A Supramolecular Hydrogel Based on Copolymers of Acrylic Acid and Maleic Anhydride Derivatives with Terpyridine Motifs
Abstract
:1. Introduction
2. Materials and Methods
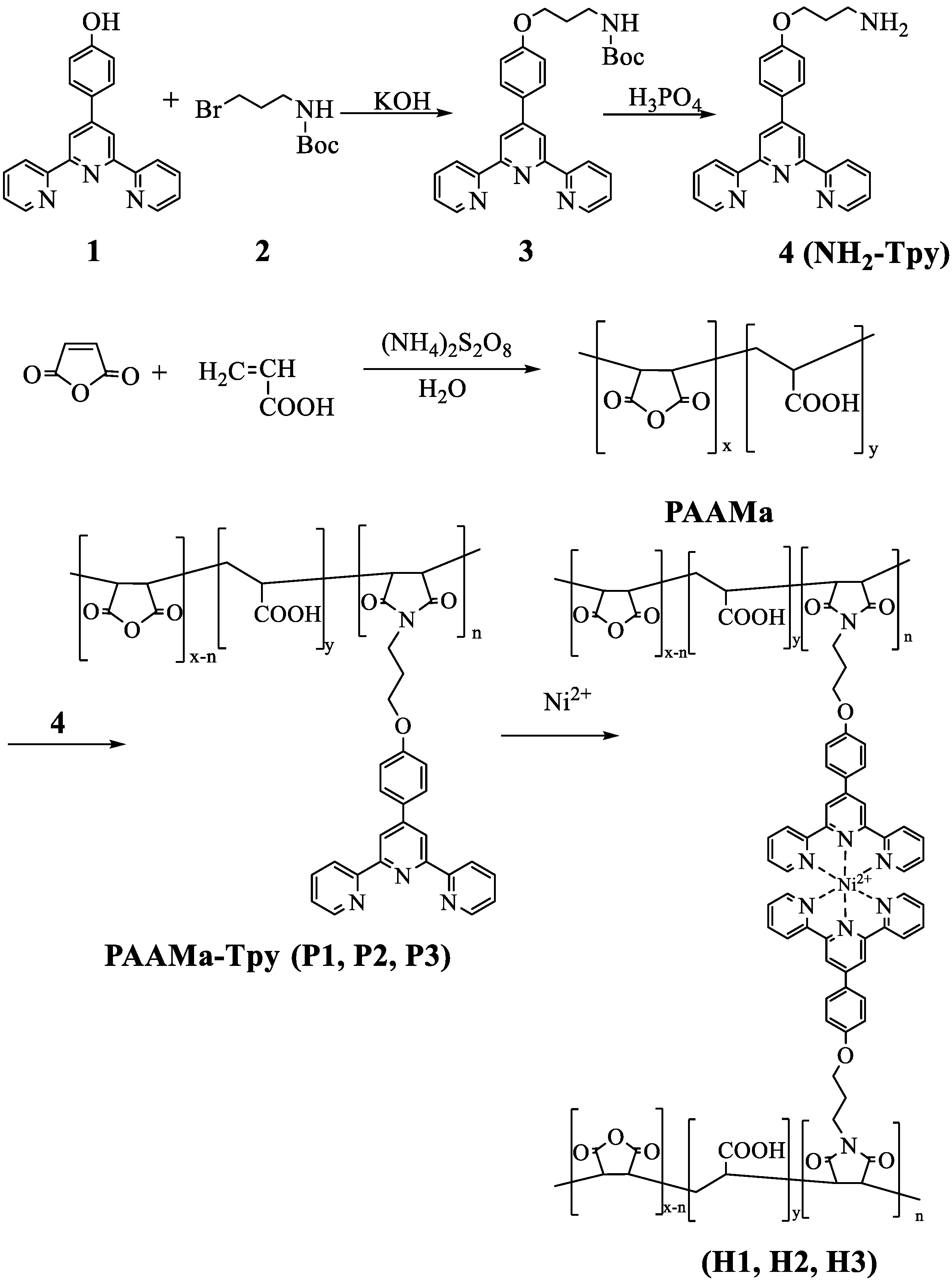
2.1. Synthesis of 4′-(p-(3-(boc-amine) propoxy)-phenyl)-2,2′:6′,2″-terpyridine (3)
2.2. Synthesis of 4′-(p-(3-amine propoxy)-phenyl)-2,2′:6′,2″-terpyridine (NH2-Tpy) (4)
2.3. Synthesis of Copolymer of Acrylic Acid and Maleic Anhydride PAAMa
2.4. General Synthetic and Purification Procedure of Gelator Polymer
2.5. Preparation of Supramolecular Hydrogels
3. Results and Discussion
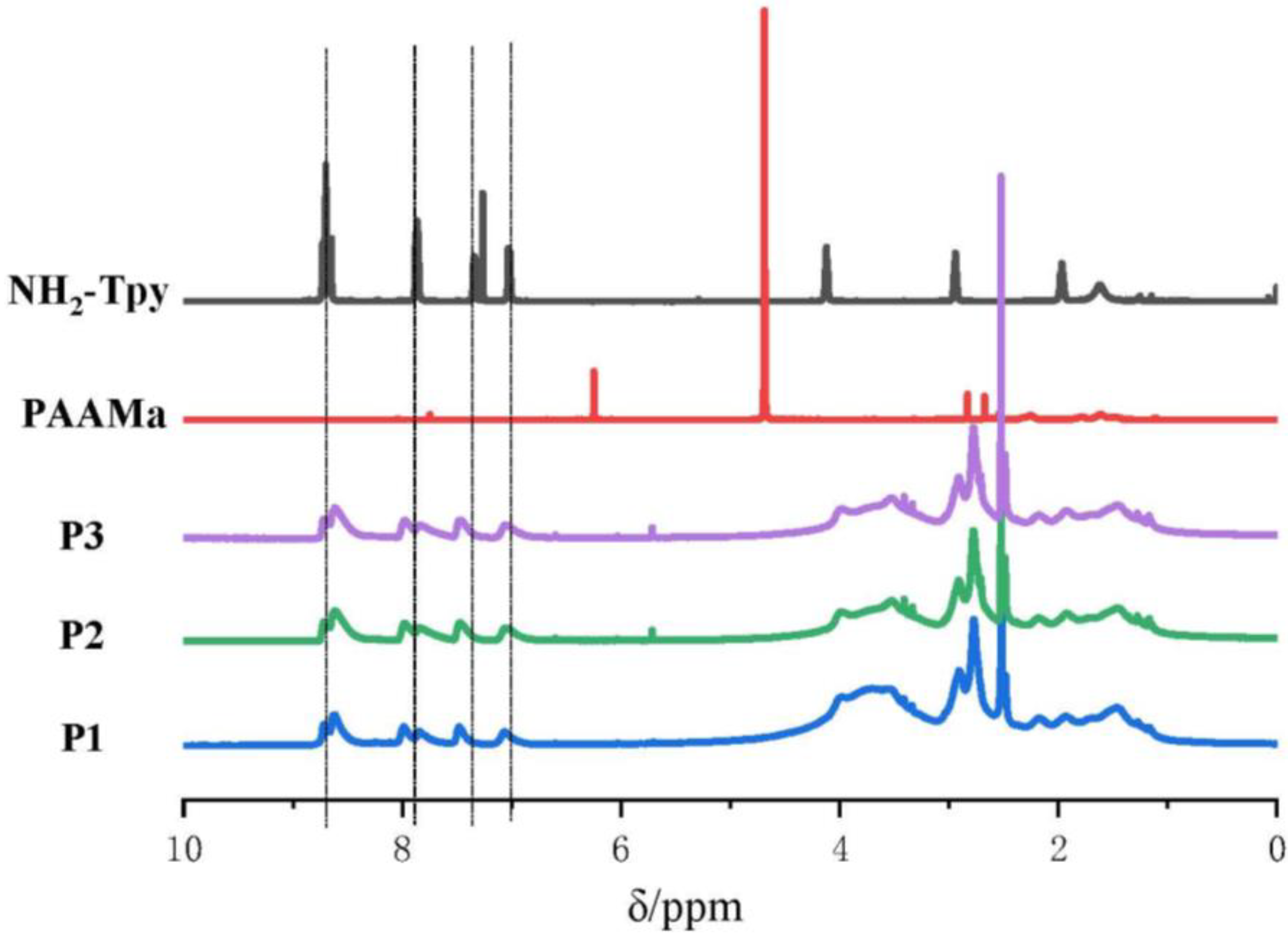
3.1. Synthesis of the Hydrophilic Polymers Containing Terpyridine Segments
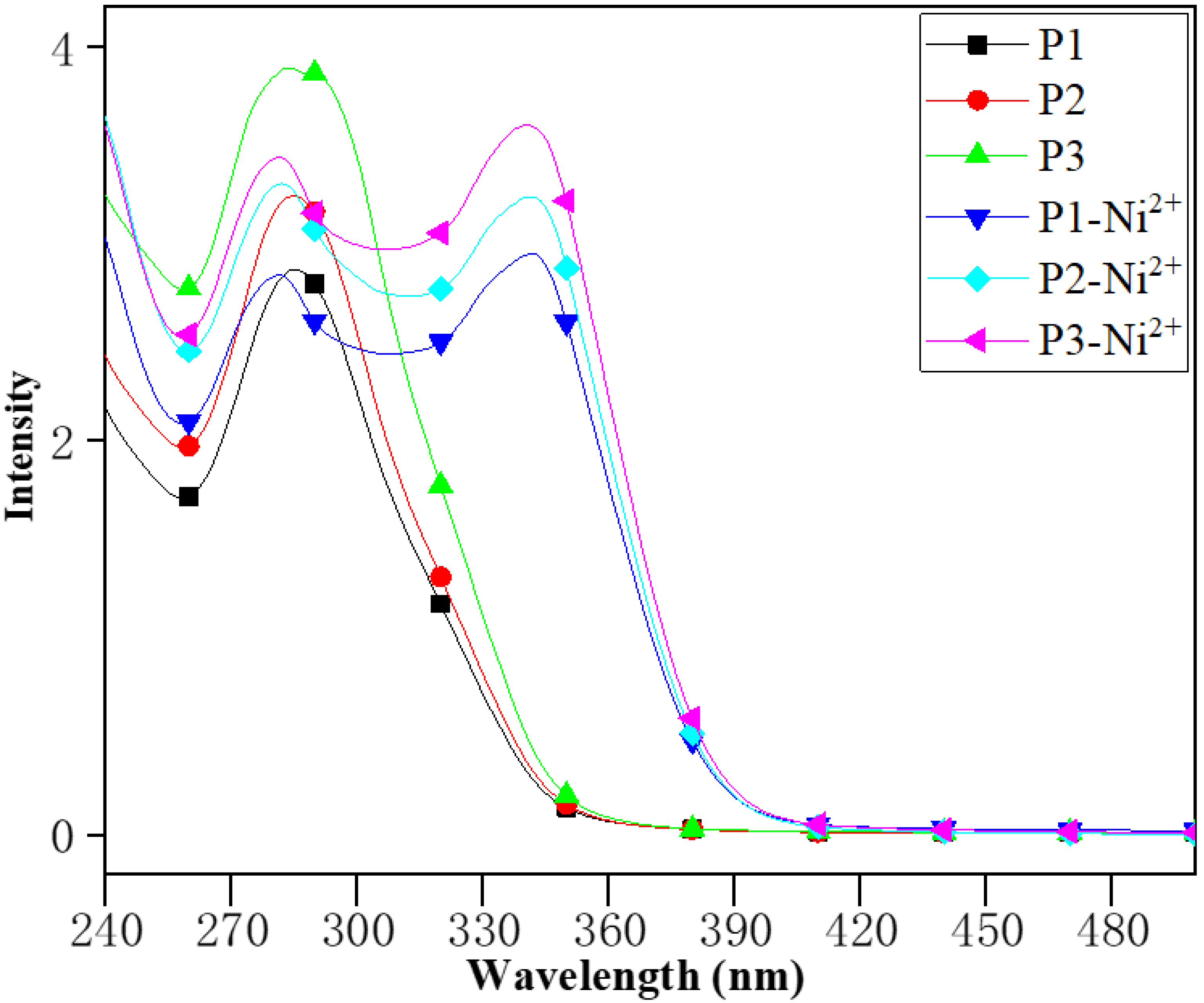
3.2. Photophysical Properties of the Linear Polymers Containing Terpyridine Segments
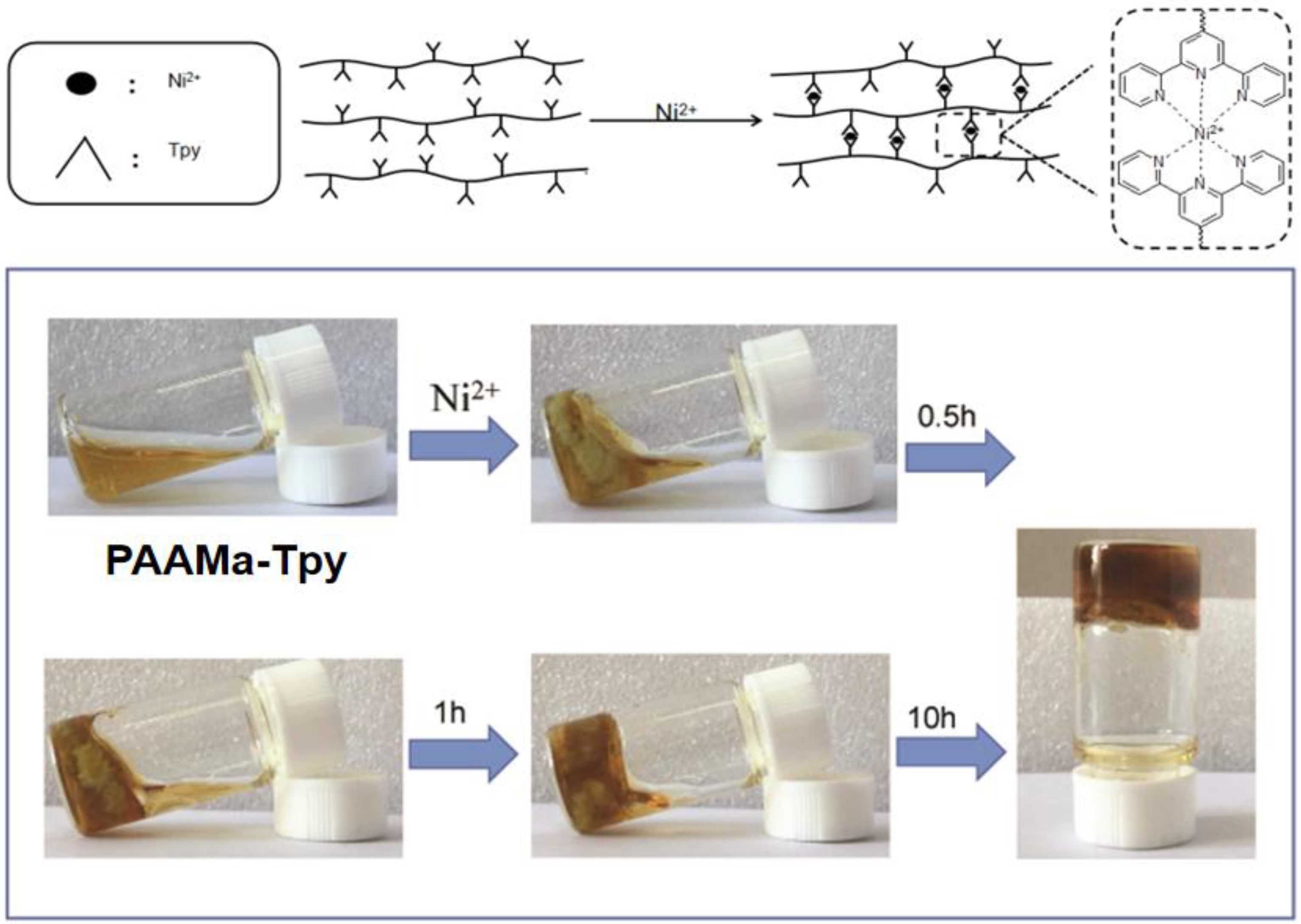
3.3. Preparation of the Metallo-Supramolecular Hydrogels
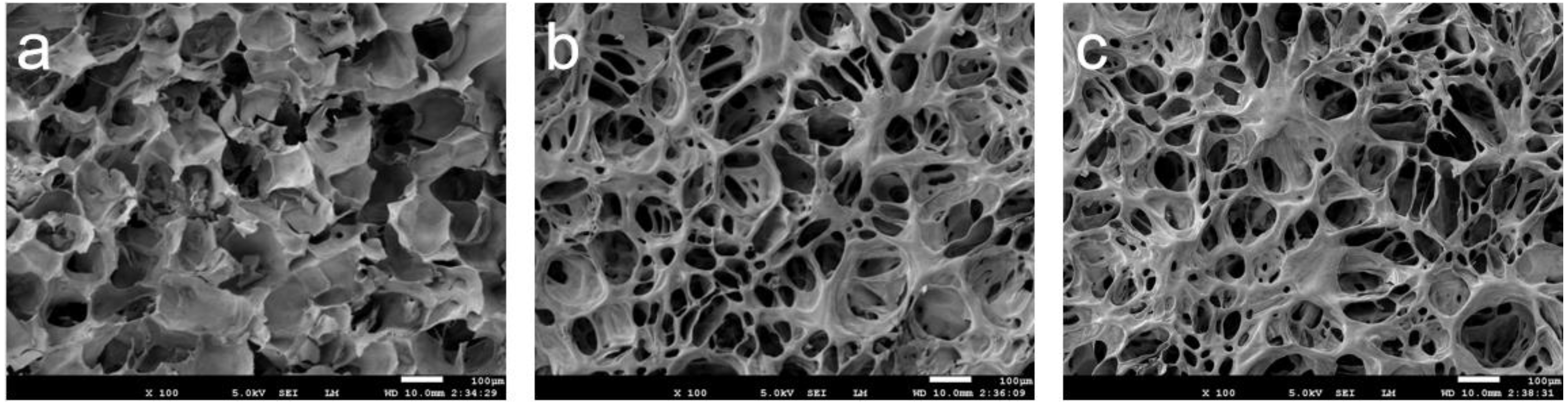
3.4. Micromorphological Study
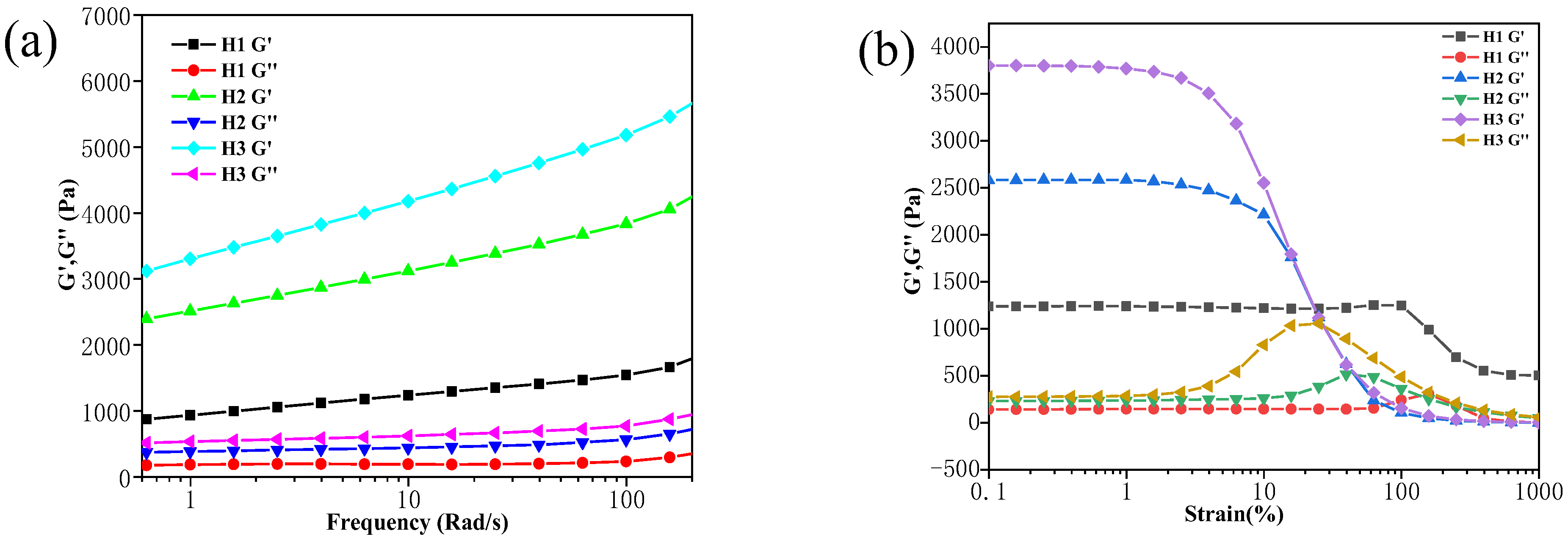
3.5. Rheological Properties
3.6. Thermo-Responsive Properties
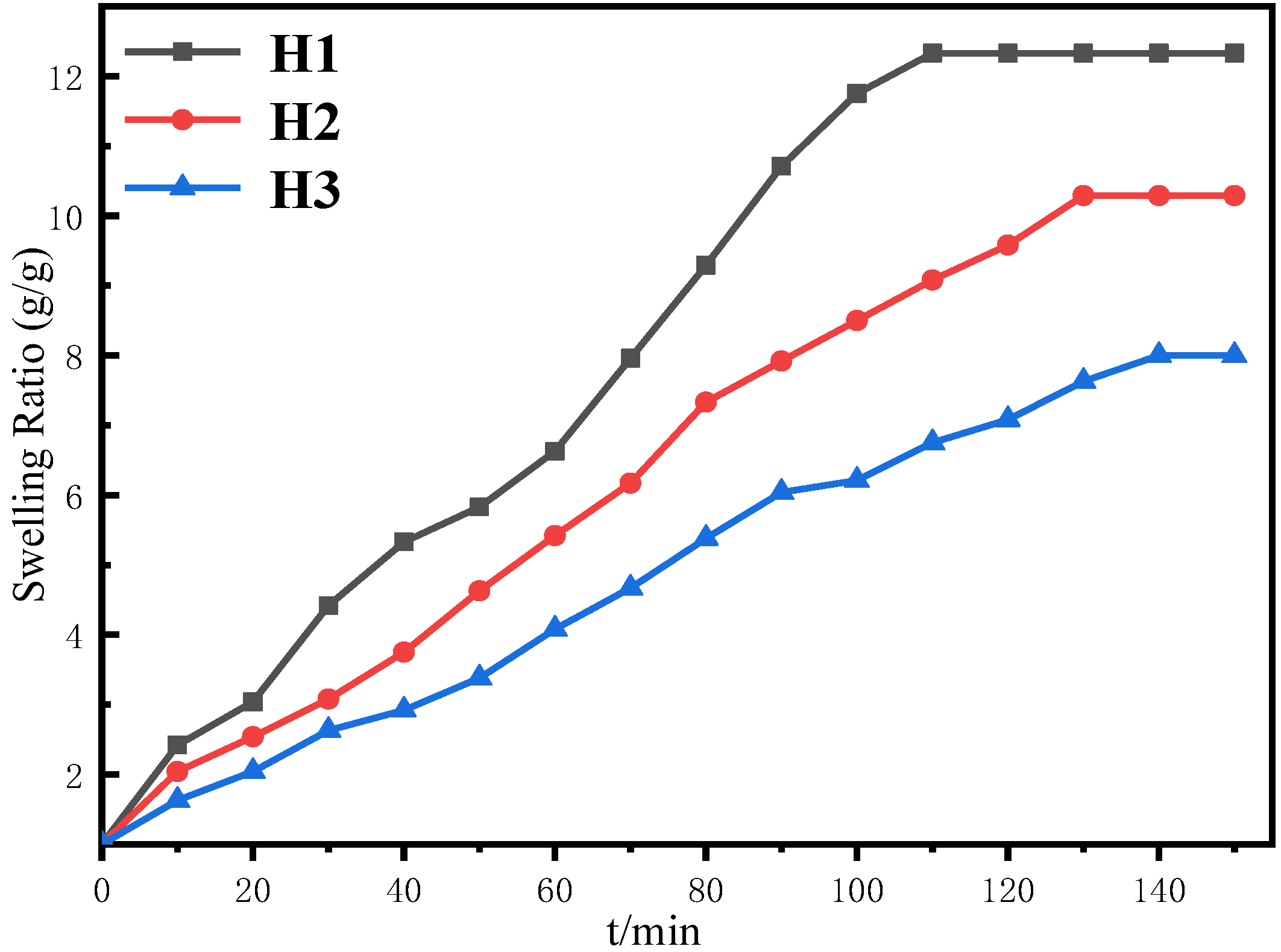
3.7. Swelling Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.K.; Wang, Z.J.; Xiao, Y.; Zhang, S.M.; Wang, J.L. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Brunsveld, L.; Folmer, B.J.B.E.; Meijer, W.; Sijbesma, R.P. Supramolecular Polymers. Chem. Rev. 2001, 101, 4071–4098. [Google Scholar] [CrossRef]
- Li, S.L.; Xiao, T.X.; Lin, C.; Wang, L.Y. Advanced Supramolecular Polymers Constructed by Orthogonal Self-Assembly. Chem. Soc. Rev. 2012, 41, 5950–5968. [Google Scholar] [CrossRef]
- Hapiot, F.; Menuel, S.; Monflier, E. Thermoresponsive Hydrogels in Catalysis. ACS Catal. 2013, 3, 1006–1010. [Google Scholar] [CrossRef]
- Ma, X.; Tian, H. Stimuli-Responsive Supramolecular Polymers in Aqueous Solution. Acc. Chem. Res. 2014, 47, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Shigemitsu, H.; Hamachi, I. Design Strategies of Stimuli-Responsive Supramolecular Hydrogels Relying on Structural Analyses and Cell-Mimicking Approaches. Acc. Chem. Res. 2017, 50, 740–750. [Google Scholar] [CrossRef]
- Shao, T.; Falcone, N.; Kraatz, H.B. Supramolecular Peptide Gels: Influencing Properties by Metal Ion Coordination and Their Wide-Ranging Applications. ACS Omega 2020, 5, 1312–1317. [Google Scholar] [CrossRef]
- Nandi, N.; Gayen, K.; Ghosh, S.; Bhunia, D.; Kirkham, S.; Sen, S.K.; Ghosh, S.; Hamley, I.W.; Banerjee, A. Amphiphilic Peptide-based Supramolecular, Non-Cytotoxic Stimuli-Responsive Hydrogels with Antibacterial Activity. Biomacromolecules 2017, 18, 3621–3629. [Google Scholar] [CrossRef]
- Hymes, J.; Wolf, B. Biotinidase and Its Role in Biotin Metabolism. Clin. Chim. Acta 1996, 255, 1–11. [Google Scholar] [CrossRef]
- Daigo, M.; Fumito, A.; Hideo, T.; Jungeun, K.; Katomasaki, T.; Takuzo, A. Ferroelectric Columnar Liquid Crystal Featuring Confined Polar Groups Within Core-Shell Architecture. Science 2012, 336, 209–213. [Google Scholar]
- Santanu, B.; Yamuna, K.G. First Report of Phase Selective Gelation of Oil from Oil/Water Mixtures. Possible Implications Toward Containing Oil Spills. Chem. Commun. 2001, 2, 185–186. [Google Scholar]
- Rowan, S.J.; Cantril, S.J.; Cousins, G.R.L.; Jeremy, K.M.; Sanders, J.K.M.; Stoddart, J.F. Dynamic Covalent Chemistry. Angew. Chem. Int. Ed. Engl. 2010, 41, 898–952. [Google Scholar] [CrossRef]
- Sun, Z.F.; Li, Z.Y.; He, Y.H.; Shen, R.J.; Deng, L.; Yang, M.H.; Liang, Y.Z.; Zhang, Y. Ferrocenoyl Phenylalanine: A New Strategy Toward Supramolecular Hydrogels with Multistimuli Responsive Properties. J. Am. Chem. Soc. 2013, 135, 13379–13386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Wang, Q.; Du, M. Metal-Directed Assembly of 1-D and 2-D Coordination Polymers with Fluconazole and Dicyanamide Co-Ligand. Inorg. Chim. Acta 2007, 360, 1970–1976. [Google Scholar] [CrossRef]
- Fullenkamp, D.E.; He, L.H.; Barrett, D.G.; Burghardt, W.R.; Messersmith, P.B. Mussel-Inspired Histidine-Based Transient Network Metal Coordination Hydrogels. Macromolecules 2013, 46, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Flynn, N.T.; Langer, R. Controlled Structure and Properties of Thermoresponsive Nanoparticle-Hydrogel Composites. Adv. Mater. 2010, 16, 1074–1079. [Google Scholar] [CrossRef]
- Khatyr, A.; Ziessel, R. Stepwise Construction of Pyrene Bridged Polytopic Ligands Carrying Acetylenic Tethers. Tetrahedron Lett. 2002, 43, 7431–7434. [Google Scholar] [CrossRef]
- Stadler, A.M.; Puntoriero, F.; Campagna, S.; Kyritsakas, N.; Welter, L.; Lehn, J.M. Synthesis, Structural Features, Absorption Spectra, Redox Behaviour and Luminescence Properties of Ruthenium(Ii) Rack-Type Dinuclear Complexes with Ditopic, Hydrazone-Based Ligands. Chemistry 2005, 11, 3997–4009. [Google Scholar] [CrossRef]
- Liu, P.; Shi, G.H.; Chen, X.G. Terpyridine-Containing π-Conjugated Polymers for Light-Emitting and Photovoltaic Materials. Front. Chem. 2020, 8, 592055. [Google Scholar] [CrossRef]
- Andreas, W.; Andreas, W.; Florian, S.; Ulrich, S.S. Advances in The Field of π-conjugated 2,2′:6′,2″-Terpyridines. Chem. Soc. Rev. 2011, 40, 1459–1511. [Google Scholar]
- Yang, H.; Ghiassinejad, S.; Ruymbeke, E.V.; Fustin, C.A. Tunable Interpenetrating Polymer Network Hydrogels Based on Dynamic Covalent Bonds and Metal–Ligand Bonds. Macromolecules 2020, 53, 6956–6967. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Li, Y.; Geng, L.; Ren, J.; Feng, G. Fluorescent and Electrochemical Supramolecular Coordination Polymer Hydrogels Formed from Ion-Tuned Self-Assembly of Small Bis-Terpyridine Monomer. Inorg. Chem. 2017, 56, 7512–7518. [Google Scholar] [CrossRef]
- Xu, Y.; Jerca, F.A.; Jerca, V.V.; Richard, H. Self-Healing and Moldable Poly (2-isopropenyl-2-oxazoline) Supramolecular Hydrogels Based on a Transient Metal Coordination Network. Macromolecules 2020, 53, 6566–6575. [Google Scholar] [CrossRef]
- Bekiari, V.; Lianos, P. Photophysical Behavior of Terpyridine-Lanthanide Ion Complexes Incorporated in A Poly (N,N-Dimethylacrylamide) Hydrogel. Langmuir 2006, 22, 8602–8606. [Google Scholar] [CrossRef] [PubMed]
- Filho, C.M.C.; Bueno, P.V.A.; Matsushita, A.F.Y.; Vilsinski, B.H.; Valente, A.J.M. Uncommon Sorption Mechanism of Aromatic Compounds onto Poly (Vinyl Alcohol)/Chitosan/Maleic Anhydride-β-Cyclodextrin Hydrogels. Polymers 2020, 12, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennig, T.L.R.; Kascholke, C.; Hoetzel, R.; Hacker, M.C. Reactive and Atimuli-Responsive Maleic Anhydride Containing Macromers–Multi-Functional Cross-Linkers and Building Blocks for Hydrogel Fabrication. React. Funct. Polym. 2013, 73, 1480–1492. [Google Scholar]
- Jakub, H.; Eva, C.K.; Miroslava, D.S.; Radka, H.; Jakub, S.; Martin, H.; Jiri, M.; Jiri, H.; Petr, L.; Roman, S. Hydrogel Tissue Expanders for Stomatology. Part II. Poly (styrene-maleic anhydride) Hydrogels. Polymers 2019, 11, 1087. [Google Scholar]
- Schmidt, U.; Zschoche, S.; Werner, C. Modification of Poly (Octadecene-alt-Maleic Anhydride) Films by Reaction with Functional Amines. J. Appl. Polym. Sci. 2010, 8, 1255–1266. [Google Scholar] [CrossRef]
- Uppu, D.S.S.M.; Akkapeddi, P.; Manjunath, G.B.; Yarlagadda, V.; Hoque, J.; Haldar, J. Polymers with Tunable Side-Chain Amphiphilicity as Non-Hemolytic Antibacterial Agents. Chem. Commun. 2013, 49, 9389–9391. [Google Scholar] [CrossRef]
- Koning, C.; Ikker, A.; Borggreve, R.; Leemans, L.; Miller, M. Reactive Blending of Poly (Styrene-co-Maleic Anhydride) with Poly (Phenylene Oxide) by Addition of (Alpha)-Amino-Polystyrene. Polymer 1993, 34, 4410–4416. [Google Scholar] [CrossRef] [Green Version]
- Hanabusa, K.; Nakamura, A.; Koyama, T.; Shirai, H. Synthesis, Polymerization, Copolymerization, and Transition-Metal Coordination of 4-(2,2′:6′,2″-terpyridin-4′-yl) styrene and its Polymers and Copolymers. Macromol. Chem. Phys. 1992, 193, 1309–1319. [Google Scholar] [CrossRef]
- Chen, X.G.; Zhou, Q.G.; Cheng, Y.X.; Geng, Y.H.; Ma, D.G. Synthesis, Structure and Luminescence Properties of Zinc (II) Complexes with Terpyridine Derivatives as Ligand. J. Lumin. 2007, 126, 81–90. [Google Scholar] [CrossRef]
- Li, B.; Berliner, M.; Buzon, R.; Chiu, C.K.F.; Colgan, S.T.; Kaneko, T.; Keene, N.; Kissel, W.; Le, T.; Leeman, K.R.; et al. Aqueous Phosphoric Acid as a Mild Reagent for Deprotection of Tert-Butyl Carbamates, Esters, and Ethers. J. Org. Chem. 2006, 71, 9045–9050. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, B.T.; Gao, G.; Liu, X.L.; Liu, F.Q. Poly (Maleic Anhydride-co-Acrylic acid)/Poly (Ethylene Glycol) Hydrogels with pH- and Ionic-Strength-Responses. Chin. J. Polym. Sci. 2010, 28, 951–959. [Google Scholar] [CrossRef]
- Barman, S.; Konai, M.M.; Samaddar, S.; Haldar, J. Amino-Acid Conjugated Polymers: Antibacterial Agents Effective against Drug-resistant A. baumannii with no Detectable Resistance. ACS Appl. Mater. Interfaces 2019, 11, 33559–33572. [Google Scholar] [CrossRef]
- Zhou, G.C.; Harruna, I.I. Synthesis and Characterization of Bis (2,2′:6′,2′′-Terpyridine) Ruthenium(II)-Connected Diblock Polymers via RAFT Polymerization. Macromolecules 2005, 38, 4114–4123. [Google Scholar] [CrossRef]
- Holyer, R.H.; Hubbard, C.D.; Kettle, S.F.A.; Wilkins, R.G. The Kinetics of Replacement Reactions of Complexes of the Transition Metals with 1,10-Phenanthroline and 2,2′-Bipyridine. Inorg. Chem. 1965, 4, 929–935. [Google Scholar] [CrossRef]
- Carlos, G.S.; Lohmeijer, B.G.G.; Meier, M.A.R.; Schubert, U.S. Synthesis of Terpyridine-Terminated Polymers by Anionic Polymerization. Macromolecules 2005, 38, 10388–10396. [Google Scholar]
- Zhang, A.J.; Chen, C.J.; Lou, D.Y.; Miao, Y.H.; Qiao, C.X.; Hu, J.B. Nickel (II), Cadmium (II), and Copper (II) Complexes Based on Ditopic Terpyridine Derivative Ligand: Syntheses, Crystal Structures, and Luminescent Properties: Nickel (II), Cadmium (II), and Copper (II). Z Anorg. Allg. Chem. 2016, 642, 817–822. [Google Scholar] [CrossRef]
- Naik, R.M.; Rai, J.; Srivastava, A.; Ratan, S.; Singh, I. Kinetic and Mechanistic Studies of Uncatalyzed Substitution of Coordinated Cyanide in Hexacyanoferrate (II) by 2,2′-Bipyridine. Int. J. Chem. Phys. Sci. 2015, 4, 105–114. [Google Scholar]
- Holyer, R.H.; Hubbard, C.D.; Kettle, S.F.A.; Wilkins, R.G. The Kinetics of Replacement Reactions of Complexes of the Transition Metals with 2,2′,2″-Terpyridine. Inorg. Chem. 1966, 5, 622–625. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, H.; Yang, Z.; Xu, B. Supramolecular Hydrogels Respond to Ligand-Receptor Interaction. J. Am. Chem. Soc. 2003, 125, 13680–13681. [Google Scholar] [CrossRef]
- Reddy, S.M.M.; Augustine, G.; Ayyadurai, N.; Shanmugam, G. Biocytin-Based pH-Stimuli Responsive Supramolecular Multivariant Hydrogelator for Potential Applications. ACS Appl. Bio. Mater. 2018, 1, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, C.J.; Chen, W. Facile Access to Guar Gum Based Supramolecular Hydrogels with Rapid Self-Healing Ability and Multi-Stimuli Responsive Gel-Sol Transitions. J. Agric. Food Chem. 2019, 11, 33559–33572. [Google Scholar]
- Appel, E.A.; Loh, X.J.; Jones, S.T.; Biedermann, F.; Dreiss, C.A.; Scherman, O.A. Ultrahigh-Water-Content Supramolecular Hydrogels Exhibiting Multistimuli Responsiveness. J. Am. Chem. Soc. 2012, 134, 11767–11773. [Google Scholar] [CrossRef]
- Song, G.S.; Zhao, Z.Y.; Peng, X.; He, C.C.; Weiss, R.A.; Wang, H.L. Rheological Behavior of Tough PVP-In Situ-PAAm Hydrogels Physically Cross-Linked by Cooperative Hydrogen Bonding. Macromolecules 2016, 49, 8265–8273. [Google Scholar] [CrossRef]
- Kitazawa, Y.; Iwata, K.; Imaizumi, S.; Ahn, H.; Sung, Y.K.; Ueno, K.; Moon, J.P.; Watanabe, M. Gelation of Solvate Ionic Liquid by Self-Assembly of Block Copolymer and Characterization as Polymer Electrolyte. Macromolecules 2014, 47, 6009–6016. [Google Scholar] [CrossRef]
- Miao, T.X.; Fenn, S.L.; Charron, P.N.; Floreani, R.A. Self-Healing and Thermoresponsive Dual-Cross-Linked Alginate Hydrogels Based on Supramolecular Inclusion Complexes. Biomacromolecules 2015, 16, 3740–3750. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, G.; Guo, M.; Jiang, M. Dual Stimuli-Responsive Supramolecular Hydrogel Based on Hybrid Inclusion Complex (HIC). Macromolecules 2010, 43, 8086–8093. [Google Scholar] [CrossRef]
- Sarkar, K.; Dastidar, P. Supramolecular Hydrogel Derived from a C3-Symmetric Boronic Acid Derivative for Stimuli Responsive Release of Insulin and Doxorubicin. Langmuir 2018, 34, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.R.; Cipriano, B.H. Gel Formation: Phase Diagrams Using Tabletop Rheology and Calorimetry. In Molecular Gels; Springer: Berlin/Heidelberg, Germany, 2006; pp. 241–252. [Google Scholar]


| Polymer | Feed Percentage of NH2-Tpy (%) | Terpyridine Content from NMR (%) | Conversion Rate (%) |
|---|---|---|---|
| P1 | 7.2 | 6.9 | 95.8 |
| P2 | 10.4 | 10.1 | 97.1 |
| P3 | 13.5 | 12.7 | 94.1 |
| Hydrogel | Transition Temperature |
|---|---|
| H1 | 55 °C |
| H2 | 60 °C |
| H3 | 75 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, Z.; Ma, C.; He, Z.; Ma, Z.; Chen, X.; Huang, Z. A Supramolecular Hydrogel Based on Copolymers of Acrylic Acid and Maleic Anhydride Derivatives with Terpyridine Motifs. Polymers 2022, 14, 2857. https://doi.org/10.3390/polym14142857
Chi Z, Ma C, He Z, Ma Z, Chen X, Huang Z. A Supramolecular Hydrogel Based on Copolymers of Acrylic Acid and Maleic Anhydride Derivatives with Terpyridine Motifs. Polymers. 2022; 14(14):2857. https://doi.org/10.3390/polym14142857
Chicago/Turabian StyleChi, Zheng, Chenchen Ma, Ziyuan He, Zihan Ma, Xuegang Chen, and Zhaoge Huang. 2022. "A Supramolecular Hydrogel Based on Copolymers of Acrylic Acid and Maleic Anhydride Derivatives with Terpyridine Motifs" Polymers 14, no. 14: 2857. https://doi.org/10.3390/polym14142857
APA StyleChi, Z., Ma, C., He, Z., Ma, Z., Chen, X., & Huang, Z. (2022). A Supramolecular Hydrogel Based on Copolymers of Acrylic Acid and Maleic Anhydride Derivatives with Terpyridine Motifs. Polymers, 14(14), 2857. https://doi.org/10.3390/polym14142857





