Simultaneous Removal of Cr(VI) and Phenol from Water Using Silica-di-Block Polymer Hybrids: Adsorption Kinetics and Thermodynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
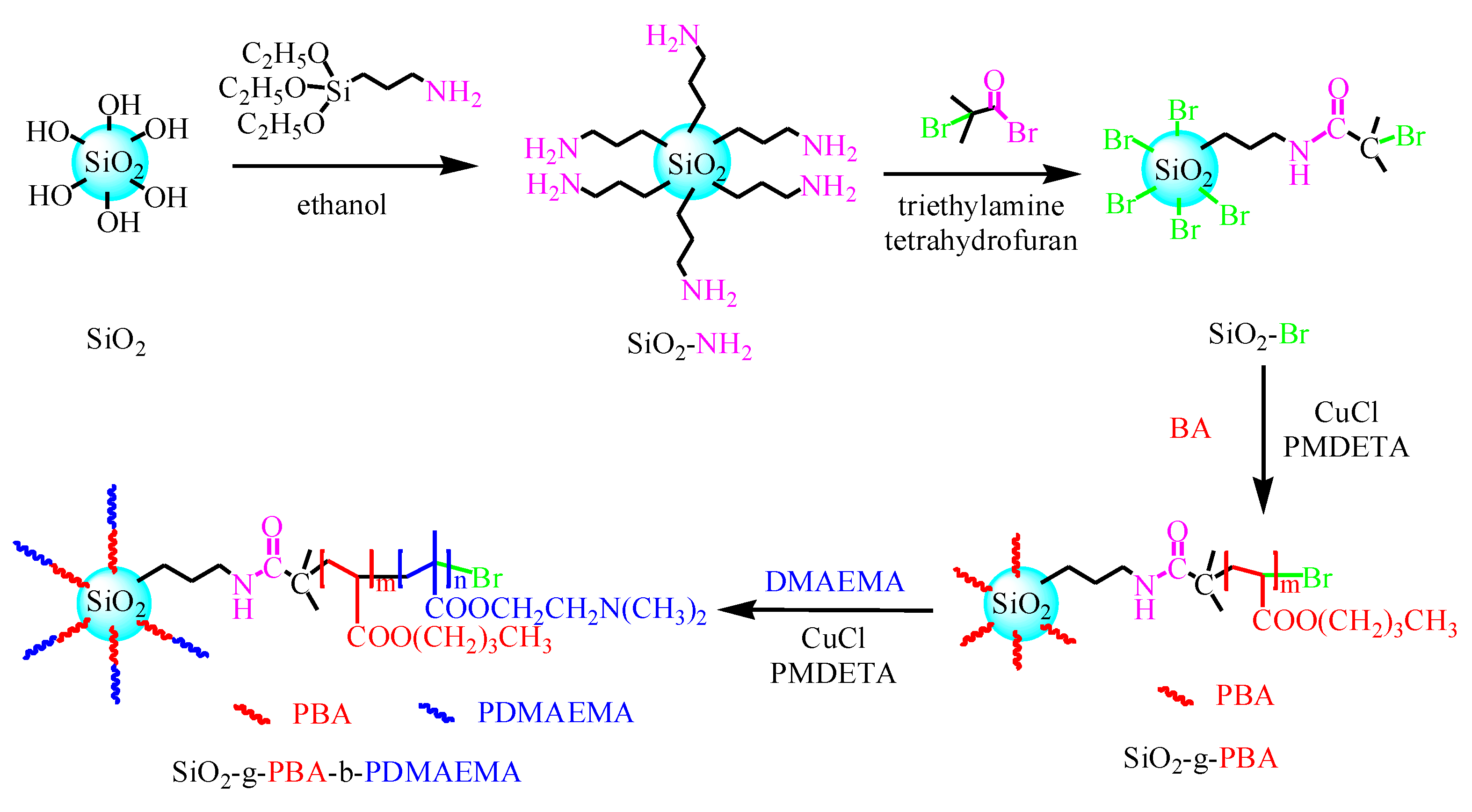
2.2. Preparation of Silica Initiator SiO2-Br
2.3. Preparation of SiO2-g-PBA-b-PDMAEMA Hybrids by SI-ATRP
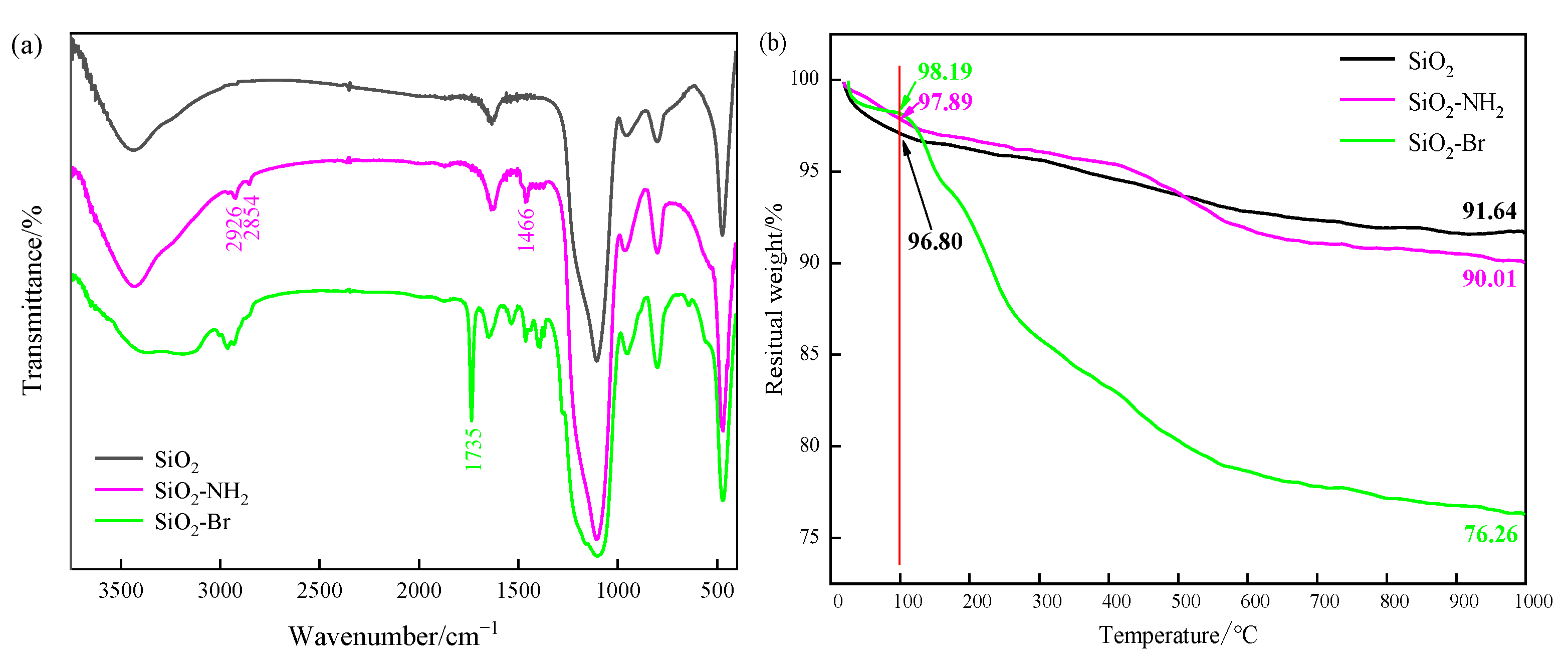
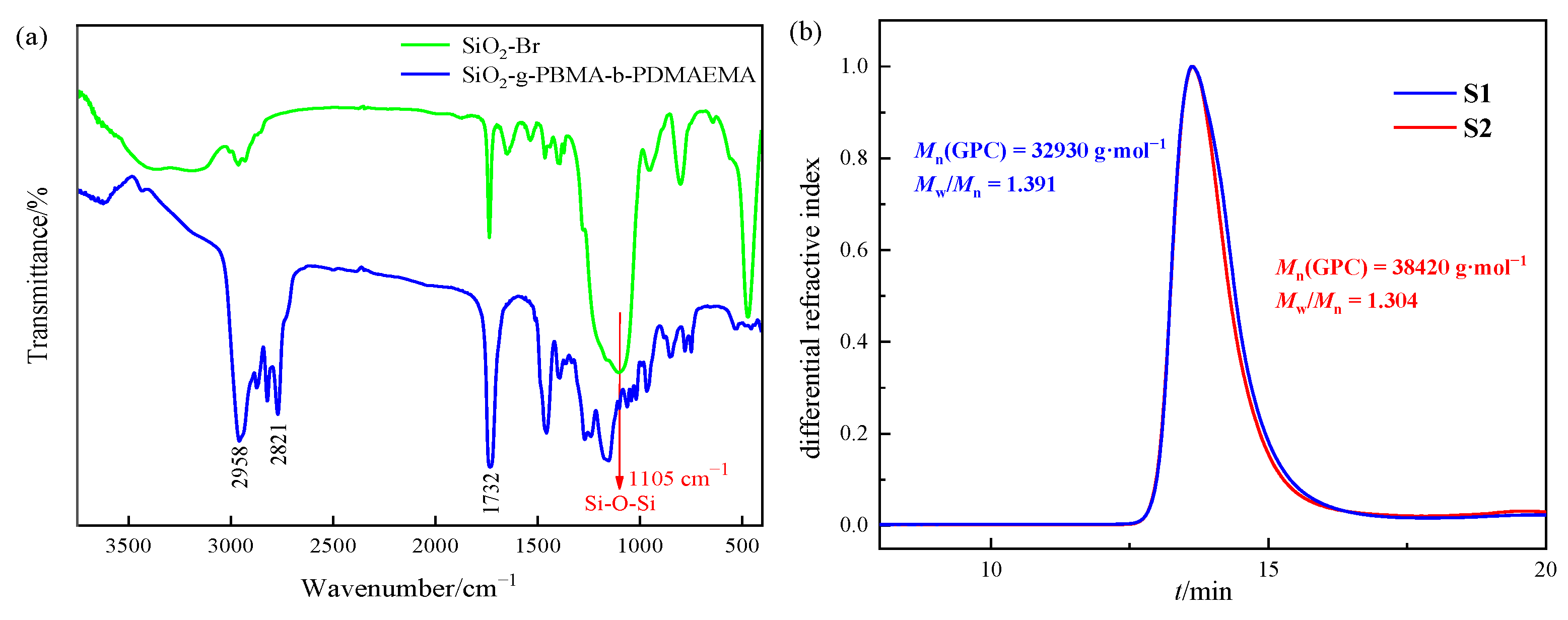
2.4. Characterization of Initiator and Hybrids
2.5. Batch Adsorption Study
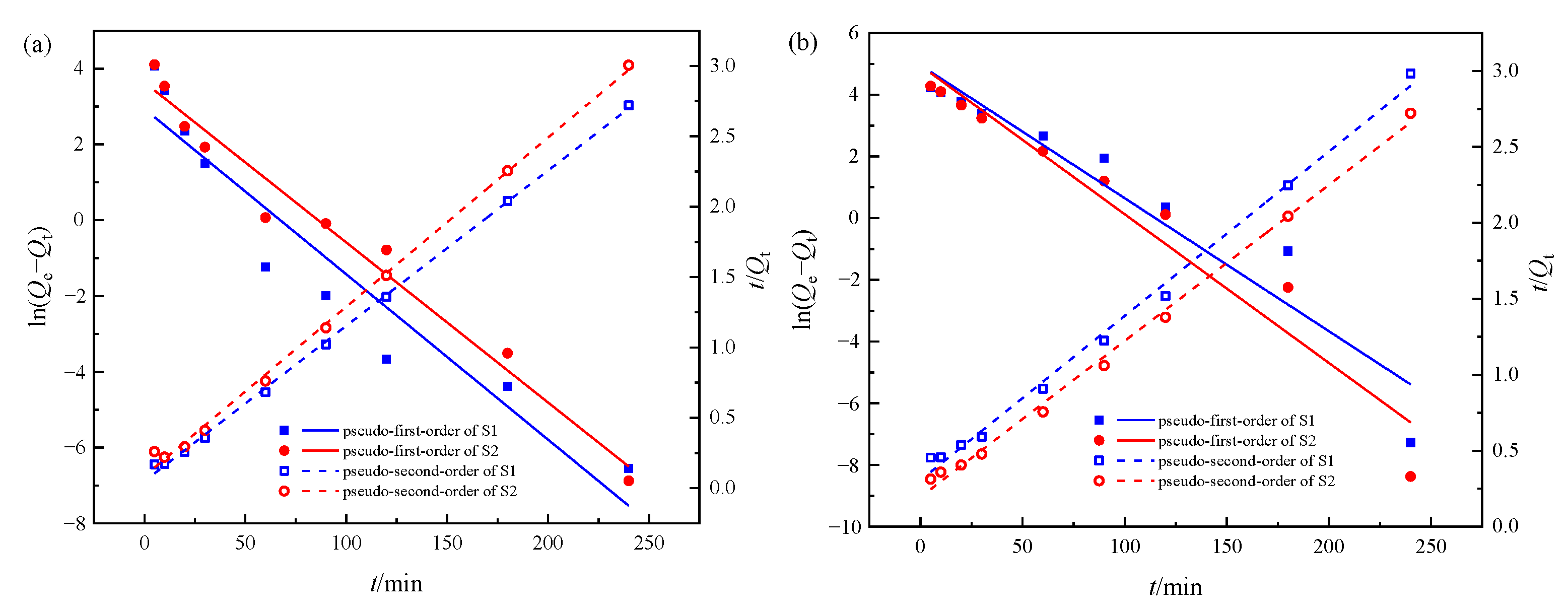
2.6. Adsorption Kinetics
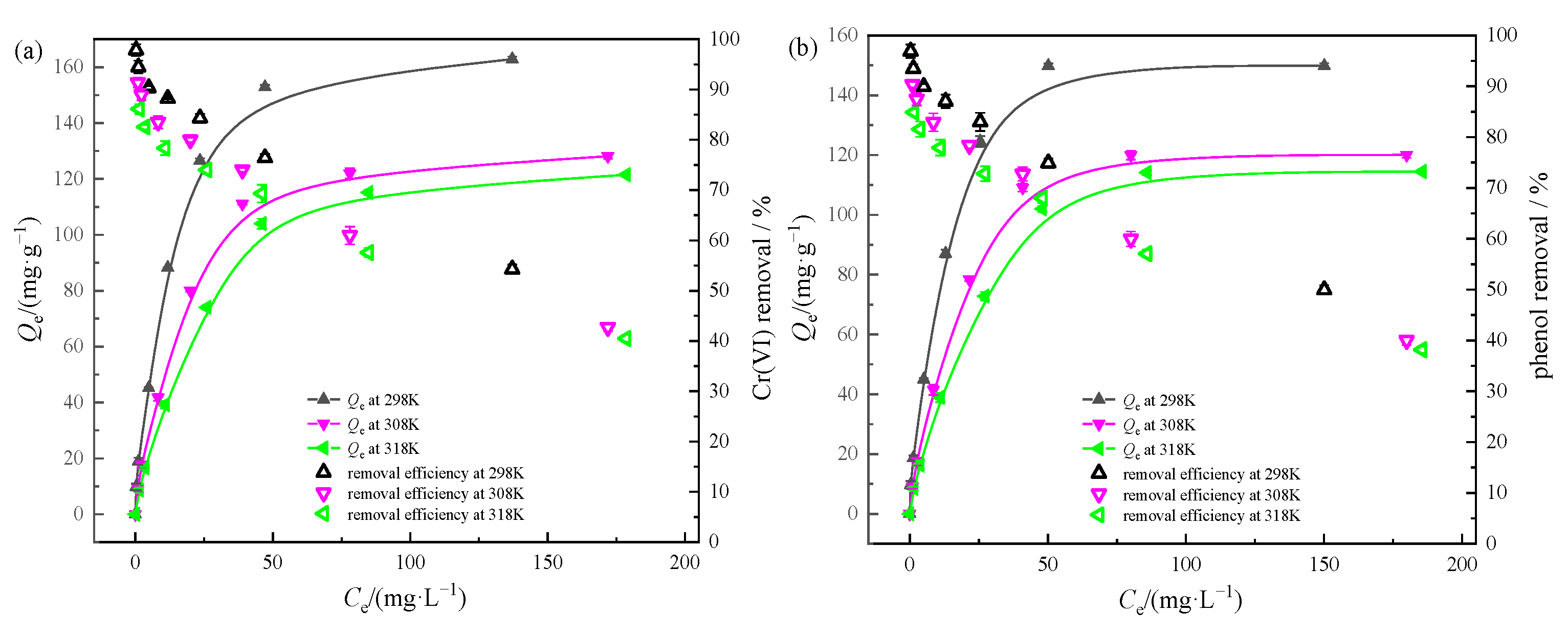
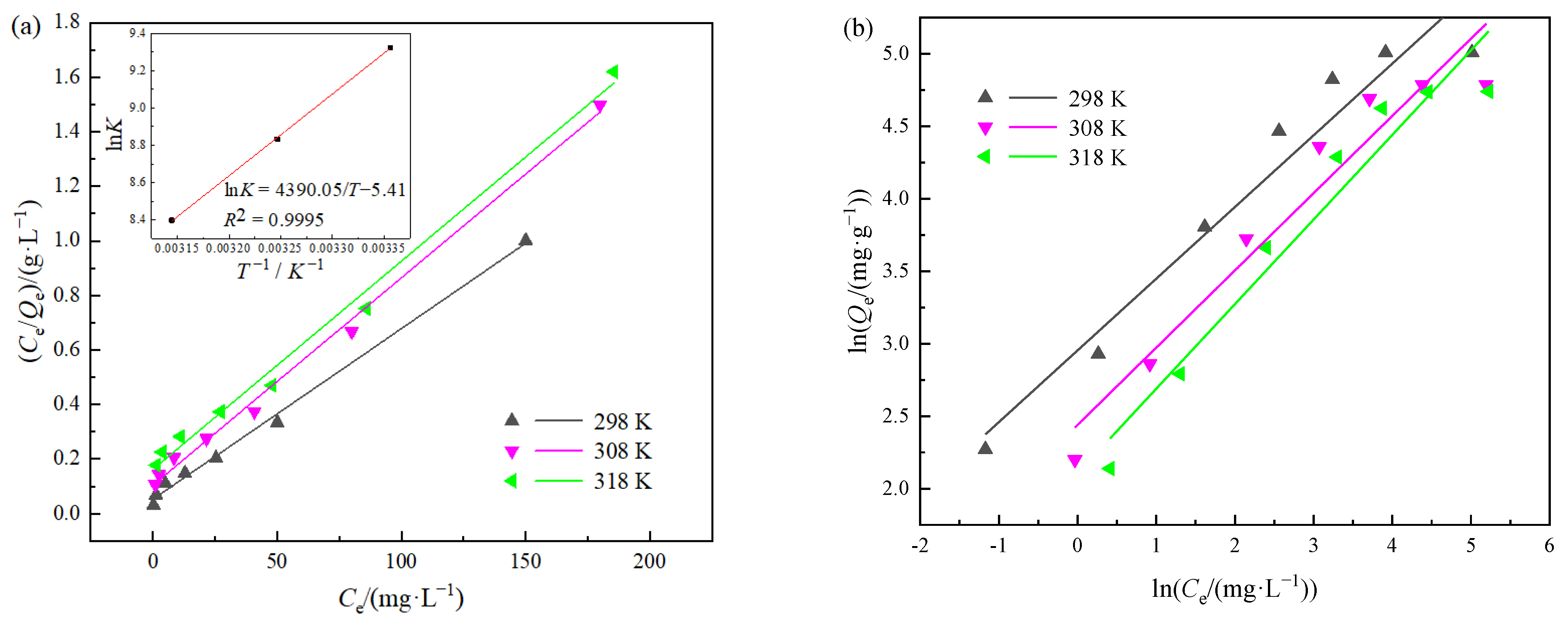
2.7. Adsorption Isotherms
2.8. Thermodynamic Study
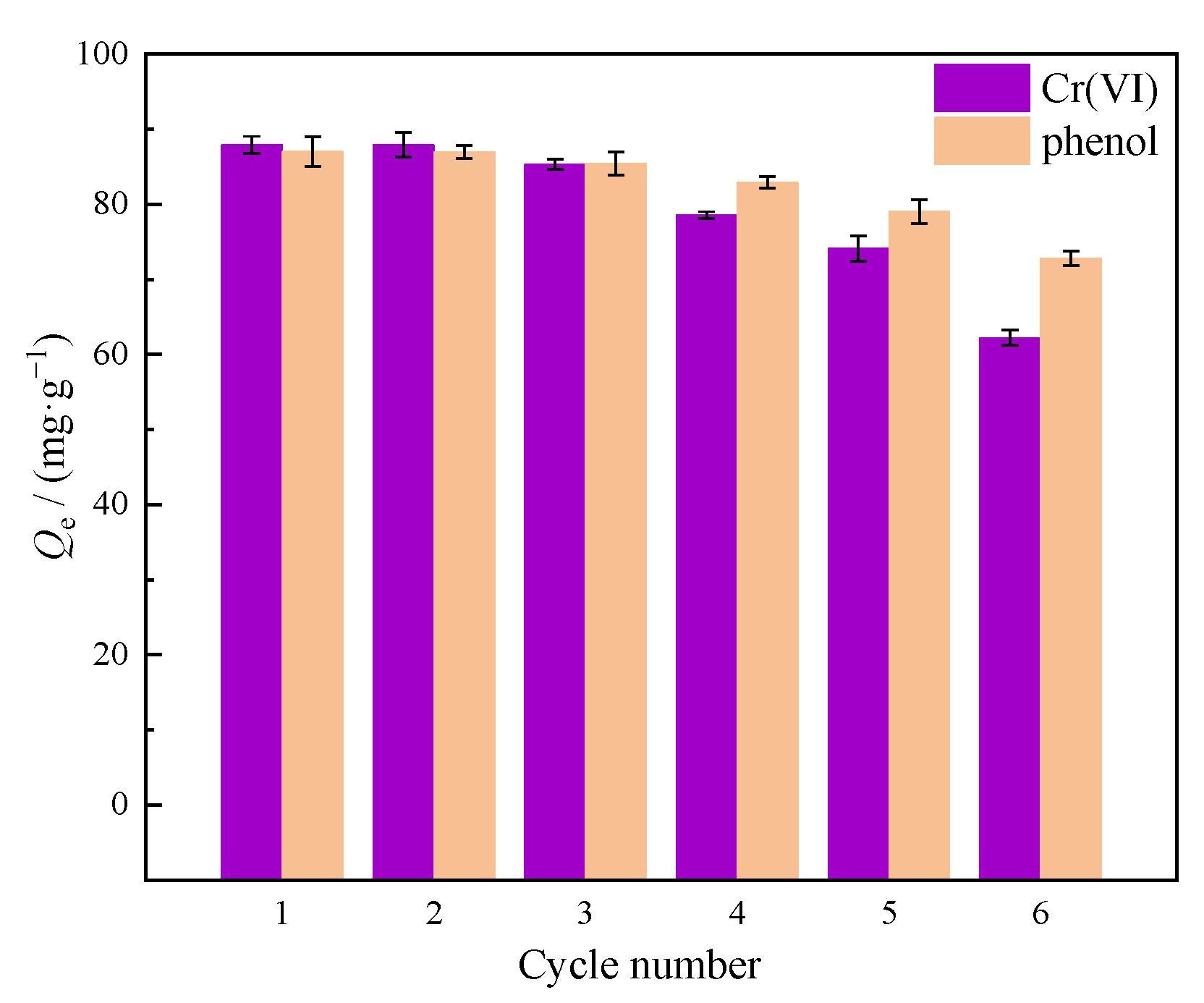
2.9. Recovery Experiments
2.10. Binary Systems Competitive Adsorption
3. Results and Discussion
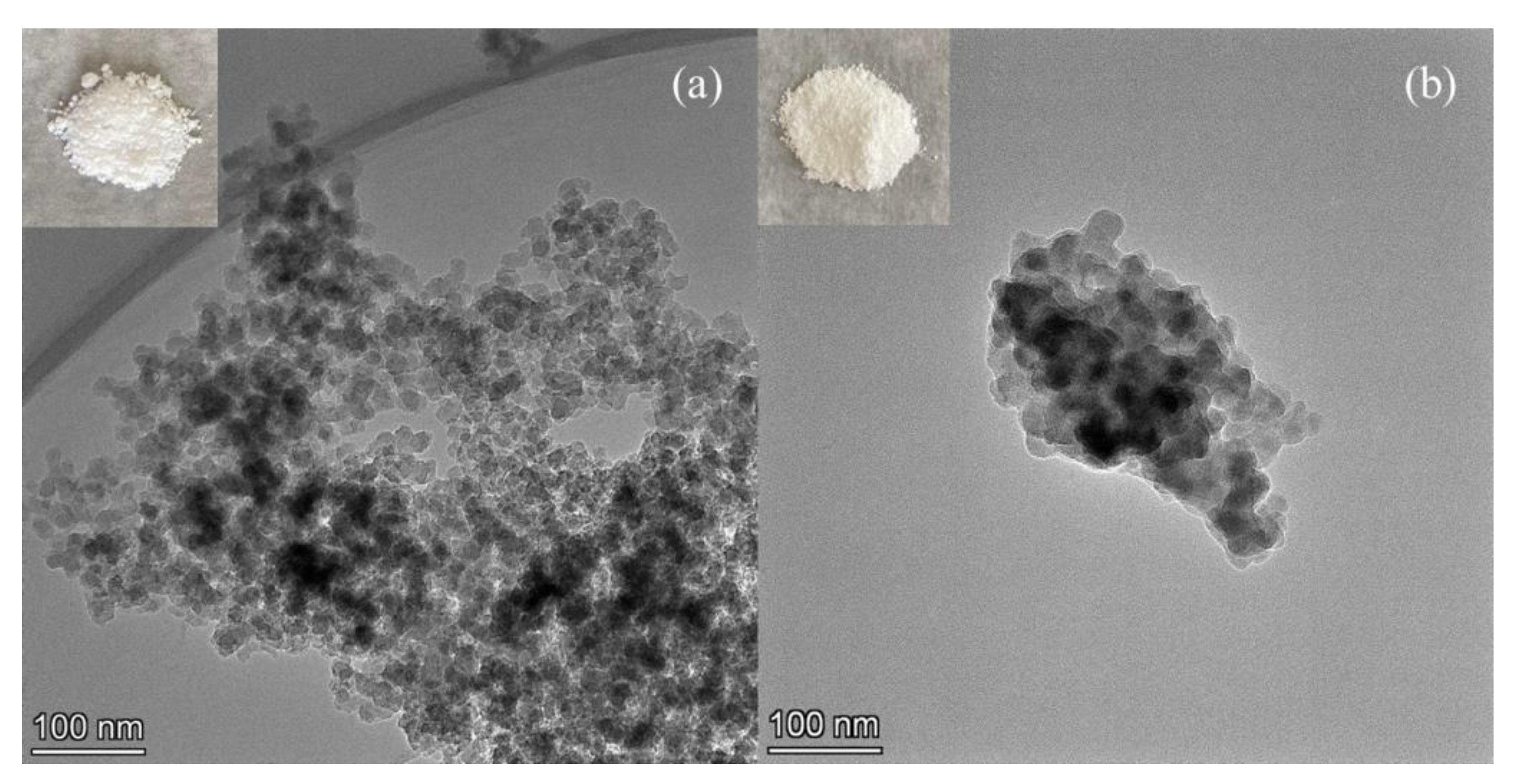
3.1. Adsorbent Characterizations
3.2. Single Systems Adsorption Kinetics
3.3. Single Systems Adsorption Isotherms and Thermodynamic Study
3.4. Recovery Performance
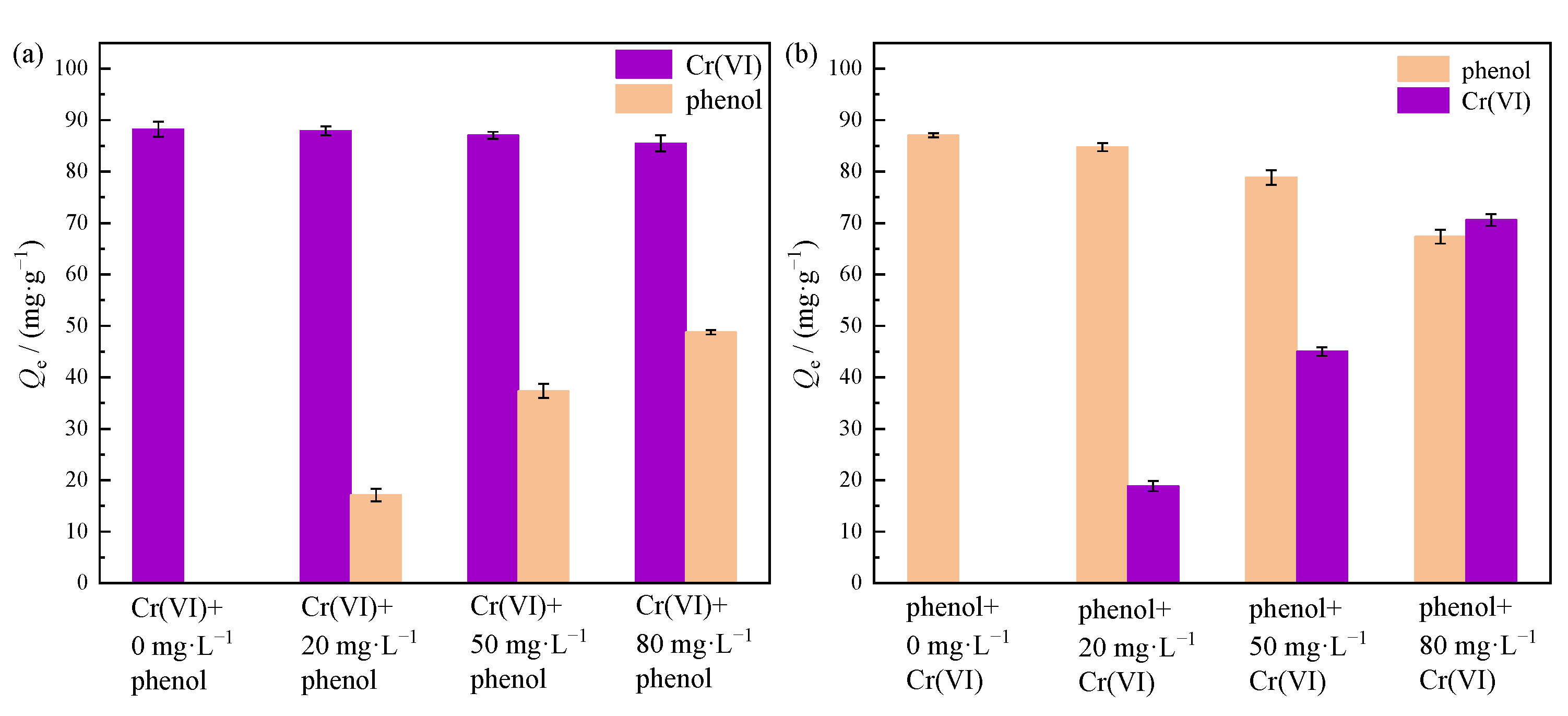
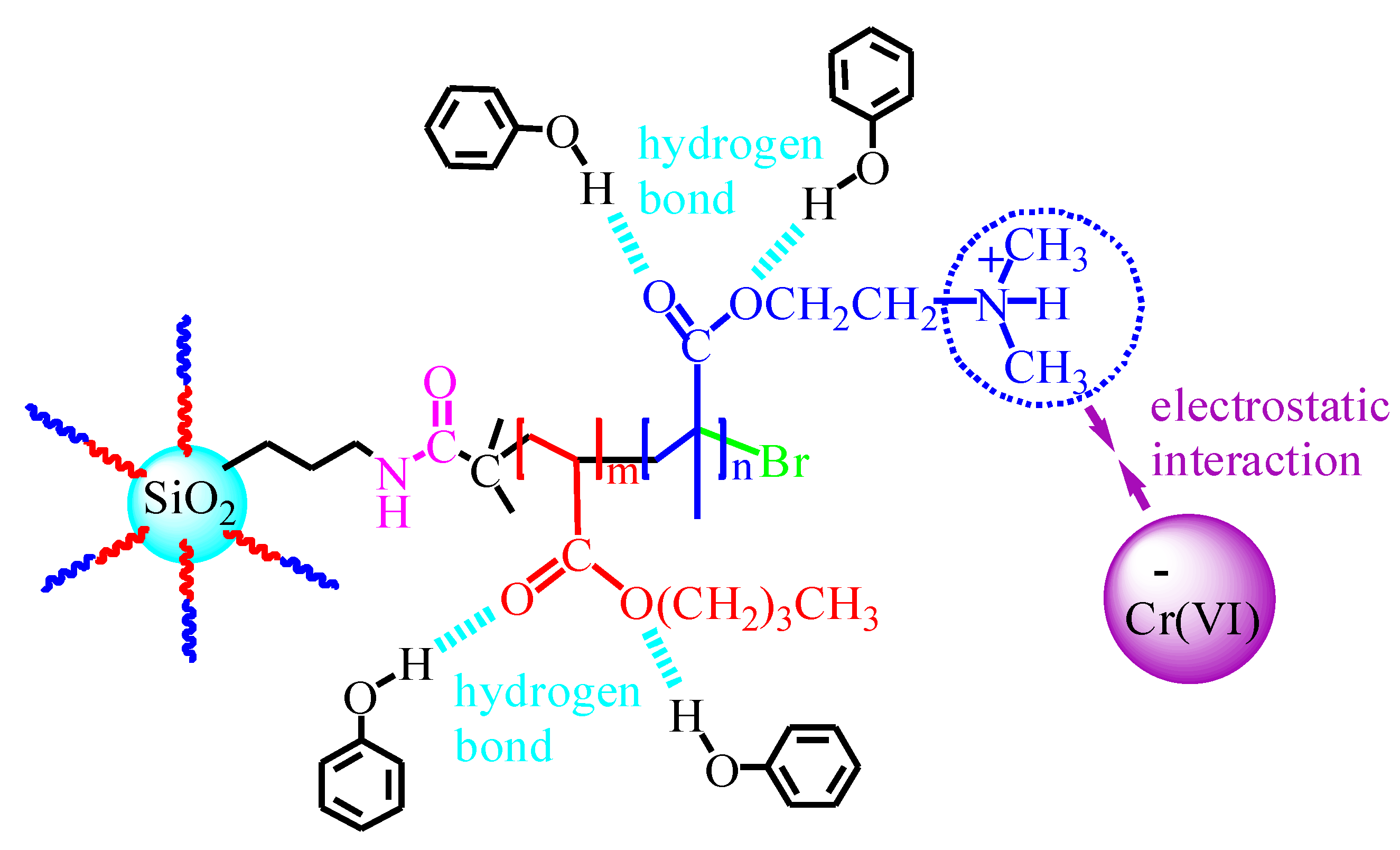
3.5. Binary Systems Competitive Adsorption Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar] [PubMed]
- Peralta-videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, A.; Rajarathinam, N.; Sivashankar, R. A review on the role of nanomaterials in the removal of organic pollutants from wastewater. Rev. Environ. Sci. Bio. Technol. 2020, 19, 751–778. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Bajpai, S.; Dey, A.; Jha, M.K.; Gupta, S.K.; Gupta, A. Removal of hazardous hexavalent chromium from aqueous solution using divinylbenzene copolymer resin. Int. J. Environ. Sci. Technol. 2012, 9, 683–690. [Google Scholar] [CrossRef][Green Version]
- Khudbudin, M.; Siona, D.; Kishor, R.; Sanjeev, T.; Nayaku, C. Adsorption of Chromium(VI) from Aqueous Solutions by Coffee Polyphenol-Formaldehyde/Acetaldehyde Resins. J. Polym. 2013, 1–10. [Google Scholar]
- Nityanandi, D.; Subbhuraam, C.V. Kinetics and thermodynamic of adsorption of chromium(VI) from aqueous solution using puresorbe. J. Hazard. Mater. 2009, 170, 876–882. [Google Scholar] [CrossRef]
- Jagirani, M.S.; Balouch, A.; Mahesar, S.A.; Kumar, A.; Bhanger, M.L. Preparation of novel arsenic imprinted polymer for the selective extraction and enhanced adsorption of toxic As3+ ions from the aqueous environment. Polym. Bull. 2020, 77, 5261–5279. [Google Scholar] [CrossRef]
- Zeng, G.M.; Liu, Y.Y.; Tang, L.; Yang, G.D.; Pang, Y.; Zhang, Y.; Zhou, Y.Y.; Li, Z.; Li, M.Y.; Lai, M.Y.; et al. Enhancement of Cd(II) adsorption by polyacrylic acid modified magnetic mesoporous carbon. Chem. Eng.J. 2015, 259, 153–160. [Google Scholar] [CrossRef]
- Ali, S.A.; Mazumder, M.A.J. A new resin embedded with chelating motifs of biogenic methionine for the removal of Hg(II) at ppb levels. J. Hazard. Mater. 2018, 350, 169–179. [Google Scholar] [CrossRef]
- Patil, S.A.; Suryawanshi, U.P.; Harale, N.S.; Patil, S.K.; Vadiyar, M.M.; Luwang, M.N.; Anuse, M.A.; Kim, J.H.; Kolekar, S.S. Adsorption of toxic Pb(II) on activated carbon derived from agriculture waste (Mahogany fruit shell): Isotherm, kinetic and thermodynamic study. Int. J. Environ. Anal. Chem. 2020, 1–17. [Google Scholar] [CrossRef]
- Rocha, C.G.; Zaia, D.A.M.; Alfaya, R.V.D.S.; Alfaya, A.A.D.S. Use of rice straw as biosorbent for removal of Cu(II), Zn(II), Cd(II) and Hg(II) ions in industrial effluents. J. Hazard. Mater. 2009, 166, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, A.O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Ahmed, M.J.; Theydan, S.K. Equilibrium isotherms, kinetics and thermodynamics studies of phenolic compounds adsorption on palm-tree fruit stones. Ecotoxicol. Environ. Saf. 2012, 84, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.L.; Moure, A.; Dominguez, H.; Parajo, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Karlowatz, M.; Kraft, M.; Mizaikoff, B. Simultaneous quantitative determination of benzene, toluene, and xylenes in water using mid-infrared evanescent field spectroscopy. Anal. Chem. 2004, 76, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Asenjo, N.G.; Alvarez, P.; Granda, M.; Blanco, C.; Santamaria, R.; Menendez, R. High performance activated carbon for benzene/toluene adsorption from industrial wastewater. J. Hazard. Mater. 2011, 192, 1525–1532. [Google Scholar] [CrossRef]
- Adachi, A.; Ikeda, C.; Takagi, S.; Fukao, N.; Yoshie, E.; Okano, T. Efficiency of rice bran for removal of organochlorine compounds and benzene from industrial wastewater. J. Agric. Food Chem. 2001, 49, 1309–1314. [Google Scholar] [CrossRef]
- Winid, B. Bromine and water quality-Selected aspects and future perspectives. Appl. Geochem. 2015, 63, 413–435. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2006, 1, 2661–2667. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Zhang, F.F.; Tang, L.; Zhang, J.C.; Zeng, G.M.; Luo, L.; Liu, Y.Y.; Wang, P.; Peng, B.; Liu, X.C. Simultaneous removal of atrazine and copper using polyacrylic acid-functionalized magnetic ordered mesoporous carbon from water: Adsorption mechanism. Sci. Rep. 2017, 7, 43831. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.L.; Li, Y.F.; Yu, C.; Ma, Y.X.; Yang, L.Q.; Hu, H.Y. Synthesis of novel inorganic–organic hybrid materials for simultaneous adsorption of metal ions and organic molecules in aqueous solution. J. Hazard. Mater. 2011, 198, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Allahverdi, A.; Ehsani, M.; Janpour, H.; Ahmadi, S. The effect of nanosilica on mechanical, thermal and morphological properties of epoxy coating. Prog. Org. Coat. 2012, 75, 543–548. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Ashekuzzaman, S.M. Development of novel inorganic adsorbent for water treatment. Curr. Opin. Chem. Eng. 2012, 1, 191–199. [Google Scholar] [CrossRef]
- Peng, H.; Zou, C.J.; Wang, C.J.; Tang, W.Y.; Zhou, J.X. The effective removal of phenol from aqueous solution via adsorption on CS/β-CD/CTA multicomponent adsorbent and its application for COD degradation of drilling wastewater. Environ. Sci. Pollut. Res. 2020, 27, 33668–33680. [Google Scholar] [CrossRef]
- Thu, P.T.T.; Dieu, H.T.; Phi, H.N.; Viet, N.N.T.; Kim, S.J.; Vo, V. Synthesis, characterization and phenol adsorption of carbonyl-functionalized mesoporous silicas. J. Porous Mater. 2012, 19, 295–300. [Google Scholar] [CrossRef]
- Huang, H.; Ren, D.Z.; Qu, J. pH and temperature-responsive POSS-based poly(2-(dimethylamino)ethyl methacrylate) for highly efficient Cr(VI) adsorption. Colloid Polym. Sci. 2020, 298, 1515–1521. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.L.; Wang, S.X.; Zhang, L.B.; Zhang, B. Efficient and Selective Adsorption of Gold Ions from Wastewater with Polyaniline Modified by Trimethyl Phosphate: Adsorption Mechanism and Application. Polymers 2019, 11, 652. [Google Scholar] [CrossRef]
- Rengaraj, S.; Moon, S.H. Kinetics of adsorption of Co(II) removal from water and wastewater by ion exchange resins. Water Res. 2002, 36, 1783–1793. [Google Scholar] [CrossRef]
- Pan, B.J.; Zhang, W.M.; Pan, B.C.; Qiu, H.; Zhang, Q.R.; Zhang, Q.X.; Zheng, S.R. Efficient removal of aromatic sulfonates from wastewater by a recyclable polymer: 2-naphthalene sulfonate as a representative pollutant. Environ. Sci. Technol. 2008, 42, 7411–7416. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.K.; Kim, K.W.; Jung, C.H.; Shul, Y.G.; Lee, E.H. Preparation of Organic-Inorganic Composite Adsorbent Beads for Removal of Radionuclides and Heavy Metal Ions. J. Radioanal. Nucl. Chem. 2000, 246, 299–307. [Google Scholar] [CrossRef]
- Meyer, T.; Prause, S.; Spange, S.; Friedrich, M. Selective Ion Pair Adsorption of Cobalt and Copper Salts on Cationically Produced Poly(1,3-divinylimidazolid-2-one)/Silica Hybrid Particles. J. Colloid Interface Sci. 2001, 236, 335–342. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.H.; Wu, J. Organic-Inorganic Hybrid Polymers as Adsorbents for Removal of Heavy Metal Ions from Solutions: A Review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.P.; Feng, Y.F.; Qu, J. Preparation and Performance of Silica-di-Block Polymer Hybrids for BSA-Resistance Coatings. Materials 2020, 13, 3478. [Google Scholar] [CrossRef]
- Tsukagoshi, T.; Kondo, Y.; Yoshino, N. Protein adsorption on polymer-modified silica particle surface. Colloids Surf. B Biointerfaces 2007, 54, 101–107. [Google Scholar] [CrossRef]
- Rao, A.V.; Kulkarni, M.M.; Amalnerkar, D.P.; Seth, T. Surface chemical modification of silica aerogels using various alkyl-alkoxy/chloro silanes. Appl. Surf. Sci. 2003, 206, 262–270. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Dhamodharan, R. A novel and simple method of preparation of poly(styrene-b-2-vinylpyridine) block copolymer of narrow molecular weight distribution: Living anionic polymerization followed by mechanism transfer to controlled/“living” radical polymerization (ATRP). J. Macromol. Sci. Part A 2000, 37, 621–631. [Google Scholar] [CrossRef]
- Leimenstoll, M.C.; Menzel, H. Behavior of ATRP-derived styrene and 4-vinylpyridine-based amphiphilic block copolymers in solution. Colloid Polym. Sci. 2018, 296, 1127–1135. [Google Scholar] [CrossRef]
- Karkare, P.; Kumar, S.; Murthy, C.N. ARGET-ATRP using β-CD as reducing agent for the synthesis of PMMA-b-PS-b-PMMA triblock copolymers. J. Appl. Polym. Sci. 2019, 136, 47117. [Google Scholar] [CrossRef]
- Huang, H.P.; He, L. Silica-diblock fluoropolymer hybrids synthesized by surface-initiated atom transfer radical polymerization. RSC Adv. 2014, 4, 13108–13118. [Google Scholar] [CrossRef]
- Pan, A.Z.; He, L. Fabrication pentablock copolymer/silica hybrids as self-assembly coatings. J. Colloid Interface Sci. 2014, 414, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; He, P.J.; Yao, Q.; Shao, L.M. Cr(VI) removal from aqueous solution by dried activated sludge biomass. J. Hazard. Mater. 2010, 176, 697–703. [Google Scholar] [CrossRef]
- Gan, W.E.; Li, Y.; He, Y.Z.; Zeng, R.H.; Guardia, M.D.L. Mechanism of porous core electroosmotic pump flow injection system and its application to determination of chromium(VI) in waste-water. Talanta 2000, 51, 667–675. [Google Scholar] [CrossRef]
- Marcu, C.; Varodi, C.; Balla, A. Adsorption Kinetics of Chromium (VI) from Aqueous Solution Using an Anion Exchange Resin. Anal. Lett. 2020, 54, 140–149. [Google Scholar] [CrossRef]
- Cherifi, H.; Hanini, S.; Bentahar, F. Adsorption of phenol from wastewater using vegetal cords as a new adsorbent. Desalination 2009, 244, 177–187. [Google Scholar] [CrossRef]
- Ye, J.J.; Feng, W.; Tian, M.M.; Zhang, J.L.; Zhou, W.H.; Jia, Q. Spectrophotometric determination of phenol by flow injection on-line preconcentration with a micro-column containing magnetic microspheres functionalized with Cyanex272. Anal. Methods 2013, 5, 1046–1051. [Google Scholar] [CrossRef]
- Konggidinata, M.I.; Chao, B.; Lian, Q.; Subramaniam, R.; Zappi, M.; Gang, D.D. Equilibrium, kinetic and thermodynamic studies for adsorption of BTEX onto Ordered Mesoporous Carbon (OMC). J. Hazard. Mater. 2017, 336, 249–259. [Google Scholar] [CrossRef]
- Attia, Y.; Yu, S. Adsorption thermodynamics of a hydrophobic polymeric flocculant on hydrophobic colloidal coal particles. Langmuir. 1991, 7, 2203–2207. [Google Scholar] [CrossRef]
- Pang, X.Y. Adsorption Kinetics and Thermodynamics Characteristics of Expanded Graphite for Polyethylene Glycol. J. Chem. 2010, 7, 1346–1358. [Google Scholar] [CrossRef]
- Malakootian, M.; Mansoorian, H.J.; Yari, A.R. Removal of reactive dyes from aqueous solutions by a non-conventional and low cost agricultural waste: Adsorption on ash of Aloe Vera plant. Iran. J. Health Saf. Environ. 2014, 1, 117–125. [Google Scholar]
- Yang, F.J.; Ma, C.H.; Yang, L.; Zhao, C.J.; Zhang, Y.; Zu, Y.G. Enrichment and Purification of Deoxyschizandrin and γ-Schizandrin from the Extract of Schisandra chinensis Fruit by Macroporous Resins. Molecules 2012, 17, 3510–3523. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gao, X. Thermodynamic study of Th ( IV) sorption on attapulgite. Appl. Radiat. Isot. 2009, 67, 1–6. [Google Scholar] [CrossRef]
- Thanapackiam, P.; Mallaiya, K.; Rameshkumar, S.; Srikandan, S.S. Inhibition of corrosion of copper in acids by norfloxacin. Anti-Corros. Methods Mater. 2017, 64, 92–102. [Google Scholar]
- Oepen, B.V.; Kördel, W.; Klein, W. Sorption of nonpolar and polar compounds to soils: Processes, measurements and experience with the applicability of the modified OECD-Guideline. Chemosphere 1991, 22, 285–304. [Google Scholar] [CrossRef]
- Li, L.; Lu, B.B.; Fan, Q.K.; Wu, J.N.; Wei, L.L.; Hou, J.; Guo, X.H.; Liu, Z.Y. Synthesis and self-assembly behavior of ph-responsive starshaped POSS-(PCL-P(DMAEMA-co-PEGMA))16 inorganic/organic hybrid block copolymer for the controlled intracellular delivery of doxorubicin. RSC Adv. 2016, 6, 61630–61640. [Google Scholar] [CrossRef]
- Pan, B.; Pan, B.; Zhang, W.; Zhang, Q.; Zhang, Q.; Zheng, S. Adsorptive removal of phenol from aqueous phase by using a porous acrylic ester polymer. J. Hazard. Mater. 2008, 157, 293–299. [Google Scholar] [CrossRef]
- Ming, Z.W.; Long, C.J.; Cai, P.B.; Xing, Z.Q.; Zhang, B. Synergistic adsorption of phenol from aqueous solution onto polymeric adsorbents. J. Hazard. Mater. 2006, 128, 123–129. [Google Scholar] [CrossRef]
- Gupta, A.; Balomajumder, C. Simultaneous Adsorption of Cr(VI) and Phenol from Binary Mixture Using Iron Incorporated Rice Husk: Insight to Multicomponent Equilibrium Isotherm. Int. J. Chem. Eng. 2016, 1–11. [Google Scholar] [CrossRef]
- Gładysz-Płaska, A.; Majdan, M.; Pikus, S.; Sternik, D. Simultaneous adsorption of chromium(VI) and phenol on natural red clay modified by HDTMA. Chem. Eng. J. 2012, 179, 140–150. [Google Scholar] [CrossRef]


| Sample (SiO2-Br:BA:DMAEMA) | SiO2-Br /mmol | BA /mmol | DMAEMA /mmol | CuCl /mmol | CuCl2 /mmol | PMDETA /mmol | CYC /g |
|---|---|---|---|---|---|---|---|
| S1 (1:50:250) | 0.1620 | 8.1000 | 40.5000 | 0.1620 | 0.0162 | 0.1620 | 11.4773 |
| S2 (1:60:240) | 0.1620 | 9.7200 | 38.8800 | 0.1620 | 0.0162 | 0.1620 | 11.4066 |
| Systems | Adsorbents | Qe,exp /mg·g−1 | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|---|
| Qe,cal /mg·g−1 | k1 /min−1 | R2 | Qe,cal /mg·g−1 | k2 /g·mg−1·min−1 | R2 | |||
| Cr(VI) | S1 | 88.25 | 18.88 | 0.04364 | 0.9055 | 90.66 | 0.00242 | 0.9988 |
| S2 | 79.88 | 37.98 | 0.04223 | 0.9688 | 83.19 | 0.00167 | 0.9971 | |
| phenol | S1 | 80.44 | 142.62 | 0.04313 | 0.9124 | 92.42 | 0.00038 | 0.9944 |
| S2 | 88.17 | 140.87 | 0.04821 | 0.9395 | 97.28 | 0.00054 | 0.9957 | |
| Systems | Temperature /K | Langmuir | Freundlich | Thermodynamic Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qm /mg·g−1 | KL /L·mg−1 | R2 | n | Kf | R2 | ΔH /(kJ·mol−1) | ΔS /(kJ·mol−1·K−1) | ΔG /(kJ·mol−1) | ||
| Cr(VI) | 298 | 174.22 | 0.1114 | 0.9947 | 2.0617 | 21.3278 | 0.9541 | −35.9 | −0.0419 | −23.4138 |
| 308 | 140.06 | 0.0701 | 0.9970 | 1.8818 | 12.2125 | 0.9341 | −22.9948 | |||
| 318 | 139.08 | 0.0447 | 0.9940 | 1.7100 | 8.5612 | 0.9395 | −22.5758 | |||
| phenol | 298 | 159.74 | 0.1191 | 0.9952 | 2.0211 | 19.1839 | 0.9385 | −36.5 | −0.0449 | −23.1198 |
| 308 | 131.58 | 0.0729 | 0.9949 | 1.8757 | 11.4701 | 0.9240 | −22.6708 | |||
| 318 | 130.89 | 0.0472 | 0.9906 | 1.7124 | 8.2102 | 0.9298 | −22.2218 | |||
| Systems | Adsorbent Material | Qm of Cr(VI) (mg·g−1) | Qm of Phenol (mg·g−1) | References |
|---|---|---|---|---|
| Cr(VI) | divinylbenzene copolymer resin | 99.91 | [5] | |
| coffee polyphenol-formaldehyde resin | 175.44 | [6] | ||
| coffee polyphenol-acetaldehyde resin | 143.32 | [6] | ||
| puresorbe | 76.92 | [7] | ||
| silica-di-block polymer hybrids | 174.22 | This study | ||
| phenol | palm-tree fruit stones | 129.56 | [14] | |
| porous acrylic ester polymer | 78.70 | [57] | ||
| polymeric adsorbents IRA-96C | 59.85 | [58] | ||
| silica-di-block polymer hybrids | 159.74 | This study | ||
| Cr(VI) and phenol | iron incorporated rice husk | 36.3817 | 6.569 | [59] |
| natural red clay modified by hexadecyltrimethylammonium bromide | 4.47 | 1.13 | [60] | |
| silica-di-block polymer hybrids | 87.02 | 37.36 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Yang, Q.; Gong, W.; Li, M.; Cao, B. Simultaneous Removal of Cr(VI) and Phenol from Water Using Silica-di-Block Polymer Hybrids: Adsorption Kinetics and Thermodynamics. Polymers 2022, 14, 2894. https://doi.org/10.3390/polym14142894
Qu J, Yang Q, Gong W, Li M, Cao B. Simultaneous Removal of Cr(VI) and Phenol from Water Using Silica-di-Block Polymer Hybrids: Adsorption Kinetics and Thermodynamics. Polymers. 2022; 14(14):2894. https://doi.org/10.3390/polym14142894
Chicago/Turabian StyleQu, Jia, Qiang Yang, Wei Gong, Meilan Li, and Baoyue Cao. 2022. "Simultaneous Removal of Cr(VI) and Phenol from Water Using Silica-di-Block Polymer Hybrids: Adsorption Kinetics and Thermodynamics" Polymers 14, no. 14: 2894. https://doi.org/10.3390/polym14142894
APA StyleQu, J., Yang, Q., Gong, W., Li, M., & Cao, B. (2022). Simultaneous Removal of Cr(VI) and Phenol from Water Using Silica-di-Block Polymer Hybrids: Adsorption Kinetics and Thermodynamics. Polymers, 14(14), 2894. https://doi.org/10.3390/polym14142894





