Multifunctional PEG Carrier by Chemoenzymatic Synthesis for Drug Delivery Systems: In Memory of Professor Andrzej Dworak
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of Compound I: Acrylate-dPEG20-Acrylate
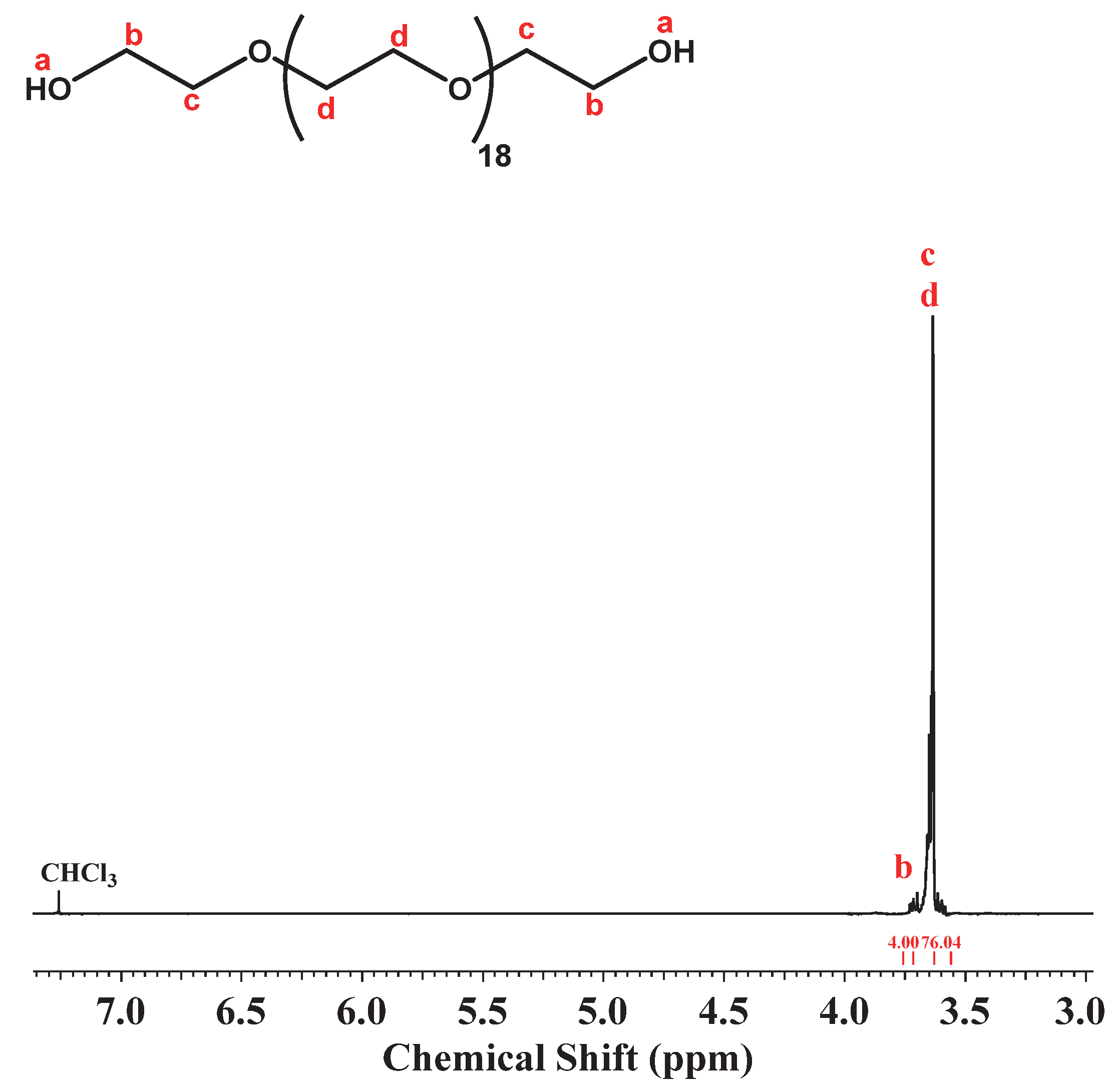
2.1.2. Synthesis of Compound II: (HO)2-dPEG20-(OH)2
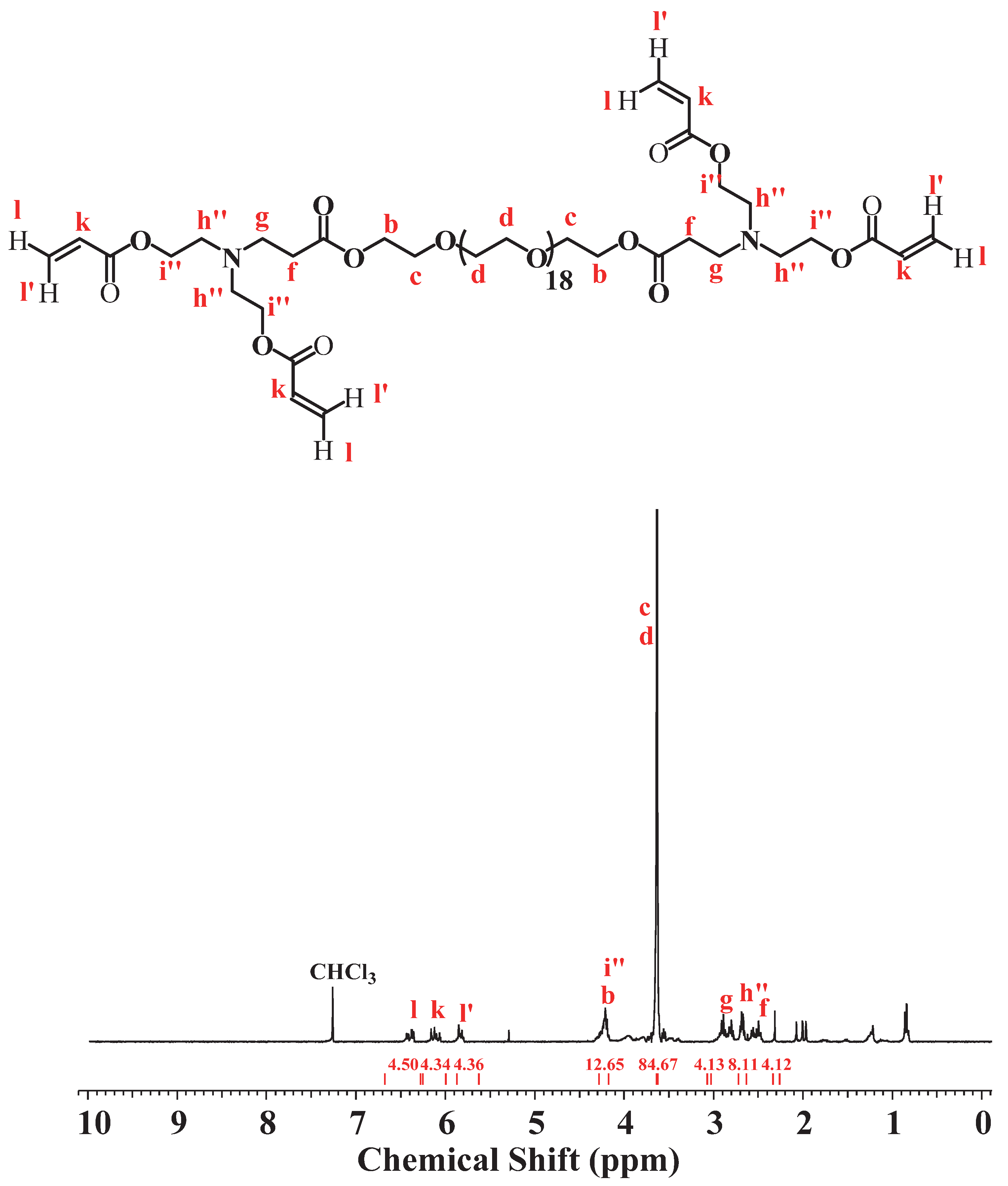
2.1.3. Synthesis of Compound III: (Acr)2-dPEG-(Acr)2
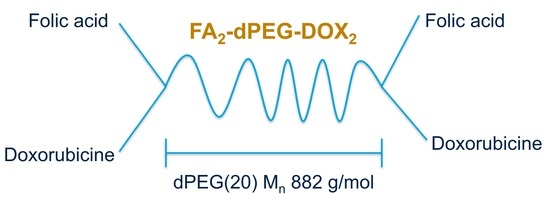
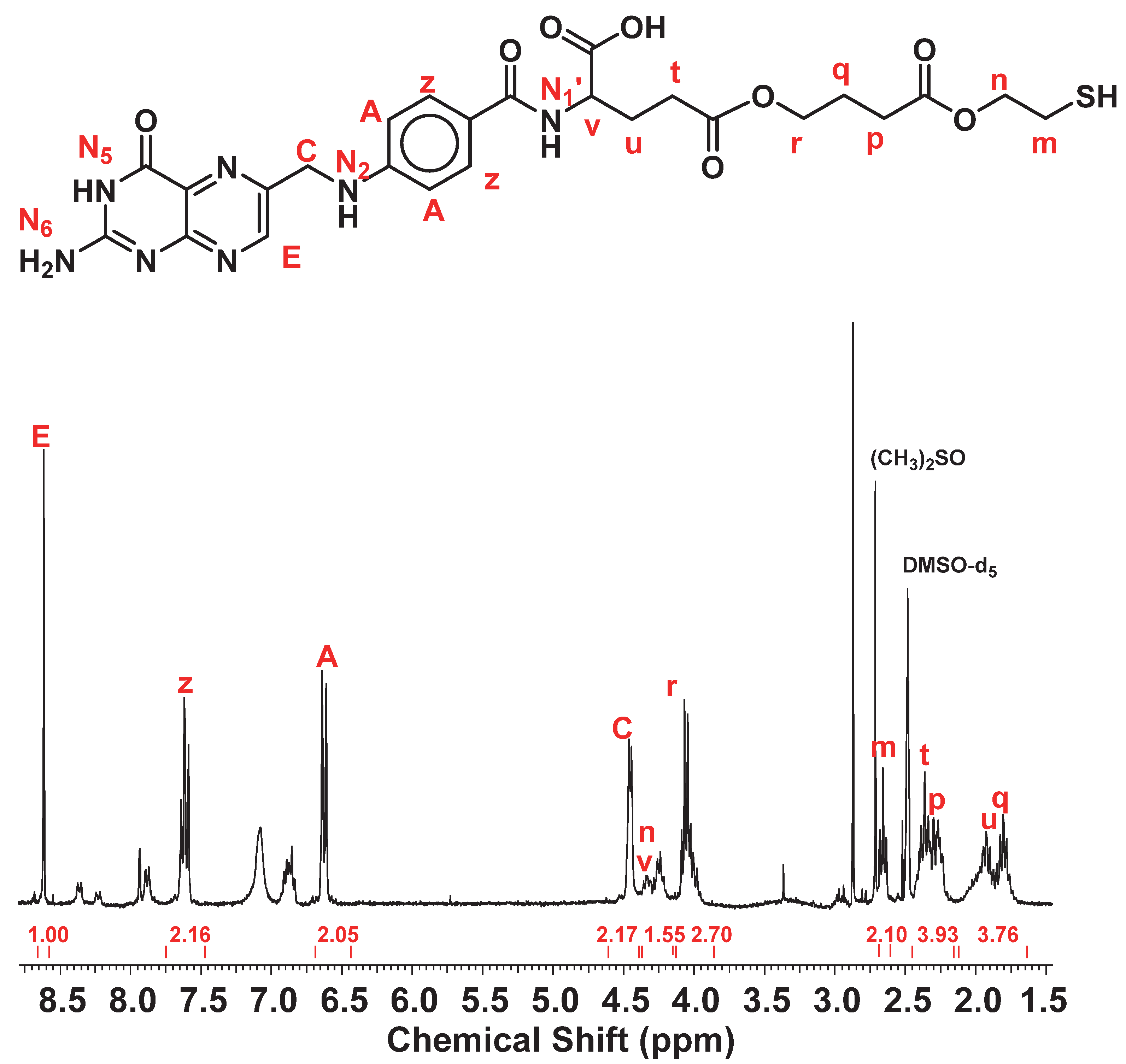
2.1.4. Synthesis of Compound IV: FA2-dPEG-DOX2
2.2. Characterization
Nuclear Magnetic Resonance (NMR) Spectroscopy
3. Results and Discussion
3.1. Synthesis of Acrylate-dPEG20-Acrylate (I)
3.2. Synthesis of (HO)2-dPEG20-(OH)2
3.3. Synthesis of (Acrylate)2-dPEG20-(Acrylate)2
3.4. Synthesis of FA2-dPEG20-DOX2
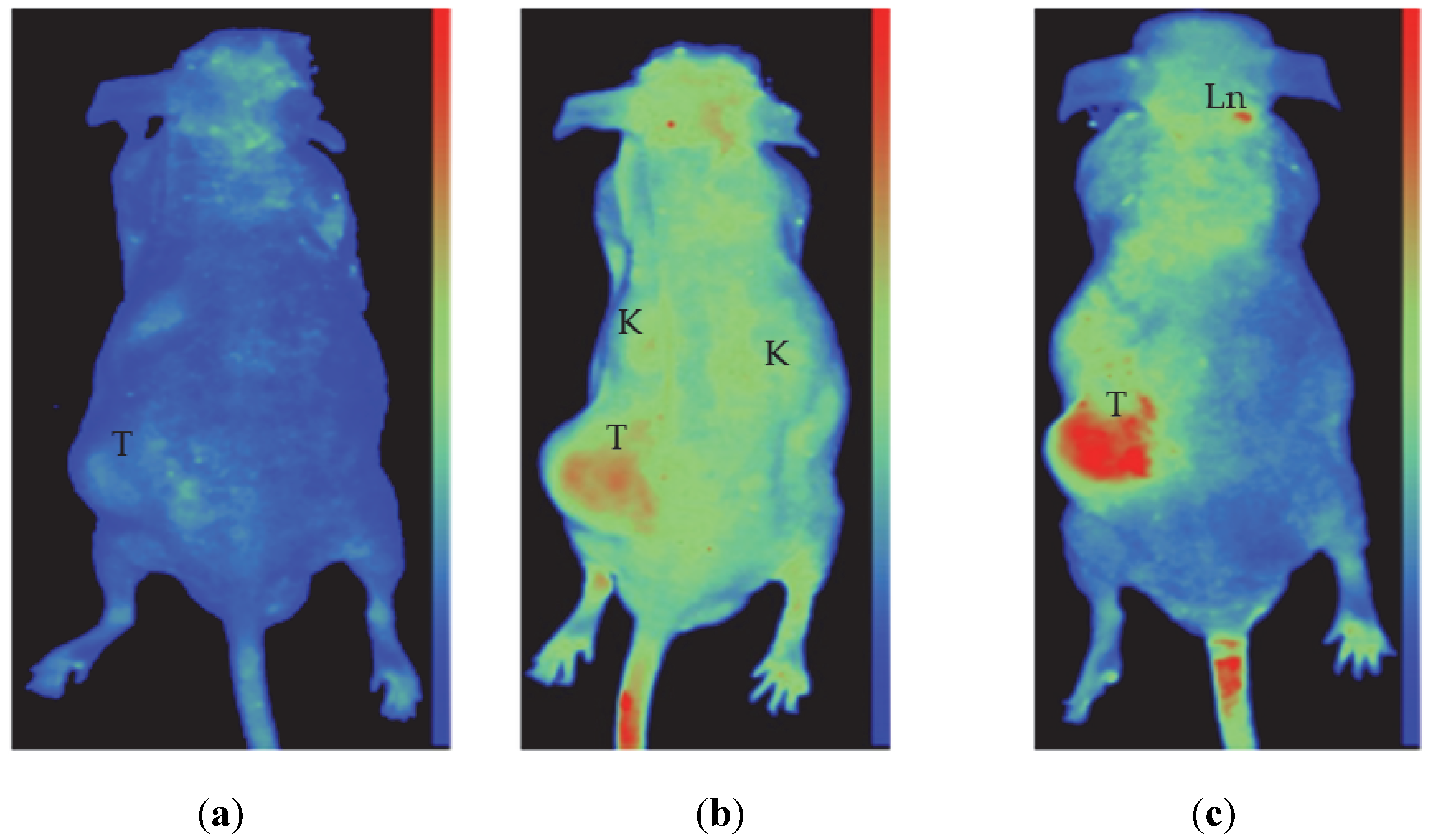
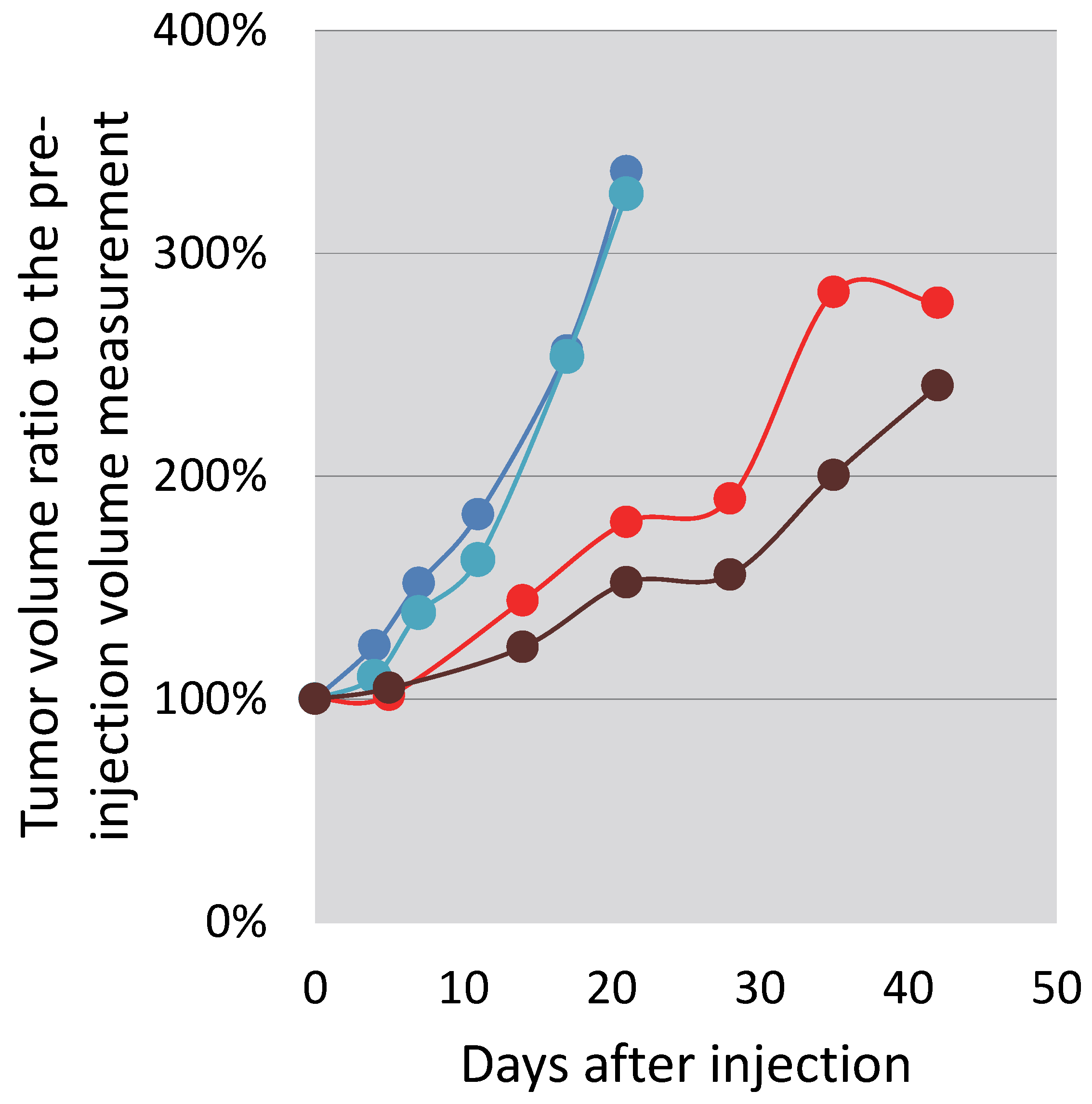
3.5. In Vitro and In Vivo Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal. Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Lipowska-Kur, D.; Szweda, R.; Trzebicka, B.; Dworak, A. Preparation and characterization of doxorubicin nanocarriers based on thermoresponsive oligo(ethylene glycol) methyl ether methacrylate polymer-drug conjugates. Eur. Polym. J. 2018, 109, 391–401. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Abdela Siraj, E. Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Low, P.S. Ligand-Targeted Drug Delivery. Chem. Rev. 2017, 117, 12133–12164. [Google Scholar] [CrossRef]
- Jurczyk, M.; Jelonek, K.; Musiał-Kulik, M.; Beberok, A.; Wrześniok, D.; Kasperczyk, J. Single- versus Dual-Targeted Nanoparticles with Folic Acid and Biotin for Anticancer Drug Delivery. Pharmaceutics 2021, 13, 326. [Google Scholar] [CrossRef]
- Frigerio, B.; Bizzoni, C.; Jansen, G.; Leamon, C.P.; Peters, G.J.; Low, P.S.; Matherly, L.H.; Figini, M. Folate receptors and transporters: Biological role and diagnostic/therapeutic targets in cancer and other diseases. J. Exp. Clin. Cancer Res. 2019, 38, 125. [Google Scholar] [CrossRef] [Green Version]
- Puskas, J.E.; Molnar, K.; Krisch, E. Toward the effective synthesis of bivalent Folate-targeted PEGylated cancer diagnostic and therapeutic agents using chemo-enzymatic processes. J. Mol. Liq. 2020, 310, 113218–113227. [Google Scholar] [CrossRef]
- Ullah, S.; Azad, A.K.; Nawaz, A.; Shah, K.U.; Iqbal, M.; Albadrani, G.M.; Al-Joufi, F.A.; Sayed, A.A.; Abdel-Daim, M.M. 5-Fluorouracil-Loaded Folic-Acid-Fabricated Chitosan Nanoparticles for Site-Targeted Drug Delivery Cargo. Polymers 2022, 14, 2010. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, M. Broad-Spectrum Theranostics and Biomedical Application of Functionalized Nanomaterials. Polymers 2022, 14, 1221. [Google Scholar] [CrossRef] [PubMed]
- Casi, G.; Neri, D. Antibody–Drug Conjugates and Small Molecule–Drug Conjugates: Opportunities and Challenges for the Development of Selective Anticancer Cytotoxic Agents. J. Med. Chem. 2015, 58, 8751–8761. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.R. Dendrimer-based nanoparticles for cancer therapy. Hematology 2009, 2009, 708–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Gupta, U.; Asthana, A.; Jain, N.K. Folate and Folate−PEG−PAMAM Dendrimers: Synthesis, Characterization, and Targeted Anticancer Drug Delivery Potential in Tumor Bearing Mice. Bioconjug. Chem. 2008, 19, 2239–2252. [Google Scholar] [CrossRef]
- Majoros, I.J.; Myc, A.; Thomas, T.; Mehta, C.B.; Baker, J.R. PAMAM Dendrimer-Based Multifunctional Conjugate for Cancer Therapy: Synthesis, Characterization, and Functionality. Biomacromolecules 2006, 7, 572–579. [Google Scholar] [CrossRef]
- van Dongen, M.A.; Dougherty, C.A.; Banaszak Holl, M.M. Multivalent Polymers for Drug Delivery and Imaging: The Challenges of Conjugation. Biomacromolecules 2014, 15, 3215–3234. [Google Scholar] [CrossRef] [Green Version]
- Marcinkowska, M.; Sobierajska, E.; Stanczyk, M.; Janaszewska, A.; Chworos, A.; Klajnert-Maculewicz, B. Conjugate of PAMAM Dendrimer, Doxorubicin and Monoclonal Antibody—Trastuzumab: The New Approach of a Well-Known Strategy. Polymers 2018, 10, 187. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, K.; Yokoyama, M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: A review. Sci. Technol. Adv. Mater. 2019, 20, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Rahme, K.; Dagher, N. Chemistry Routes for Copolymer Synthesis Containing PEG for Targeting, Imaging, and Drug Delivery Purposes. Pharmaceutics 2019, 11, 327. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Koirala, N.; Li, X.; Khan, N.; Dong, F.; Zhang, W.; Mulay, P.; Shrikhande, G.; Puskas, J.; Drazba, J.; et al. Screening of Polymer-Based Drug Delivery Vehicles Targeting Folate Receptors in Triple-Negative Breast Cancer. J. Vasc. Interv. Radiol. 2020, 31, 1866–1873.e2. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Das, D.; Fayazzadeh, E.; Sen, S.; McClain, A.; Puskas, J.E.; Drazba, J.A.; McLennan, G. Folic acid conjugated polymeric drug delivery vehicle for targeted cancer detection in hepatocellular carcinoma. J. Biomed. Mater. Res. Part A 2019, 107, 2522–2535. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.S.; Toth, K.; Pallinger, E.; Takacs, A.; Kohidai, L.; Jedlovszky-Hajdu, A.; Mathe, D.; Kovacs, N.; Veres, D.S.; Szigeti, K.; et al. Folate-Targeted Monodisperse PEG-Based Conjugates Made by Chemo-Enzymatic Methods for Cancer Diagnosis and Treatment. Int. J. Mol. Sci. 2021, 22, 10347. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.C.; Woodle, M.; Mixson, A.J. Advances in Delivery Systems for Doxorubicin. J. Nanomed. Nanotechnol. 2018, 9, 519. [Google Scholar] [CrossRef]
- Psarrou, M.; Kothri, M.G.; Vamvakaki, M. Photo- and Acid-Degradable Polyacylhydrazone–Doxorubicin Conjugates. Polymers 2021, 13, 2461. [Google Scholar] [CrossRef]
- Xue, X.; Wu, Y.; Xu, X.; Xu, B.; Chen, Z.; Li, T. pH and Reduction Dual-Responsive Bi-Drugs Conjugated Dextran Assemblies for Combination Chemotherapy and In Vitro Evaluation. Polymers 2021, 13, 1515. [Google Scholar] [CrossRef]
- Puskas, J.E.; Castano, M.; Mulay, P.; Dudipala, V.; Wesdemiotis, C. Method for the Synthesis of γ-PEGylated Folic Acid and Its Fluorescein-Labeled Derivative. Macromolecules 2018, 51, 9069–9077. [Google Scholar] [CrossRef]
- Piorecka, K.; Stanczyk, W.; Florczak, M. NMR analysis of antitumor drugs: Doxorubicin, daunorubicin and their functionalized derivatives. Tetrahedron Lett. 2017, 58, 152–155. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Haladjova, E.; Toncheva-Moncheva, N.; Apostolova, M.D.; Trzebicka, B.; Dworak, A.; Petrov, P.; Dimitrov, I.; Rangelov, S.; Tsvetanov, C.B. Polymeric Nanoparticle Engineering: From Temperature-Responsive Polymer Mesoglobules to Gene Delivery Systems. Biomacromolecules 2014, 15, 4377–4395. [Google Scholar] [CrossRef] [PubMed]




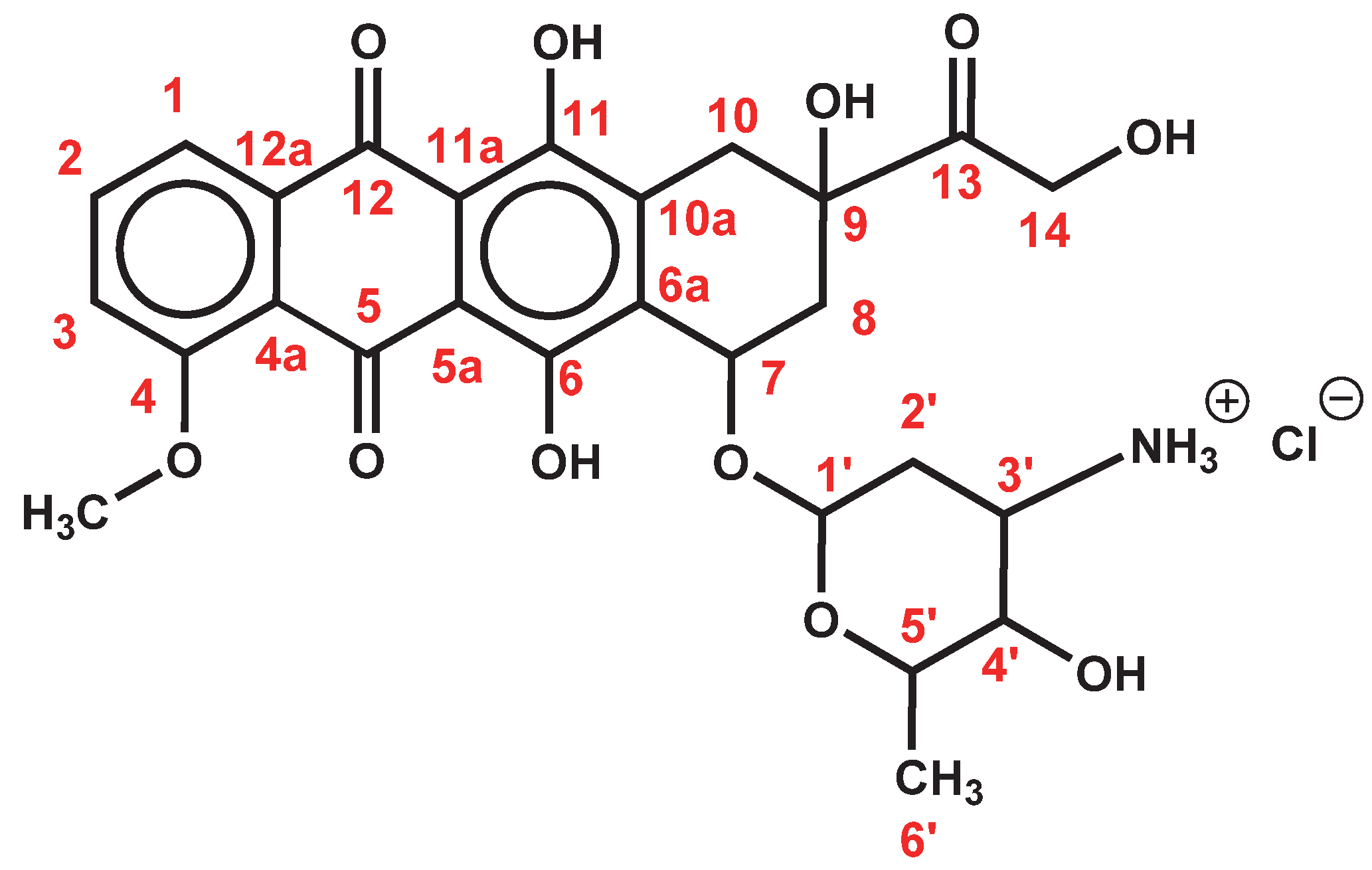
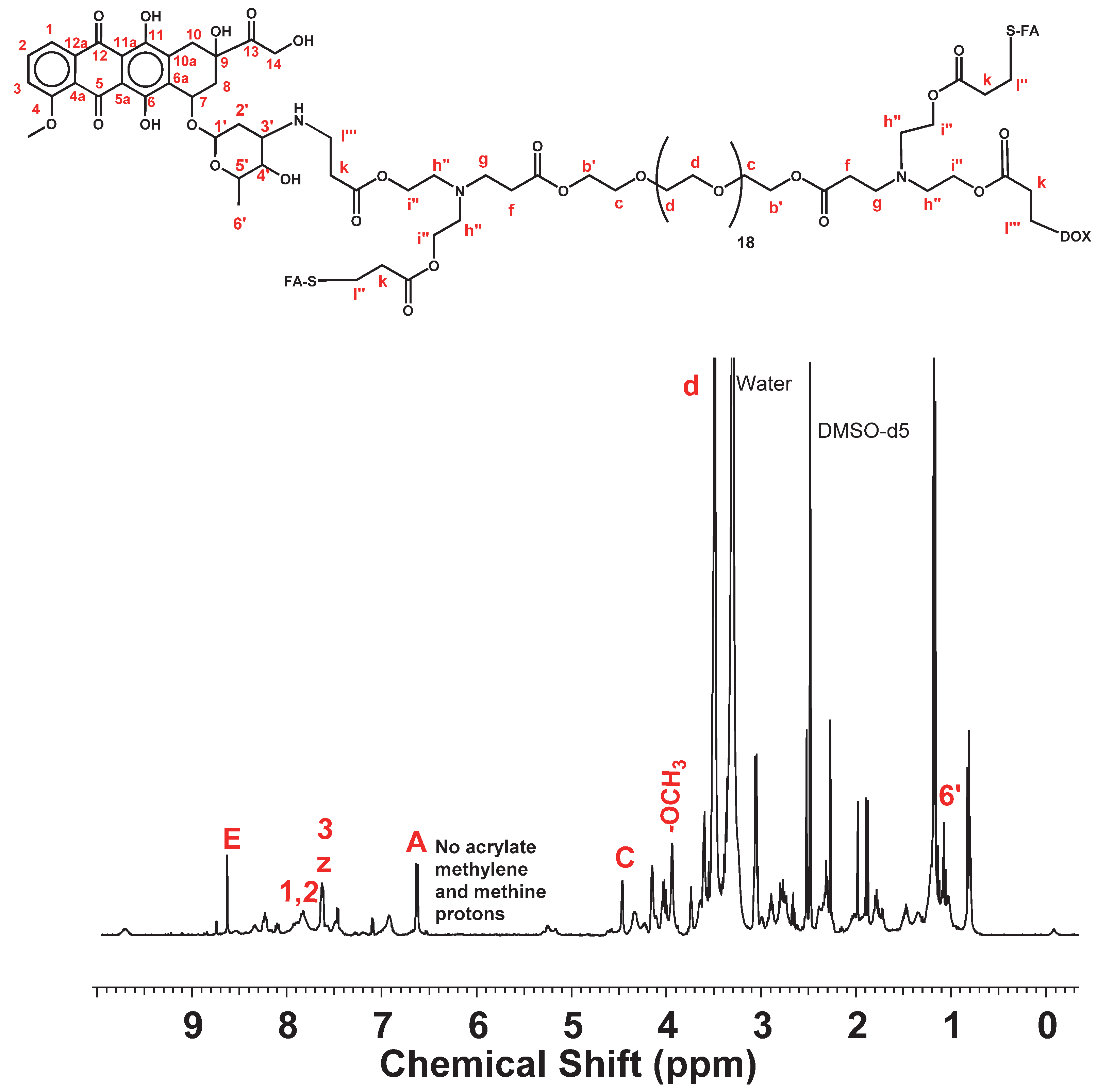
| DOX.HCl | DOC-NH-(C = O)-R | FA2-dPEG20-DOX2 | ||||
|---|---|---|---|---|---|---|
| Group | H | C | H | C | H | C |
| OCH3 (4) | 3.94 | 57.0 | 3.94 | 57.0 | 3.94 | 56.6 |
| 1′ | 5.25 | 99.7 | 5.18 | 100.9 | 5.18 | - |
| 2′ | 1.67; 1.87 | 28.6 | (1.38); 1.79 | 30.2 | 1.79 | 30.2 |
| 3′ | 3.37 | 46.9 | 3.94 | 45.3 | 3.94 | 45.9 |
| 4′ | 3.61 | 66.6 | 3.37 | 68.6 | 3.35 | 69.7 |
| 4′-OH | 5.46 | - | 4.70 | - | 4.46 | - |
| 5′ | 4.19 | 66.5 | 4.12 | 67.2 | 4.16 | 68.1 |
| 6′ | 1.14 | 17.2 | 1.19 | 17.6 | 1.17 | 19.6 |
| FA-SH | FA2-dPEG20-DOX2 | |||||
| E | 8.6 | 8.63 | ||||
| z | 7.6 | 7.63 | ||||
| A | 6.6 | 6.63 | ||||
| C | 4.4 | 4.47 | ||||
| m | 2.7 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puskas, J.E.; Shrikhande, G.; Krisch, E.; Molnar, K. Multifunctional PEG Carrier by Chemoenzymatic Synthesis for Drug Delivery Systems: In Memory of Professor Andrzej Dworak. Polymers 2022, 14, 2900. https://doi.org/10.3390/polym14142900
Puskas JE, Shrikhande G, Krisch E, Molnar K. Multifunctional PEG Carrier by Chemoenzymatic Synthesis for Drug Delivery Systems: In Memory of Professor Andrzej Dworak. Polymers. 2022; 14(14):2900. https://doi.org/10.3390/polym14142900
Chicago/Turabian StylePuskas, Judit E., Gayatri Shrikhande, Eniko Krisch, and Kristof Molnar. 2022. "Multifunctional PEG Carrier by Chemoenzymatic Synthesis for Drug Delivery Systems: In Memory of Professor Andrzej Dworak" Polymers 14, no. 14: 2900. https://doi.org/10.3390/polym14142900
APA StylePuskas, J. E., Shrikhande, G., Krisch, E., & Molnar, K. (2022). Multifunctional PEG Carrier by Chemoenzymatic Synthesis for Drug Delivery Systems: In Memory of Professor Andrzej Dworak. Polymers, 14(14), 2900. https://doi.org/10.3390/polym14142900