Effect of TiO2 Nanoparticles on Capillary-Driven Flow in Water Nanofilters Based on Chitosan Cellulose and Polyvinylidene Fluoride Nanocomposites: A Theoretical Study
Abstract
:1. Introduction
2. Materials and Methods
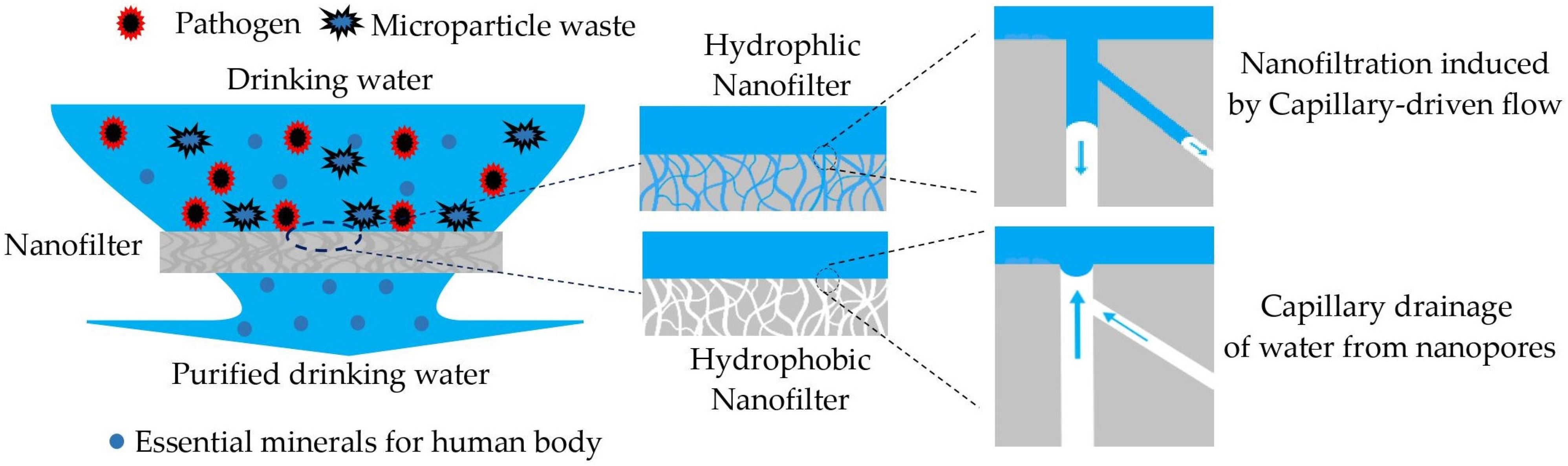
2.1. Capillary Nanofiltration
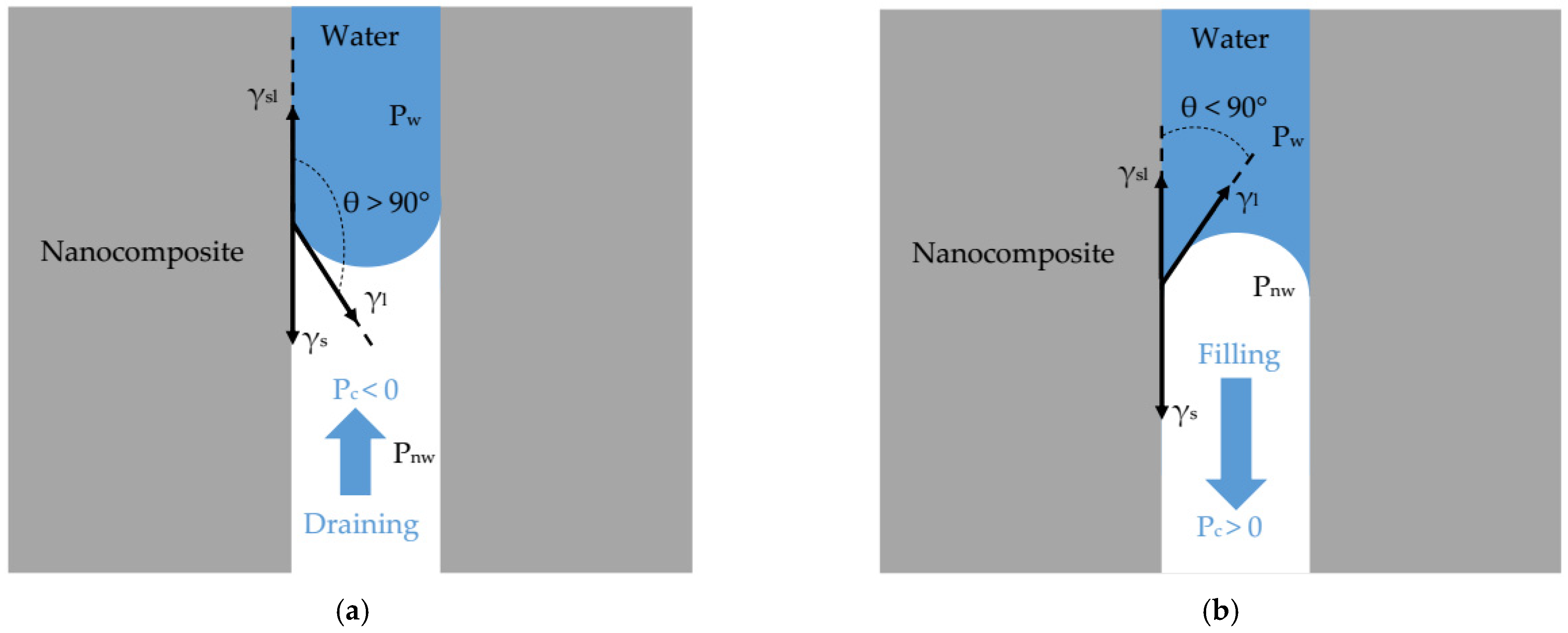
2.1.1. Nanofiltration and Capillary Pressure
2.1.2. Capillary Rise in Nanopore
2.1.3. Modified Lucas–Washburn for Predicting Capillary-Driven Water Volume
2.1.4. Wettability: Contact Angle and Surface Energy
- Contact angle
- Surface energy
2.2. Method for Calculation of the Hamaker Constant
2.3. Model for Calculation the Dielectric Constant of Nanocomposites
2.4. Models for Calculation of the Refractive Index
2.5. Materials
3. Results and Discussion
3.1. Effect of TiO2 NPs Filling on Surface Energy
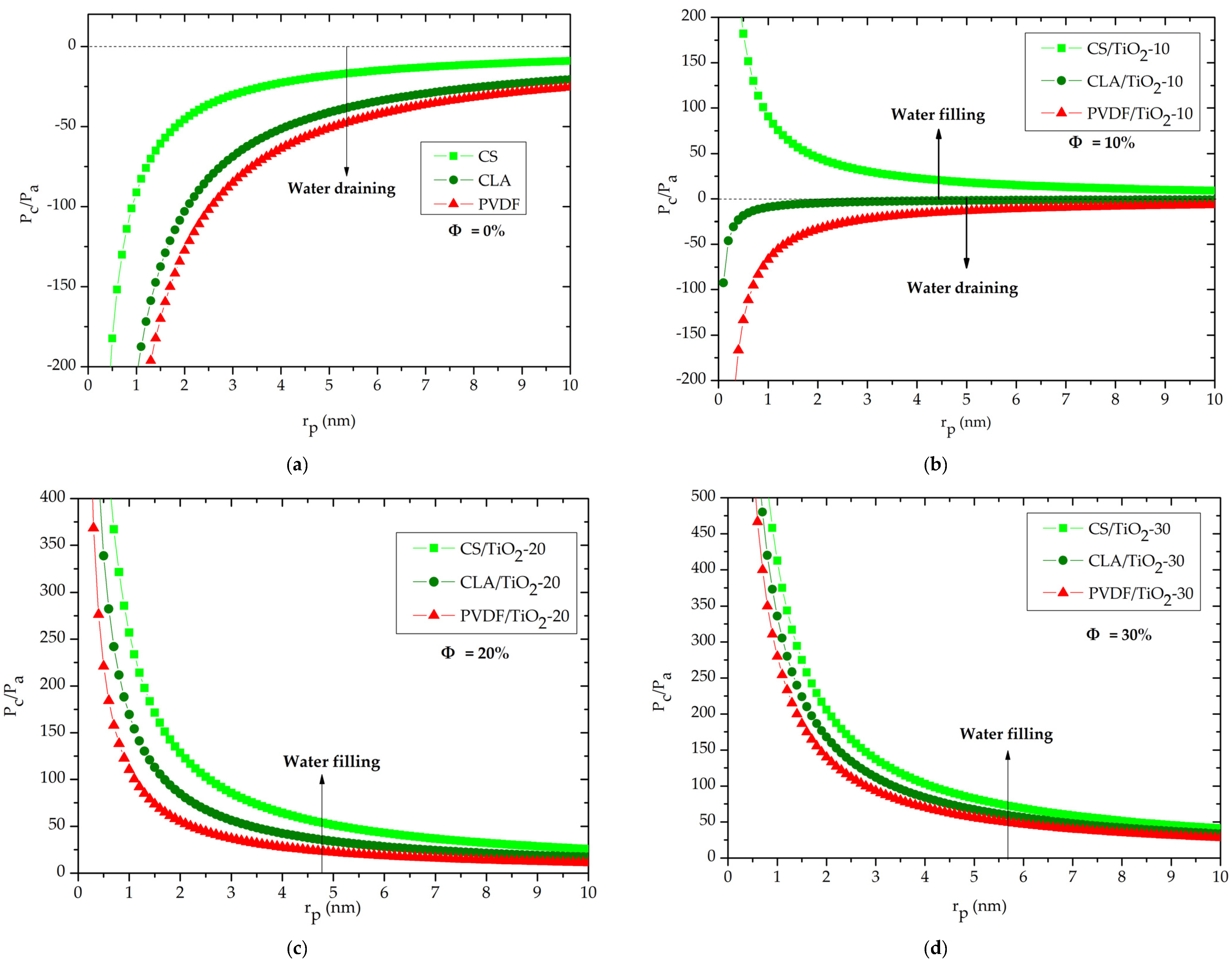
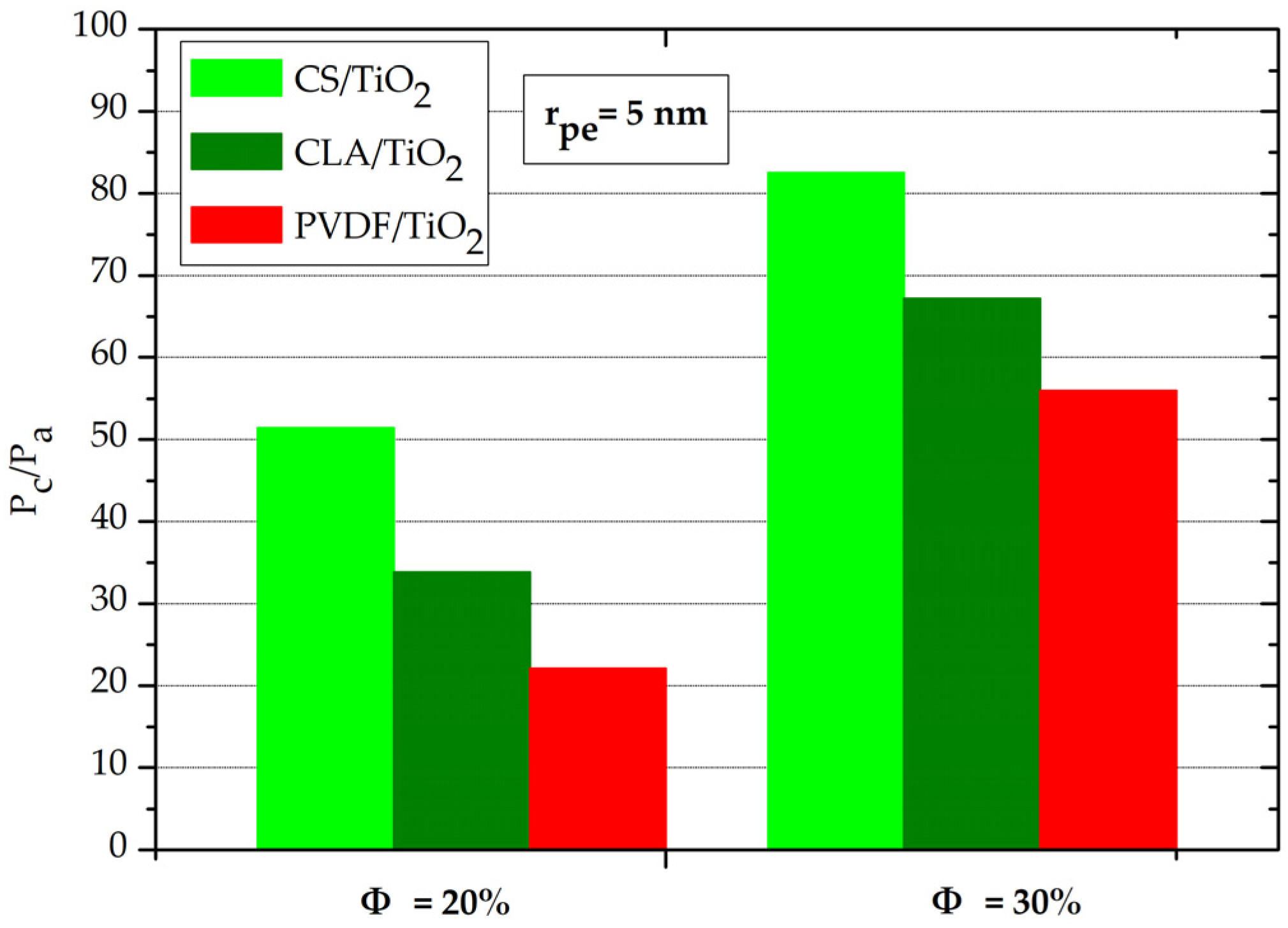
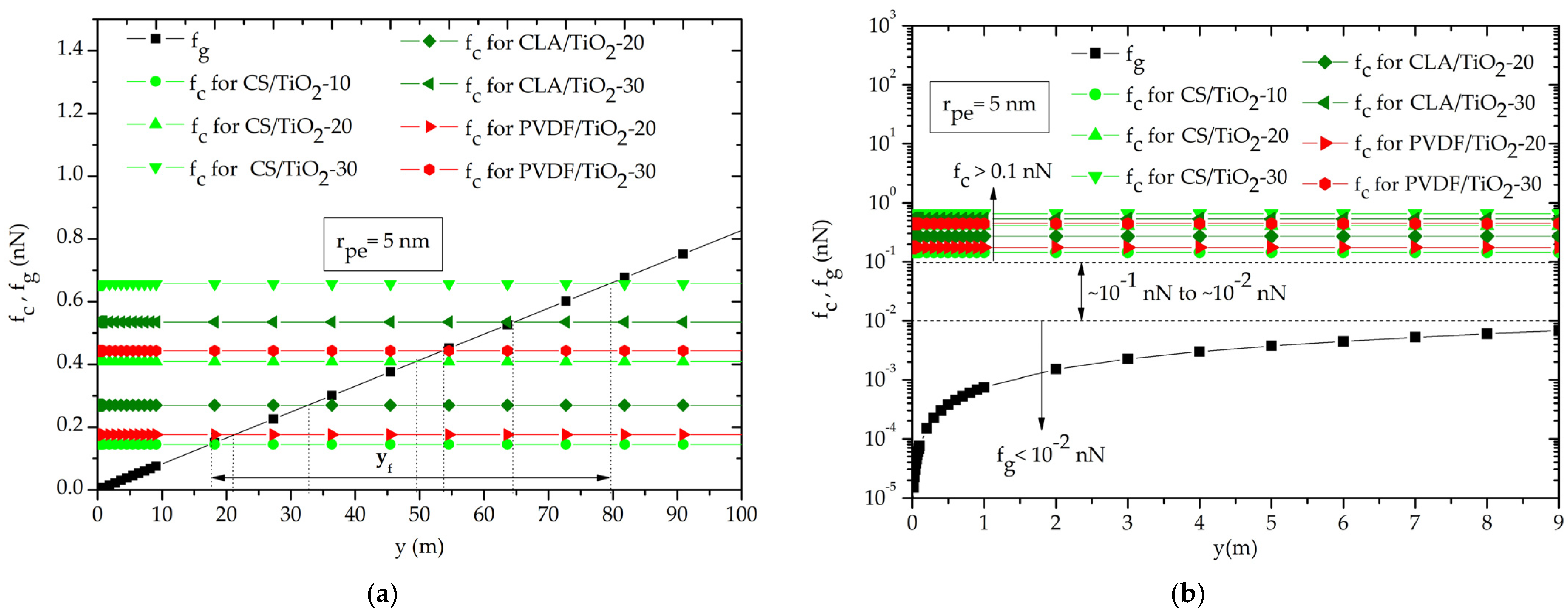
3.2. Contact Angle and Capillary Pressure
3.3. Capillary Rise
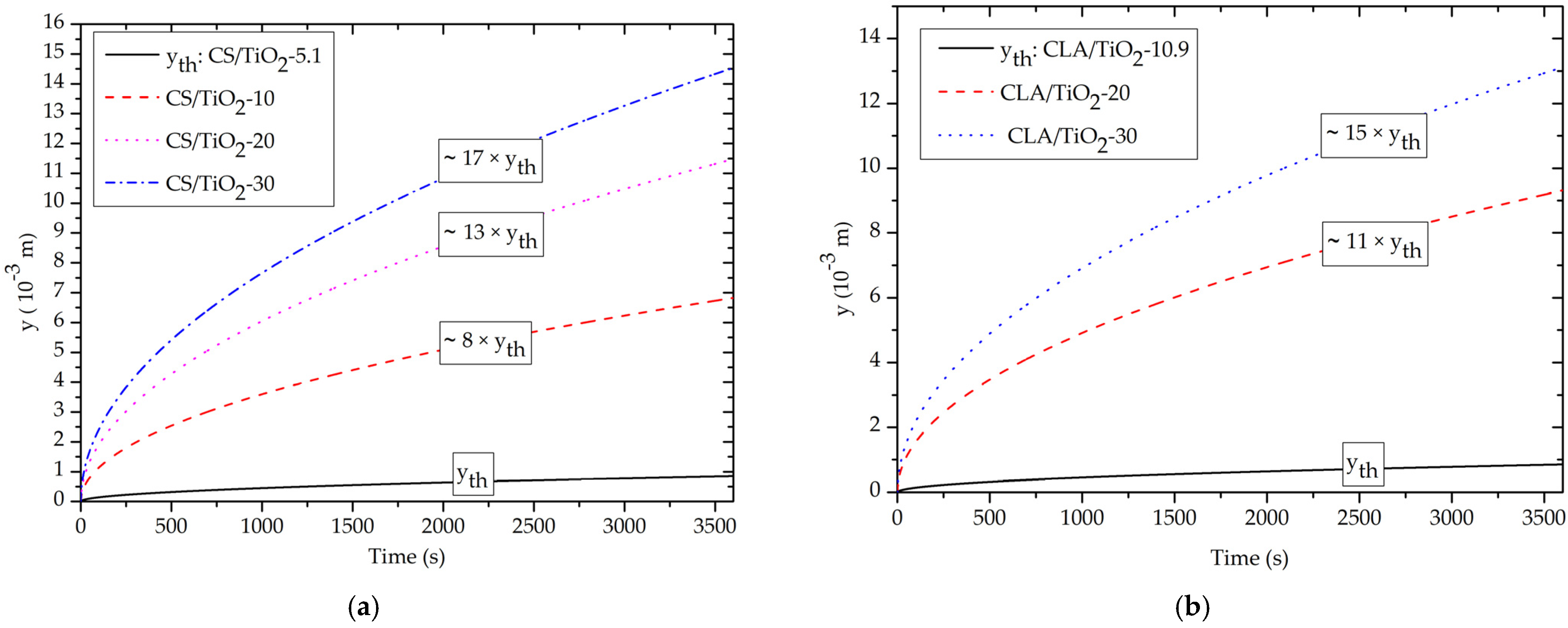
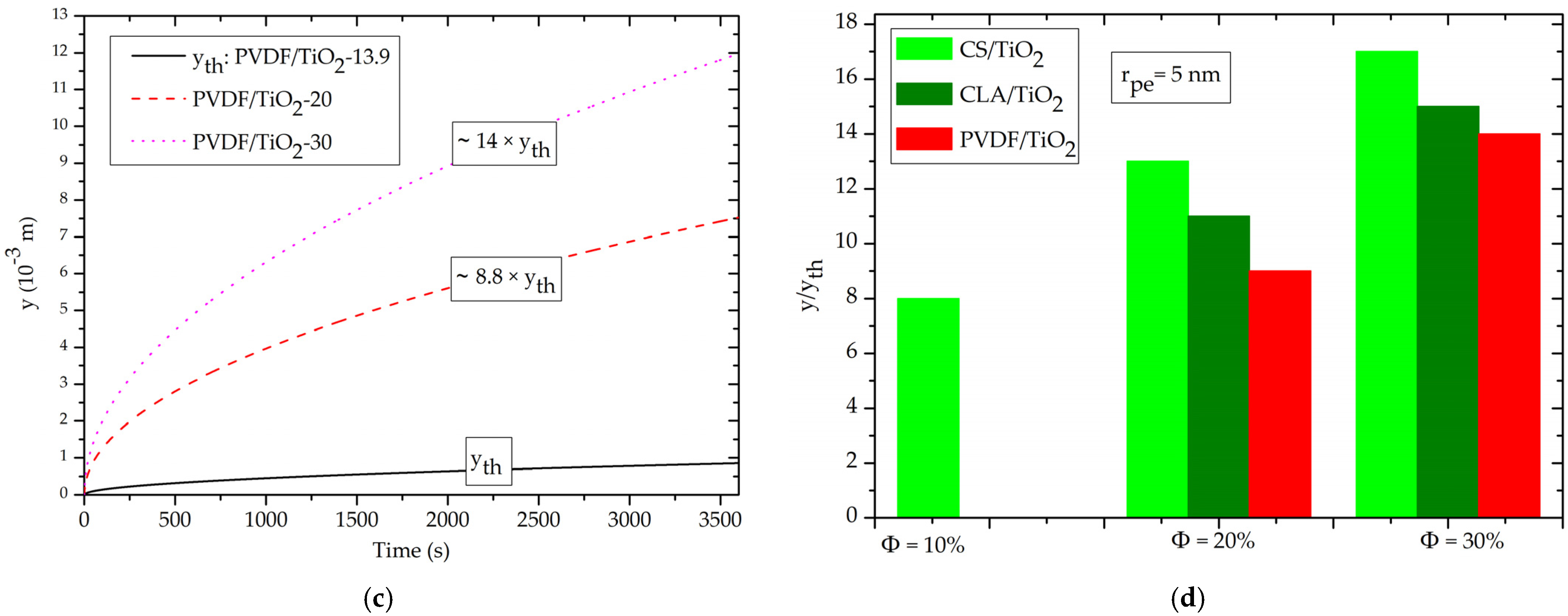
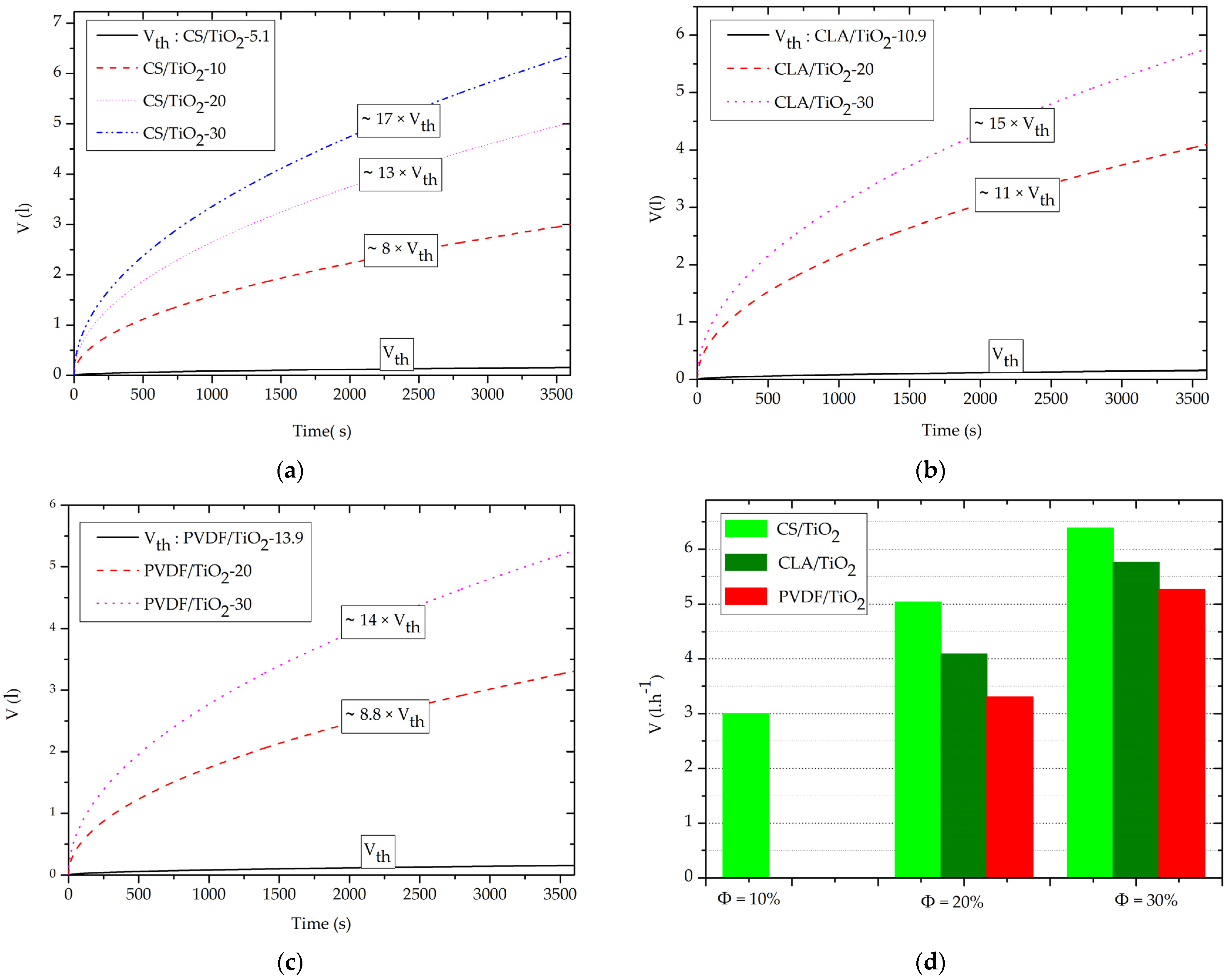
3.4. Effect of Volume Fraction of TiO2 NPs on Capillary-Driven Water Volume
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lytle, D.A.; Schock, M.R.; Formal, C.; Bennett-Stamper, C.; Harmon, S.; Nadagouda, M.N.; Williams, D.; DeSantis, M.K.; Tully, J.; Pham, M. Lead Particle Size Fractionation and Identification in Newark, New Jersey’s Drinking Water. Environ. Sci. Technol. 2020, 54, 13672–13679. [Google Scholar] [CrossRef] [PubMed]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mazumder, M.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Mraz, A.L.; Tumwebaze, I.K.; McLoughlin, S.R.; McCarthy, M.E.; Verbyla, M.E.; Hofstra, N.; Rose, J.B.; Murphy, H.M. Why pathogens matter for meeting the united nations’ sustainable development goal 6 on safely managed water and sanitation. Water Res. 2021, 189, 116591. [Google Scholar] [CrossRef]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of drinking water: A systematic review. PLoS ONE 2020, 15, e0236838. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lü, Z.; Chen, Z.; Yu, S.; Gao, C. Comparison of reverse osmosis and nanofiltration membranes in the treatment of biologically treated textile effluent for water reuse. Desalination 2011, 281, 372–378. [Google Scholar] [CrossRef]
- Naidu, L.D.; Saravanan, S.; Chidambaram, M.; Goel, M.; DAS, A.; Babu, J.S.C. Nanofiltration in Transforming Surface Water into Healthy Water: Comparison with Reverse Osmosis. J. Chem. 2015, 2015, 326869. [Google Scholar] [CrossRef]
- Bouchareb, R.; Derbal, K.; Özay, Y.; Bilici, Z.; Dizge, N. Combined natural/chemical coagulation and membrane filtration for wood processing wastewater treatment. J. Water Process Eng. 2020, 37, 101521. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Peña-Bahamonde, J.; Bandara, P.C.; Rodrigues, D.F. Nano-based adsorbent and photocatalyst use for pharmaceutical contaminant removal during indirect potable water reuse. NPJ Clean Water 2020, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Liosis, C.; Karvelas, E.G.; Karakasidis, T.; Sarris, I.E. Numerical study of magnetic particles mixing in waste water under an external magnetic field. J. Water Supply: Res. Technol. 2020, 69, 266–275. [Google Scholar] [CrossRef]
- Karvelas, E.G.; Lampropoulos, N.K.; Karakasidis, T.E.; Sarris, I.E. A computational tool for the estimation of the optimum gradient magnetic field for the magnetic driving of the spherical particles in the process of cleaning water. Desalination Water Treat. 2017, 99, 27–33. [Google Scholar] [CrossRef]
- Karvelas, E.; Liosis, C.; Benos, L.; Karakasidis, T.; Sarris, I. Micromixing Efficiency of Particles in Heavy Metal Removal Processes under Various Inlet Conditions. Water 2019, 11, 1135. [Google Scholar] [CrossRef] [Green Version]
- Hafiz, M.; Hawari, A.H.; Alfahel, R.; Hassan, M.K.; Altaee, A. Comparison of Nanofiltration with Reverse Osmosis in Reclaiming Tertiary Treated Municipal Wastewater for Irrigation Purposes. Membranes 2021, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, E. Chapter 15—Nanofiltration. In Basic Equations of Mass Transport Through a Membrane Layer, 2nd ed.; Elsevier: Boston, MA, USA, 2019; pp. 417–428. [Google Scholar] [CrossRef]
- Khan, A.A.; Boddu, S. Chapter 13—Hybrid membrane process: An emerging and promising technique toward industrial wastewater treatment. In Membrane-Based Hybrid Processes for Wastewater Treatment; Shah, M.P., Rodriguez-Couto, S., Eds.; Elsevier: Boston, MA, USA, 2021; pp. 257–277. [Google Scholar] [CrossRef]
- Futselaar, H.; Schonewille, H.; van der Meer, W. Direct capillary nanofiltration—A new high-grade purification concept. Desalination 2002, 145, 75–80. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, J.; Werber, J.R.; Elimelech, M. Capillary-driven desalination in a synthetic mangrove. Sci. Adv. 2020, 6, eaax5253. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Jin, T.; Kou, J.; Zou, S.; Xiao, J.; Meng, Q. Lucas–Washburn Equation-Based Modeling of Capillary-Driven Flow in Porous Systems. Langmuir 2021, 37, 1623–1636. [Google Scholar] [CrossRef]
- Heiranian, M.; Aluru, N.R. Modified Lucas-Washburn theory for fluid filling in nanotubes. Phys. Rev. E 2022, 105, 055105. [Google Scholar] [CrossRef]
- Barrat, J.-L.; Bocquet, L. Influence of wetting properties on hydrodynamic boundary conditions at a fluid/solid interface. Faraday Discuss. 1999, 112, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Sharma, V.; Borkute, G.; Gumfekar, S.P. Biomimetic nanofiltration membranes: Critical review of materials, structures, and applications to water purification. Chem. Eng. J. 2021, 433, 133823. [Google Scholar] [CrossRef]
- Johnson, D.J.; Hilal, N. Nanocomposite nanofiltration membranes: State of play and recent advances. Desalination 2022, 524, 115480. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, X.; Yang, W.; Yao, Z.; Mei, Y.; Peng, L.E.; Yang, Z.; Shao, S.; Tang, C.Y. Nanofiltration for drinking water treatment: A review. Front. Chem. Sci. Eng. 2021, 16, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Park, M.J.; Seo, D.H.; Drioli, E.; Matsuyama, H.; Shon, H. Recent advances in nanomaterial-incorporated nanocomposite membranes for organic solvent nanofiltration. Sep. Purif. Technol. 2021, 268, 118657. [Google Scholar] [CrossRef]
- Khraisheh, M.; Elhenawy, S.; AlMomani, F.; Al-Ghouti, M.; Hassan, M.K.; Hameed, B.H. Recent Progress on Nanomaterial-Based Membranes for Water Treatment. Membranes 2021, 11, 995. [Google Scholar] [CrossRef]
- Lakhotia, S.R.; Mukhopadhyay, M.; Kumari, P. Cerium oxide nanoparticles embedded thin-film nanocomposite nanofiltration membrane for water treatment. Sci. Rep. 2018, 8, 4976. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.U.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef]
- Thomas, M.S.; Koshy, R.R.; Mary, S.K.; Thomas, S.; Pothan, L.A. Starch, Chitin and Chitosan Based Composites and Nanocomposites; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–65. [Google Scholar] [CrossRef]
- Pandey, J.K.; Takagi, H.; Nakagaito, A.N.; Kim, H.-y. Processing, Performance and Application: Volume C: Polymer Nanocomposites of Cellulose Nanoparticles. In Handbook of Polymer Nanocomposites; Springer: Heidelberg, Germany, 2015; pp. 1–518. [Google Scholar] [CrossRef]
- Drobny, J.G. Electron Beam Processing of Commercial Polymers, Monomers, and Oligomers. In Plastics Design Library, Ionizing Radiation and Polymers; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 101–147. [Google Scholar] [CrossRef]
- Jye, L.W.; Ismail, A.F. Nanofiltration Membranes Synthesis, Characterization, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2017; pp. 1–184. ISBN 9781498751377. [Google Scholar]
- Jährig, J.; Vredenbregt, L.; Wicke, D.; Miehe, U.; Sperlich, A. Capillary Nanofiltration under Anoxic Conditions as Post-Treatment after Bank Filtration. Water 2018, 10, 1599. [Google Scholar] [CrossRef] [Green Version]
- de Gennes, P.-G.; Brochard-Wyart, F.; Quere, D. Capillarity and Gravity. In Capillarity and Wetting Phenomena Drops, Bubbles, Pearls, Waves, 1st ed.; Springer Science & Business Media: New York, NY, USA, 2003; p. 33. [Google Scholar] [CrossRef]
- Collins, R.E.; Cooke, C.E. Fundamental basis for the contact angle and capillary pressure. Trans. Faraday Soc. 1959, 55, 1602–1606. [Google Scholar] [CrossRef]
- Jurin, J., II. An account of some experiments shown before the Royal Society; with an enquiry into the cause of the ascent and suspension of water in capillary tubes. Philos. Trans. R. Soc. Lond. 1719, 30, 739–747. [Google Scholar] [CrossRef]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Benavente, D.; Lock, P.; García del Cura, M.Á.; Ordóñez, S. Predicting the Capillary Imbibition of Porous Rocks from Microstructure. Transp. Porous Media 2002, 49, 59–76. [Google Scholar] [CrossRef]
- Matyka, M.; Khalili, A.; Koza, Z. Tortuosity-porosity relation in porous media flow. Phys. Rev. E 2008, 78, 026306. [Google Scholar] [CrossRef] [Green Version]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Burlington, NJ, USA, 2011; p. 196. ISBN 978-0-12-375182-9. [Google Scholar]
- Keesom, W.H. The cohesion forces in the theory of Van Der Waals. Phys. Z. 1921, 22, 129–141. [Google Scholar]
- Debye, P. Molecular forces and their electric explanation. Phys. Z. 1921, 22, 302–308. [Google Scholar]
- London, F. The general theory of molecular forces. Trans. Faraday Soc. 1937, 33, 8–26. [Google Scholar] [CrossRef]
- Karkkainen, K.K.; Sihvola, A.H.; Nikoskinen, K.I. Effective permittivity of mixtures: Numerical validation by the FDTD method. IEEE Trans. Geosci. Remote Sens. 2000, 38, 1303–1308. [Google Scholar] [CrossRef] [Green Version]
- Garnett, J.C.M. VII—Colours in Metal Glasses, in Metallic Films, and in Metallic Solutions–II; Series A Containing Papers of a Mathematical or Physical Character. Philos. Trans. Royal Soc. Lond. 1906, 205, 237–288. [Google Scholar]
- Leite, F.L.; Bueno, C.C.; Da Róz, A.L.; Ziemath, E.C.; Oliveira, O.N., Jr. Theoretical Models for Surface Forces and Adhesion and Their Measurement Using Atomic Force Microscopy. Int. J. Mol. Sci. 2012, 13, 12773–12856. [Google Scholar] [CrossRef] [PubMed]
- Mahdhi, N.; Alsaiari, N.S.; Alzahrani, F.M.; Katubi, K.M.; Amari, A.; Hammami, S. Theoretical Investigation of the Adsorption of Cadmium Iodide from Water Using Polyaniline Polymer Filled with TiO2 and ZnO Nanoparticles. Water 2021, 13, 2591. [Google Scholar] [CrossRef]
- Drummond, C.J.; Chan, D.Y.C. van der Waals Interaction, Surface Free Energies, and Contact Angles: Dispersive Polymers and Liquids. Langmuir 1997, 13, 3890–3895. [Google Scholar] [CrossRef]
- Aishwarya, S.; Shanthi, J.; Swathi, R. Surface energy calculation using Hamaker’s constant for polymer/silane hydrophobic thin films. Mater. Lett. 2019, 253, 409–411. [Google Scholar] [CrossRef]
- Saud, A.; Saleem, H.; Zaidi, S.J. Progress and Prospects of Nanocellulose-Based Membranes for Desalination and Water Treatment. Membranes 2022, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Matei, E.; Predescu, A.M.; Râpă, M.; Țurcanu, A.A.; Mateș, I.; Constantin, N.; Predescu, C. Natural Polymers and Their Nanocomposites Used for Environmental Applications. Nanomaterials 2022, 12, 1707. [Google Scholar] [CrossRef] [PubMed]
- Divya, S.; Oh, T.H. Polymer Nanocomposite Membrane for Wastewater Treatment: A Critical Review. Polymers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef] [Green Version]
- Ekambaram, K.; Doraisamy, M. Surface modification of PVDF nanofiltration membrane using Carboxymethylchitosan-Zinc oxide bionanocomposite for the removal of inorganic salts and humic acid. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 525, 49–63. [Google Scholar] [CrossRef]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with different dosage of nano-TiO2. J. Membr. Sci. 2012, 389, 522–531. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, H.; Chen, Y.; Ni, Y.; Huang, L.; Mondal, A.K.; Lin, S.; Huang, F.; Zhang, H. A cellulose-based nanofiltration membrane with a stable three-layer structure for the treatment of drinking water. Cellulose 2020, 27, 8237–8253. [Google Scholar] [CrossRef]
- Bonardd, S.; Robles, E.; Barandiaran, I.; Saldías, C.; Leiva, A.; Kortaberria, G. Biocomposites with increased dielectric constant based on chitosan and nitrile-modified cellulose nanocrystals. Carbohydr. Polym. 2018, 199, 20–30. [Google Scholar] [CrossRef]
- Azofeifa, D.E.; Arguedas, H.J.; Vargas, W.E. Optical properties of chitin and chitosan biopolymers with application to structural color analysis. Opt. Mater. 2012, 35, 175–183. [Google Scholar] [CrossRef]
- Diantoro, M.; A Mustikasari, A.; Wijayanti, N.; Yogihati, C.; Taufiq, A. Microstructure and dielectric properties of cellulose acetate-ZnO/ITO composite films based on water hyacinth. J. Physics: Conf. Ser. 2017, 853, 012047. [Google Scholar] [CrossRef]
- Sultanova, N.G.; Kasarova, S.N.; Nikolov, I.D. Characterization of optical properties of optical polymers. Opt. Quantum Electron. 2013, 45, 221–232. [Google Scholar] [CrossRef]
- Arshad, A.N.; Wahid, M.H.M.; Rusop, M.; Majid, W.H.A.; Subban, R.H.Y.; Rozana, M.D. Dielectric and Structural Properties of Poly(vinylidene fluoride) (PVDF) and Poly(vinylidene fluoride-trifluoroethylene) (PVDF-TrFE) Filled with Magnesium Oxide Nanofillers. J. Nanomater. 2019, 2019, 5961563. [Google Scholar] [CrossRef]
- Gibbons, J.; Patterson, S.B.; Zhakeyev, A.; Vilela, F.; Marques-Hueso, J. Spectroscopic ellipsometric study datasets of the fluorinated polymers: Bifunctional urethane methacrylate perfluoropolyether (PFPE) and polyvinylidene fluoride (PVDF). Data Brief 2021, 39, 107461. [Google Scholar] [CrossRef]
- Manke, F.; Frost, J.M.; Vaissier, V.; Nelson, J.; Barnes, P.R.F. Influence of a nearby substrate on the reorganization energy of hole exchange between dye molecules. Phys. Chem. Chem. Phys. 2015, 17, 7345–7354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, D.P.; Mulev, Y.; Goodwin, A.R.H.; Sengers, J.M.H.L. A Database for the Static Dielectric Constant of Water and Steam. J. Phys. Chem. Ref. Data 1995, 24, 33–70. [Google Scholar] [CrossRef]
- Cunha, A.G.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. What Is the Real Value of Chitosan’s Surface Energy? Biomacromolecules 2008, 9, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Lepoittevin, B.; Elzein, T.; Dragoe, D.; Bejjani, A.; Lemée, F.; Levillain, J.; Bazin, P.; Roger, P.; Dez, I. Hydrophobization of chitosan films by surface grafting with fluorinated polymer brushes. Carbohydr. Polym. 2019, 205, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Tungprapa, S.; Puangparn, T.; Weerasombut, M.; Jangchud, I.; Fakum, P.; Semongkhol, S.; Meechaisue, C.; Supaphol, P. Electrospun cellulose acetate fibers: Effect of solvent system on morphology and fiber diameter. Cellulose 2005, 14, 563–575. [Google Scholar] [CrossRef]
- Hamilton, W. A technique for the characterization of hydrophilic solid surfaces. J. Colloid Interface Sci. 1972, 40, 219–222. [Google Scholar] [CrossRef]
- Wu, Q.; Tiraferri, A.; Li, T.; Xie, W.; Chang, H.; Bai, Y.; Liu, B. Superwettable PVDF/PVDF-g-PEGMA Ultrafiltration Membranes. ACS Omega 2020, 5, 23450–23459. [Google Scholar] [CrossRef]
- Deka, B.J.; Guo, J.; Khanzada, N.K.; An, A.K. Omniphobic re-entrant PVDF membrane with ZnO nanoparticles composite for desalination of low surface tension oily seawater. Water Res. 2019, 165, 114982. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Y.; Liu, Y.; Li, S.; Sun, C. Capillary imbibition and flow of wetting liquid in irregular capillaries: A 100-year review. Adv. Colloid Interface Sci. 2022, 304, 102654. [Google Scholar] [CrossRef]
- Jamil, T.S.; Dijkstra, I.; Sayed, S. Usage of permeate water for treated domestic wastewater by direct capillary nanofiltration membrane in agriculture reuse. Desalination Water Treat. 2013, 51, 2584–2591. [Google Scholar] [CrossRef]

| Φ% of TiO2 NPs | Nanocomposite | ||
|---|---|---|---|
| CS/TiO2 | CLA/TiO2 | PVDF/TiO2 | |
| 0 | CS | CLA | PVDF |
| 10 | CS/TiO2-10 | CLA/TiO2-10 | PVDF/TiO2-10 |
| 20 | CS/TiO2-20 | CLA/TiO2-20 | PVDF/TiO2-20 |
| 30 | CS/TiO2-30 | CLA/TiO2-30 | PVDF/TiO2-30 |
| Material | ε at 1 MHz | n at 600 nm | |
|---|---|---|---|
| Bio-based polymer | CS | 5.5 [61] | 1.53 [62] |
| CLA | 5 [63] | 1.47 [64] | |
| Synthetic polymer | PVDF | 8.5 [65] | 1.42 [66] |
| Filler | TiO2 (Anatase) | 86 [67] | 2.60 [67] |
| Air | 1 [43] | 1 [43] | |
| Water | 78.4 [68] | 1.33 [68] | |
| Φ% (TiO2) | n | ε | ||||
|---|---|---|---|---|---|---|
| CS/TiO2 | CLA/TiO2 | PVDF/TiO2 | CS/TiO2 | CLA/TiO2 | PVDF/TiO2 | |
| 0 | 1.53 | 1.47 | 1.42 | 5.5 | 5 | 8.5 |
| 10 | 1.61 | 1.56 | 1.51 | 8.36 | 7.76 | 11.82 |
| 20 | 1.70 | 1.65 | 1.61 | 12.08 | 11.40 | 15.91 |
| 30 | 1.80 | 1.75 | 1.70 | 16.77 | 16.02 | 20.86 |
| Φ% (TiO2) | CS/TiO2 | CLA/TiO2 | PVDF/TiO2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HP (10−21 J) | HD (10−20 J) | H (10−20 J) | HP (10−21 J) | HD (10−20 J) | H (10−20 J) | HP (10−21 J) | HD (10−20 J) | H (10−20 J) | |
| 0 | 2.08 | 5.11 | 5.325 | 2.00 | 4.62 | 4.82 | 2.37 | 4.19 | 4.42 |
| 10 | 2.36 | 5.81 | 6.049 | 2.32 | 5.36 | 5.59 | 2.53 | 4.98 | 5.23 |
| 20 | 2.54 | 6.48 | 6.738 | 2.52 | 6.09 | 6.34 | 2.65 | 5.75 | 6.01 |
| 30 | 2.670 | 7.13 | 7.399 | 2.65 | 6.78 | 7.04 | 2.73 | 6.48 | 6.75 |
| Φ% (TiO2) | CS/TiO2 | CLA/TiO2 | PVDF/TiO2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.72 | 37.82 | 38.54 | 0.66 | 30.80 | 31.47 | 0.93 | 25.33 | 26.26 |
| 10 | 0.93 | 48.91 | 49.84 | 0.89 | 41.68 | 42.57 | 1.07 | 35.87 | 36.94 |
| 20 | 1.08 | 61.04 | 62.12 | 1.05 | 53.78 | 54.84 | 1.17 | 47.83 | 49.00 |
| 30 | 1.18 | 74.15 | 75.33 | 1.17 | 67.05 | 68.22 | 1.24 | 61.16 | 62.40 |
| Polymer | ||
|---|---|---|
| This Study | Literature | |
| CS | 38.54 | 34–43 [69,70] |
| CLA | 31.47 | 30–34 [71,72] |
| PVDF | 26.26 | 22–29 [73,74] |
| Φ% (TiO2) | γsl (10−3 J·m−2) | ||
|---|---|---|---|
| CS/TiO2 | CLA/TiO2 | PVDF/TiO2 | |
| 0 | 43.16 | 41.92 | 39.18 |
| 10 | 45.23 | 43.04 | 40.32 |
| 20 | 49.09 | 46.25 | 43.40 |
| 30 | 54.43 | 51.20 | 48.22 |
| Nanocomposite | CS/TiO2 | CLA/TiO2 | PVDF/TiO2 |
|---|---|---|---|
| Фth (%) | 5.10 | 10.90 | 13.90 |
| nth | 1.57 | 1.57 | 1.55 |
| εth | 6.83 | 7.92 | 13.24 |
| (10−20 J) | 9.08 | 8.97 | 8.55 |
| γth (10−3 J·m−2) | 43.83 | 42.62 | 41.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdhi, N.; Alsaiari, N.S.; Amari, A.; Chakhoum, M.A. Effect of TiO2 Nanoparticles on Capillary-Driven Flow in Water Nanofilters Based on Chitosan Cellulose and Polyvinylidene Fluoride Nanocomposites: A Theoretical Study. Polymers 2022, 14, 2908. https://doi.org/10.3390/polym14142908
Mahdhi N, Alsaiari NS, Amari A, Chakhoum MA. Effect of TiO2 Nanoparticles on Capillary-Driven Flow in Water Nanofilters Based on Chitosan Cellulose and Polyvinylidene Fluoride Nanocomposites: A Theoretical Study. Polymers. 2022; 14(14):2908. https://doi.org/10.3390/polym14142908
Chicago/Turabian StyleMahdhi, Noureddine, Norah Salem Alsaiari, Abdelfattah Amari, and Mohamed Ali Chakhoum. 2022. "Effect of TiO2 Nanoparticles on Capillary-Driven Flow in Water Nanofilters Based on Chitosan Cellulose and Polyvinylidene Fluoride Nanocomposites: A Theoretical Study" Polymers 14, no. 14: 2908. https://doi.org/10.3390/polym14142908
APA StyleMahdhi, N., Alsaiari, N. S., Amari, A., & Chakhoum, M. A. (2022). Effect of TiO2 Nanoparticles on Capillary-Driven Flow in Water Nanofilters Based on Chitosan Cellulose and Polyvinylidene Fluoride Nanocomposites: A Theoretical Study. Polymers, 14(14), 2908. https://doi.org/10.3390/polym14142908







