester-urethane)s obtained using bio-based and petrochemical 1,3-propanediol and 1,4-butanediol. J. Therm. Anal. Calorim. 2017, 130, 261–276. [Google Scholar] [CrossRef]
- Lei, W.; Fang, C.; Zhou, X.; Li, J.; Yang, R.; Zhang, Z.; Liu, D. Thermal properties of polyurethane elastomer with different flexible molecular chain based on para-phenylene diisocyanate. J. Mater. Sci. Technol. 2017, 33, 1424–1432. [Google Scholar] [CrossRef]
- Hou, X.; Sun, L.; Wei, W.; Taylor, D.K.; Su, S.; Yu, H. Structure and performance control of high-damping bio-based thermoplastic polyurethane. J. Appl. Polym. Sci. 2022, 139, e52059. [Google Scholar] [CrossRef]
- Špírková, M.; Hodan, J.; Kobera, L.; Kredatusová, J.; Kubies, D.; Machová, L.; Poręba, R.; Serkis, M.; Zhigunov, A.; Kotek, J. The influence of the length of the degradable segment on the functional properties and hydrolytic stability of multi-component polyurethane elastomeric films. Polym. Deg. Stab. 2017, 137, 216–228. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, X.; Zhao, Z.; Mao, L.; Zhang, L.; Coates, P. Effects of wide-range γ-irradiation doses on the structures and properties of 4,4′-dicyclohexyl methane diisocyanate based poly(carbonate urethane)s. J. Appl. Polym. Sci. 2014, 131, 41049. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Nachman, M. The Abrasive Wear Resistance of the Segmented Linear Polyurethane Elastomers Based on a Variety of Polyols as Soft Segments. Polymers 2017, 9, 705. [Google Scholar] [CrossRef] [Green Version]
- Kull, K.L.; Bass, R.W.; Craft, G.; Julien, T.; Marangon, E.; Marrouat, C.; Harmon, J.P. Synthesis and characterization of an ultra-soft poly(carbonate urethane). Eur. Polym. J. 2015, 71, 510–522. [Google Scholar] [CrossRef]
- Patti, A.; Acierno, D. The Puncture and Water Resistance of Polyurethane- Impregnated Fabrics after UV Weathering. Polymers 2020, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patti, A.; Costa, F.; Perrotti, M.; Barbarino, D.; Acierno, D. Polyurethane Impregnation for Improving the Mechanical and the Water Resistance of Polypropylene-Based Textiles. Materials 2021, 14, 1951. [Google Scholar] [CrossRef]
- Samaniego-Aguilar, K.; Sánchez-Safont, E.; Arrillaga, A.; Anakabe, J.; Gamez-Perez, J.; Cabedo, L. In Service Performance of Toughened PHBV/TPU Blends Obtained by Reactive Extrusion for Injected Parts. Polymers 2022, 14, 2337. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, L.; Chen, A.; Wu, F. Improvement of Mechanical Property for PLA/TPU Blend by Adding PLA-TPU Copolymers Prepared via In Situ Ring-Opening Polymerization. Polymers 2022, 14, 1530. [Google Scholar] [CrossRef] [PubMed]
- Formela, K.; Kurańska, M.; Barczewski, M. Recent Advances in Development of Waste-Based Polymer Materials: A Review. Polymers 2022, 14, 1050. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-G.; Jeong, J.-O.; Jeong, S.-I.; Park, J.-S. Radiation-Based Crosslinking Technique for Enhanced Thermal and Mechanical Properties of HDPE/EVA/PU Blends. Polymers 2021, 13, 2832. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Heinrich, G.; Mahmood, N.; Boldt, R.; Wießner, S.; Stöckelhuber, K.W. Blending In Situ Polyurethane-Urea with Different Kinds of Rubber: Performance and Compatibility Aspects. Materials 2018, 11, 2175. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wu, H.; Zhang, R.; Deng, K.; Li, Y.; Liu, Z.; Zhong, Q.; Kang, Y. Effect of Graphene/Spherical Graphite Ratio on the Properties of PLA/TPU Composites. Polymers 2022, 14, 2538. [Google Scholar] [CrossRef]
- Shiu, B.-C.; Hsu, P.-W.; Lin, J.-H.; Chien, L.-F.; Lin, J.-H.; Lou, C.-W. A Study on Preparation and Property Evaluations of Composites Consisting of TPU/Triclosan Membranes and Tencel®/LMPET Nonwoven Fabrics. Polymers 2022, 14, 2514. [Google Scholar] [CrossRef]
- Luo, Z.; Li, X.; Zhao, S.; Xu, L.; Liu, L. Structure and Dielectric Properties of TPU Composite Filled with CNTs@PDA Nanofibers and MXene Nanosheets. Polymers 2022, 14, 2157. [Google Scholar] [CrossRef]
- Zafar, K.; Zia, K.M.; Alzhrani, R.M.; Almalki, A.H.; Alshehri, S. Biocompatibility and Hemolytic Activity Studies of Synthesized Alginate-Based Polyurethanes. Polymers 2022, 14, 2091. [Google Scholar] [CrossRef]
- Brzeska, J.; Jasik, G.; Sikorska, W.; Mendrek, B.; Karczewski, J.; Kowalczuk, M.; Rutkowska, M. Susceptibility to Degradation in Soil of Branched Polyesterurethane Blends with Polylactide and Starch. Polymers 2022, 14, 2086. [Google Scholar] [CrossRef]
- De Keer, L.; Kilic, K.I.; Van Steenberge, P.H.M.; Daelemans, L.; Kodura, D.; Frisch, H.; De Clerck, K.; Reyniers, M.F.; Barner-Kowollik, C.; Dauskardt, R.H.; et al. Computational prediction of the molecular configuration of three-dimensional network polymers. Nat. Mater. 2021, 20, 1422–1430. [Google Scholar] [CrossRef]
- De Keer, L.; Cavallli, F.; Estupii, D.; Krüger, A.J.D.; Rocha, S.; Van Steenberge, P.H.M.; Reyniers, M.-F.; De Laporte, L.; Hofkens, J.; Barner, L.; et al. Synergy of Advanced Experimental and Modeling Tools to Underpin the Synthesis of Static Step-Growth-Based Networks Involving Polymeric Precursor Building Blocks. Macromolecules 2021, 54, 9280–9298. [Google Scholar] [CrossRef]
- Schollenberger, C.S.; Stewart, F.D. Thermoplastic Polyurethane Hydrolysis Stability. J. Elastoplast. 1971, 3, 28–56. [Google Scholar] [CrossRef]
- Tanzi, M.C.; Mantovani, D.; Petrini, P.; Guidoin, R.; Laroche, G. Chemical stability of polyether urethanes versus polycarbonate urethanes. J. Biomed. Mater. Res. 1997, 36, 550–559. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Jeong, H.M. Properties of polythiourethanes prepared by thiol–isocyanate click reaction. J. Appl. Polym. Sci. 2017, 135, 46070. [Google Scholar] [CrossRef]
- Rahmawati, R.; Nozaki, S.; Kojio, K.; Takahara, A.; Shinohara, N.; Yamasaki, S. Microphase-separated structure and mechanical properties of cycloaliphatic diisocyanate-based thiourethane elastomers. Polym. J. 2019, 51, 265–273. [Google Scholar] [CrossRef]
- Ellson, G.; Carrier, X.; Walton, J.; Mahmood, S.F.; Yang, K.; Salazar, J.; Voit, W.E. Tough thiourethane thermoplastics for fused filament fabrication. J. Appl. Polym. Sci. 2017, 135, 45574. [Google Scholar] [CrossRef]
- Shin, J.; Matsushima, H.; Chan, J.W.; Hoyle, C.E. Segmented Polythiourethane Elastomers through Sequential Thiol−Ene and Thiol−Isocyanate Reactions. Macromolecules 2009, 42, 3294–3301. [Google Scholar] [CrossRef]
- Kultys, A.; Puszka, A. Transparent poly(thiourethane-urethane)s based on dithiol chain extender. J. Therm. Anal. Calorim. 2014, 117, 1427–1439. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Olszewska, E. New thermoplastic poly(thiourethane-urethane) elastomers based on hexane-1,6-diyldiisocyanate (HDI). J. Therm. Anal. Calorim. 2013, 114, 903–916. [Google Scholar] [CrossRef] [Green Version]
- Puszka, A. Thermal and Mechanical Behavior of New Transparent Thermoplastic Polyurethane Elastomers Derived from Cycloaliphatic Diisocyanate. Polymers 2018, 10, 537. [Google Scholar] [CrossRef] [Green Version]
- Puszka, A.; Kultys, A.; Rogulska, M. Influence of DMPA content on the properties of new thermoplastic poly(ether-urethane) elastomers. J. Elastom. Plast. 2018, 50, 140–150. [Google Scholar] [CrossRef]
- Puszka, A.; Kultys, A. The influence of soft segments on some properties of new transparent segmented polyurethanes. Polym. Adv. Technol. 2017, 28, 1937–1944. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Pikus, S. The effect of chain extender structure on the properties of new thermoplastic poly(carbonate–urethane)s derived from MDI. J. Therm. Anal. Calorim. 2017, 127, 2325–2339. [Google Scholar] [CrossRef] [Green Version]
- Puszka, A.; Kultys, A. New thermoplastic polyurethane elastomers based on aliphatic diisocyanate. J. Therm. Anal. Calorim. 2017, 128, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Rogulska, M.; Kultys, A.; Puszka, A. New thermoplastic poly(carbonate-urethane)s based on chain extenders with sulfur atoms. Chem. Pap. 2017, 71, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Kultys, A.; Rogulska, M.; Pikus, S. New thermoplastic segmented polyurethanes with hard segment derived from 4,4′-diphenylmethane diisocyanate and methylenebis(1,4-phenylenemethylenethio)dialcanols. J. Appl. Polym. Sci. 2012, 123, 331–346. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M.; Gluchowska, H. The effect of soft segment structure on the properties of novel thermoplastic polyurethane elastomers based on an unconventional chain extender. Polym. Int. 2011, 60, 652–659. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M. New thermoplastic poly(carbonate-urethane) elastomers. Pol. J. Chem. Technol. 2011, 13, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Kultys, A.; Rogulska, M.; Pikus, S.; Skrzypiec, K. The synthesis and characterization of new thermoplastic poly(carbonate-urethane) elastomers derived from HDI and aliphatic-aromatic chain extenders. Eur. Polym. J. 2009, 45, 2629–2643. [Google Scholar] [CrossRef]
- Kultys, A.; Pikus, S. Polyurethanes containing sulfur. III. New thermoplastic HDI-based segmented polyurethanes with diphenylmethane unit in their structure. J. Polym. Sci. Pol. Chem. 2001, 39, 1733–1742. [Google Scholar] [CrossRef]
- Kultys, A.; Podkoscielny, W.; Majewski, W. Polyurethanes containing sulfur. II. New thermoplastic nonsegmented polyurethanes with diphenylmethane unit in their structure. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1767–1773. [Google Scholar] [CrossRef]
- Kultys, A.; Puszka, A. New thermoplastic polyurethane elastomers based on sulfur-containing chain extenders. Pol. J. Chem. Technol. 2013, 15, 65–70. [Google Scholar] [CrossRef]
- Rogulska, M. New thermoplastic poly(carbonate-urethane)s based on diphenylethane-derivative chain extenders—the effect of chain extender structure on thermal and mechanical properties. J. Therm. Anal. Calorim. 2020, 139, 3107–3121. [Google Scholar] [CrossRef] [Green Version]
- Rogulska, M.; Maciejewska, M.; Olszewska, E. New thermoplastic poly(carbonate-urethane)s based on diphenylethane derivative chain extender. J. Therm. Anal. Calorim. 2020, 139, 1049–1068. [Google Scholar] [CrossRef] [Green Version]
- Rogulska, M.; Podkoscielny, W.; Kultys, A.; Pikus, S.; Pozdzik, E. Studies on thermoplastic polyurethanes based on new diphenylethane-derivative diols. I. Synthesis and characterization of nonsegmented polyurethanes from HDI and MDI. Eur. Polym. J. 2006, 42, 1786–1797. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Podkoscielny, W. Studies on thermoplastic polyurethanes based on new diphenylethane-derivative diols. II. Synthesis and characterization of segmented polyurethanes from HDI and MDI. Eur. Polym. J. 2007, 43, 1402–1414. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Pikus, S. Studies on thermoplastic Polyurethanes based on new diphenylethane-derivative diols. III. The effect of molecular mass and structure of soft segment on some properties of segmented polyurethanes. J. Appl. Polym. Sci. 2008, 110, 1677–1689. [Google Scholar] [CrossRef]
- Kultys, A.; Podkoscielny, W.; Pikus, S. Polyurethanes containing sulfur. I. New thermoplastic polyurethanes with benzophenone unit in their structure. J. Polym. Sci. Pol. Chem. 1999, 37, 4140–4150. [Google Scholar] [CrossRef]
- Kultys, A. Polyesters containing sulfur. VIII. New benzophenone derivative thiopolyesterdiols synthesis and characterization. Their use for thermoplastic polyurethanes preparation. J. Polym. Sci. Pol. Chem. 2000, 38, 3977–3983. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M.; Pikus, S. The synthesis and characterization of new thermoplastic poly(thiourethane-urethane)s. J. Polym. Sci. Pol. Chem. 2008, 46, 1770–1782. [Google Scholar] [CrossRef]
- Wdowicka, D.; Podkoscielny, W.; Kultys, A. Polyurethanes (III): Polyaddition products of 4,4′-Bis(2-hydroxyethylenethio) diphenyl ether and 2,4-tolylene diisocyanate. Iran. Polym. J. 2000, 9, 97–103. [Google Scholar]
- Rogulska, M.; Kultys, A.; Lubczak, J. New thermoplastic polyurethane elastomers based on aliphatic-aromatic chain extenders with different content of sulfur atoms. J. Therm. Anal. Calorim. 2015, 121, 397–410. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A. Aliphatic polycarbonate-based thermoplastic polyurethane elastomers containing diphenyl sulfide units. J. Therm. Anal. Calorim. 2016, 126, 225–243. [Google Scholar] [CrossRef] [Green Version]
- Rogulska, M. Transparent sulfur-containing thermoplastic polyurethanes with polyether and polycarbonate soft segments. Polym. Bull. 2018, 75, 1211–1235. [Google Scholar] [CrossRef] [Green Version]
- Rogulska, M. Polycarbonate-based thermoplastic polyurethane elastomers modified by DMPA. Polym. Bull. 2019, 76, 4719–4733. [Google Scholar] [CrossRef] [Green Version]
- Wnuczek, K.; Puszka, A.; Podkościelna, B. Synthesis and Spectroscopic Analyses of New Polycarbonates Based on Bisphenol A-Free Components. Polymers 2021, 13, 4437. [Google Scholar] [CrossRef] [PubMed]
- Stepto, R.F.T. Dispersity in polymer science. Pure Appl. Chem. 2009, 81, 351–353. [Google Scholar] [CrossRef] [Green Version]
- Król, P.; Uram, ł.; Król, B.; Pielichowska, K.; Walczak, M. Study of chemical, physico-mechanical and biological properties of 4,4′-methylenebis(cyclohexyl isocyanate)-based polyurethane films. Mater. Sci. Eng. C 2018, 93, 483–494. [Google Scholar] [CrossRef] [PubMed]
- ISO 11357:2016; Plastics—Differential Scanning Calorimetry (DSC). International Organization of Standardization: Geneva, Switzerland, 2016.
- ISO 527-2:2012; Plastics—Determination of Tensile Properties—Part 2. International Organization of Standardization: Geneva, Switzerland, 2012.
- EN ISO. 868:2003; Plastics—Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (ShoreHardness). International Organization of Standardization: Geneva, Switzerland, 2003.
- PN EN 1465:2009; Adhesives. Determination of Tensile Lap-Shear Strength of Bonded Assemblies. European Committee for Standardization: Brussels, Belgium, 2009.
- PN-EN 13887:2005; Structural Adhesives. Guidelines for Surface Preparation of Metals and Plastics Prior to Adhesive Bonding. European Committee for Standardization: Brussels, Belgium, 2005.
- ISO 489:2022; Plastics—Determination of Refractive Index. International Organization of Standardization: Geneva, Switzerland, 2022.
- ASTM E308; Standard Practice for Computing the Colour of Objects by Using the CIE System. ASTM International (ASTM): London, UK, 2018.
- Baier, R.E. The role of surface energy in thrombogenesis. Bull. N. Y. Acad. Med. 1972, 48, 257–272. [Google Scholar] [PubMed]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 2004, 25, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.C.; Pagani, R.; Vallet-Regi, M.; Pena, J.; Ramila, A.; Izquierdo, I.; Portoles, M.T. In vitro biocompatibility assessment of poly(ε-caprolactone) films using L929 mouse fibroblasts. Biomaterials 2004, 25, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 23, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
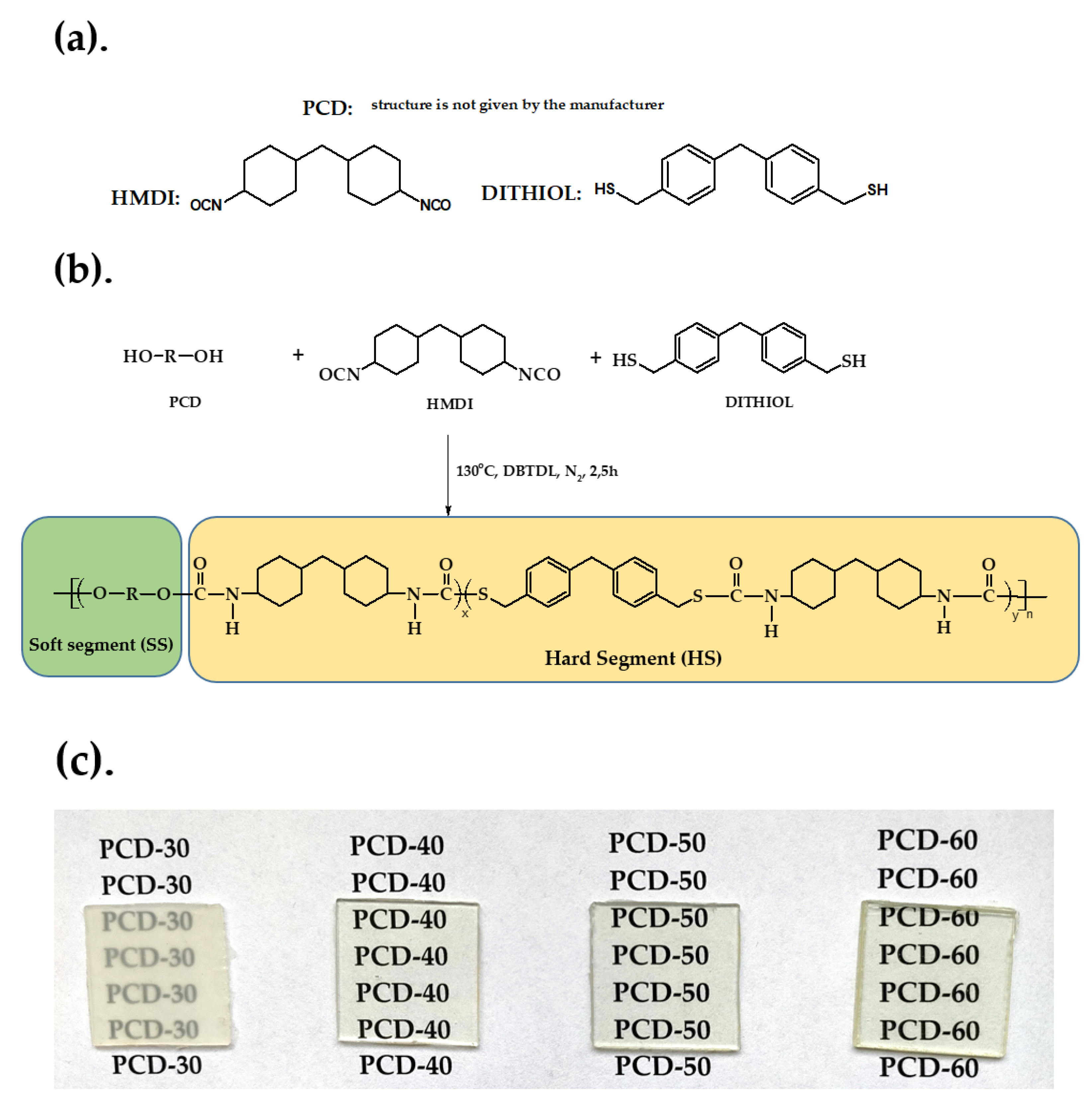
| PTUR | Amount of Dithiol (mol %) | Amount of Soft Segment (mol %) | 1 Hard-Segment Content (wt%) |
|---|---|---|---|
| PCD-30 | 53 | 47 | 29.87 |
| PCD-40 | 67 | 33 | 39.83 |
| PCD-50 | 77 | 23 | 50.15 |
| PCD-60 | 85 | 15 | 59.88 |
| Types of Vibrations [cm−1] | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTUR | νN–H | νC–H CH2 | νC=O Carbonyl | νC=O Thiour. | δN–H | δC–H and CH2 | ν O–CO–O Carbonyl | νC–N + δN–H | δ O–CO–O Carbonyl | |||||
| νas | νsym | H-Bonded | Free | νas | νsym | νas | νsym | Out of Plane | in pl. | |||||
| PCD-30 | 3351 | 2933 | 2859 | 1738 | - | 1676 | 1511 | 1465 | 1404 | 1245 | 957 | - | 791 | 731 |
| PCD-40 | 3351 | 2930 | 2857 | 1740 | - | 1676 | 1510 | 1458 | 1403 | 1245 | 901 | - | 792 | 730 |
| PCD-50 | 3338 | 2928 | 2856 | 1740 | 1654 | 1672 | 1510 | 1458 | 1403 | 1246 | 900 | 1195 | 792 | 729 |
| PCD-60 | 3309 | 2926 | 2854 | 1741 | 1654 | - | 1509 | 1450 | 1404 | 1249 | 900 | 1194 | 791 | 727 |
| PTUR | ηred (dL/g) | ÐM | ||
|---|---|---|---|---|
| PCD-30 | 3.63 | 41,900 | 60,100 | 1.43 |
| PCD-40 | 1.90 | 32,000 | 53,200 | 1.66 |
| PCD-50 | 1.54 | 42,000 | 62,000 | 1.48 |
| PCD-60 | 0.62 | 21,700 | 33,000 | 1.52 |
| PTUR | Tg [°C] | Tm [°C] | ΔH [J/g] | |||
|---|---|---|---|---|---|---|
| I a | II b | I a | II b | I a | II b | |
| PCD-30 | −2 | −15 | 50 | - | 34.1 | - |
| PCD-40 | 1 | −12 | 47; 132 | - | 0.7; 0.6 | - |
| PCD-50 | 32 | 20 | 52; 146 | - | 1.9; 2.2 | - |
| PCD-60 | 44 | 33 | 55 | - | 2.6 | - |
| PCD | −40 | −50 | 21; 56 | 5.6; 40.1; 53.3 | 6.6; 64.8 | 3.6; 3.3; 53.0 |
| PTUR | T5 1 (°C) | T10 2 (°C) | T50 3 (°C) | Tmax4 (°C) |
|---|---|---|---|---|
| PCD-30 | 281 | 295 | 343 | 286; 333; 360; 429 |
| PCD-40 | 279 | 289 | 341 | 292; 339; 362; 444 |
| PCD-50 | 278 | 288 | 337 | 292; 334; 367; 446 |
| PCD-60 | 275 | 285 | 333 | 295; 342; 451 |
| PTUR | E′onset (°C) | E′20 (MPa) | E″max (°C) | T tanδmax (°C) | tanδmax | FWHM (°C) |
|---|---|---|---|---|---|---|
| PCD-30 | −6.69 | 52 | −5.81 | 21.07 | 0.459 | 48.45 |
| PCD-40 | 10.55 | 418 | 5.81 | 46.19 | 0.448 | 40.23 |
| PCD-50 | 36.98 | 910 | 27.42 | 66.37 | 0.461 | 31.43 |
| PCD-60 | 61.41 | 1704 | 50.95 | 86.87 | 0.535 | 24.48 |
| PTUR | Hardness (Sh) | Tensile Strength (MPa) | Elongation at Break (%) | Modulus of Elasticity (MPa) | Lap Shear Strength (MPa) | |
|---|---|---|---|---|---|---|
| A | D | |||||
| PCD-30 | 71.75 ± 1.50 | 25.00 ± 0.82 | 34.43 ± 0.23 | 350 ± 0 | 1.82 ± 0.13 | 4.32 ± 0.33 |
| PCD-40 | 84.75 ± 0.50 | 34.25 ± 2.99 | 45.76 ± 2.71 | 275 ± 0 | 4.35 ± 0.56 | 14.70 ± 0.51 |
| PCD-50 | 96.25 ± 0.50 | 53.00 ± 1.87 | 46.54 ± 4.06 | 223 ± 2.89 | 69.51 ± 2.19 | 15.93 ± 0.64 |
| PCD-60 | 91.50 ± 1.15 | 66.00 ± 1.41 | 51.11 ± 2.37 | 75 ± 0 | 289.88 ± 2.88 | 18.05 ± 0.44 |
| PTUR | Refractive Index | Transmittance (%) | |
|---|---|---|---|
| T500 1 | T800 2 | ||
| PCD-30 | - 3 | 63.41 ± 0.012 | 71.78 ± 0.009 |
| PCD-40 | 1.5135 ± 0.002 | 76.52 ± 0.008 | 82.91 ± 0.014 |
| PCD-50 | 1.5355 ± 0.003 | 76.05 ± 0.007 | 82.89 ± 0.011 |
| PCD-60 | 1.5615 ± 0.003 | 72.67 ± 0.010 | 80.15 ± 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puszka, A.; Sikora, J.W. Synthesis and Characterization of New Polycarbonate-Based Poly(thiourethane-urethane)s. Polymers 2022, 14, 2933. https://doi.org/10.3390/polym14142933
Puszka A, Sikora JW. Synthesis and Characterization of New Polycarbonate-Based Poly(thiourethane-urethane)s. Polymers. 2022; 14(14):2933. https://doi.org/10.3390/polym14142933
Chicago/Turabian StylePuszka, Andrzej, and Janusz W. Sikora. 2022. "Synthesis and Characterization of New Polycarbonate-Based Poly(thiourethane-urethane)s" Polymers 14, no. 14: 2933. https://doi.org/10.3390/polym14142933
APA StylePuszka, A., & Sikora, J. W. (2022). Synthesis and Characterization of New Polycarbonate-Based Poly(thiourethane-urethane)s. Polymers, 14(14), 2933. https://doi.org/10.3390/polym14142933







