Nanoparticles and Plant By-Products for Edible Coatings Production: A Case Study with Zinc, Titanium, and Silver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Packaging Preparation
2.1.1. Production of Packaging with the Addition of Nanoparticles
2.1.2. Production of Packaging with the Addition of Colloidal Silver
2.1.3. Grape Marc Preparation
2.1.4. Extract Preparation
2.2. Sensory Analysis
2.2.1. Quantitative Descriptive Analysis
2.2.2. Hedonic Analysis
2.2.3. Assessment of the Probability of Purchasing Food Commodities in Edible Packaging
2.3. Evaluation of Color and Color Properties of Packaging
2.3.1. Opacity
2.3.2. Whiteness Index
2.3.3. Yellowness Index
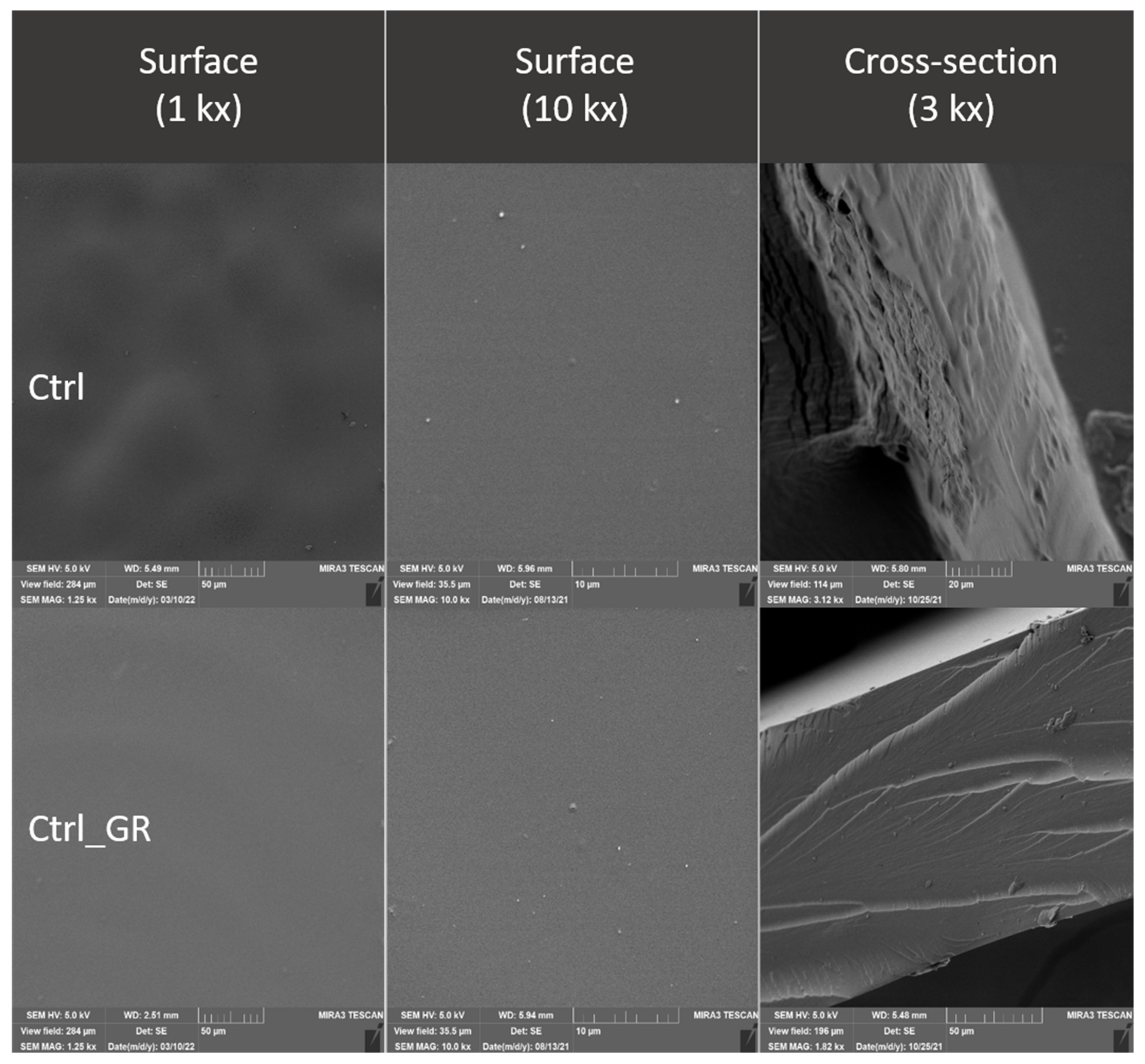
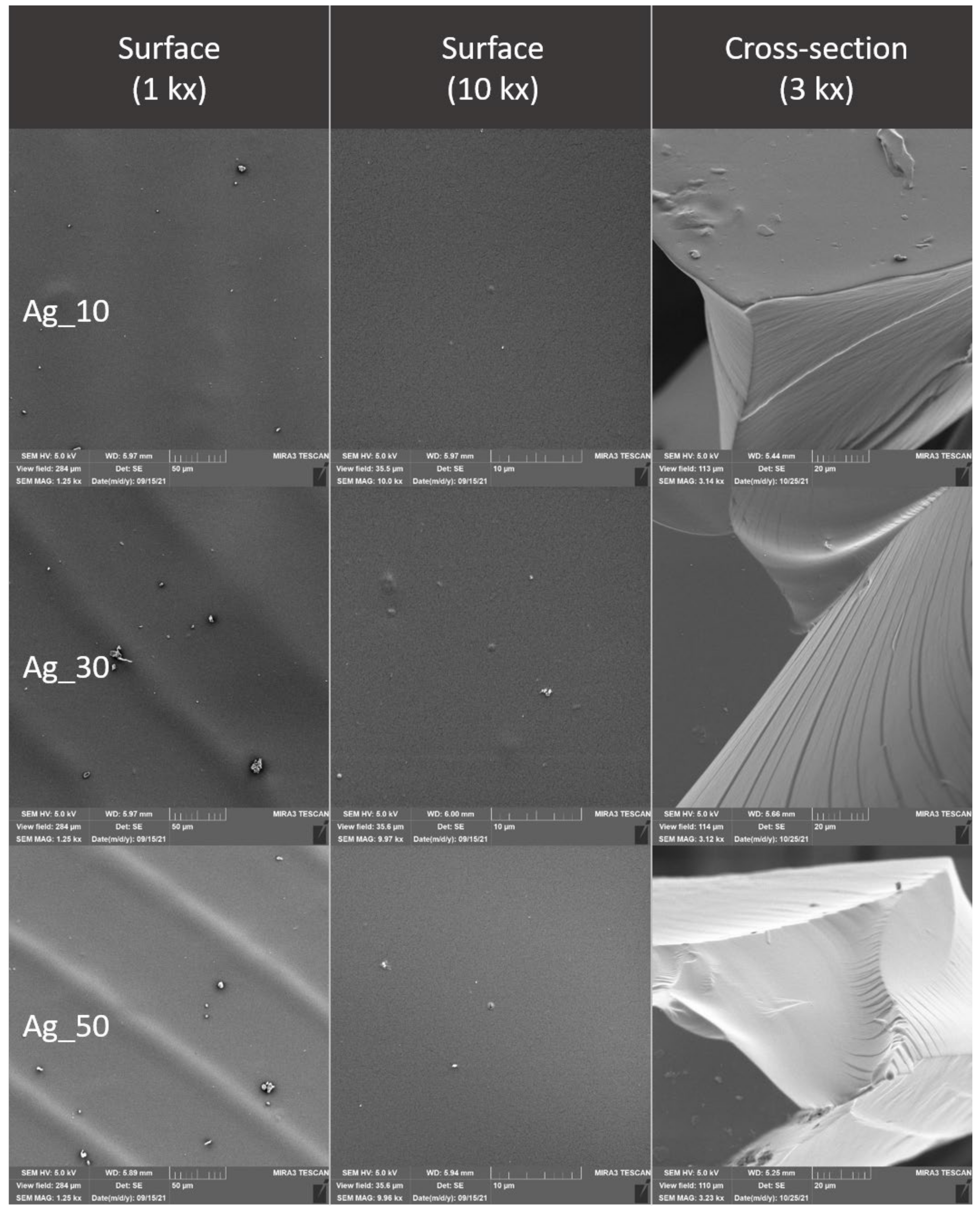
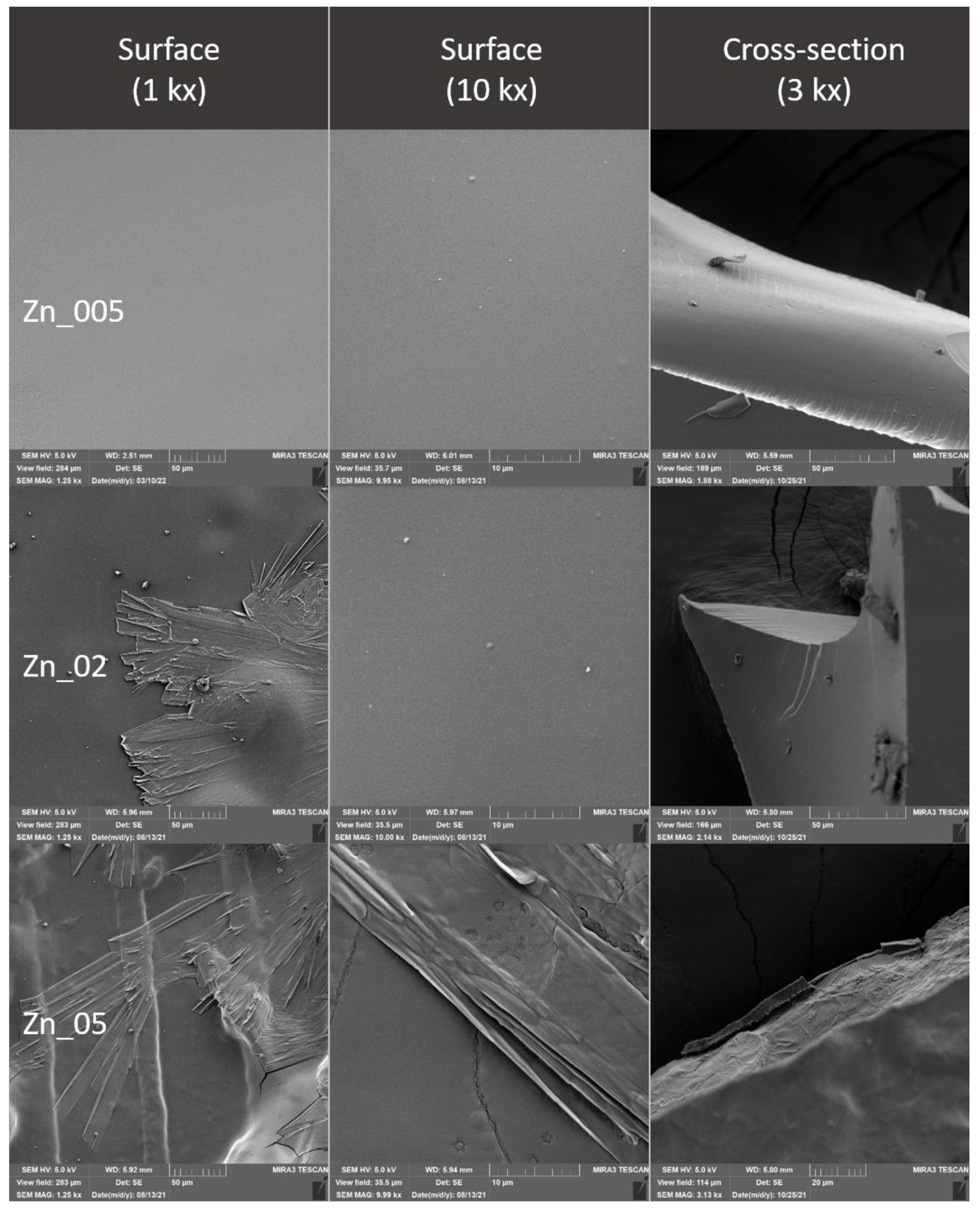
2.4. Evaluation of the Surface of Edible Packaging by SEM
2.5. Statistical Analysis
3. Results and Discussion
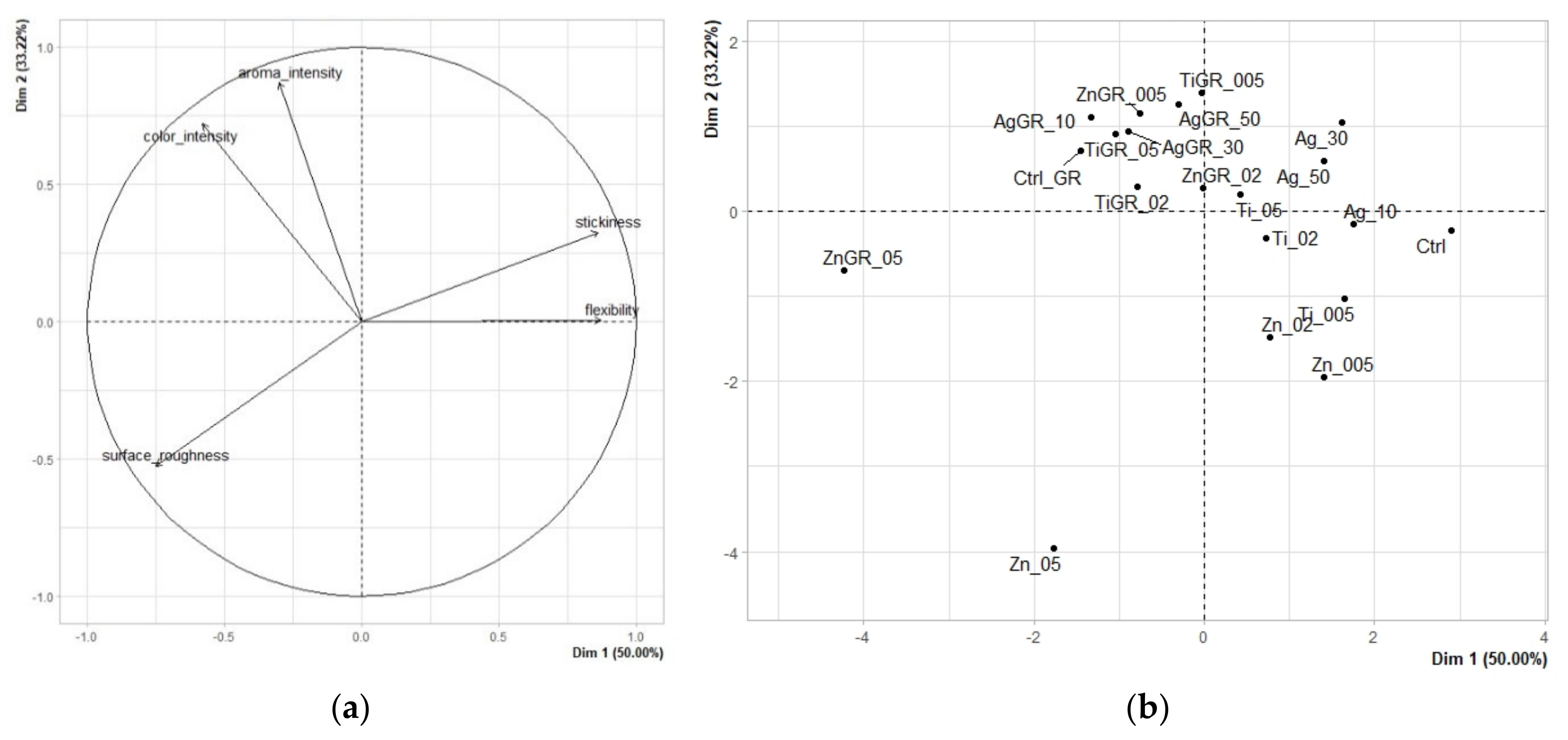
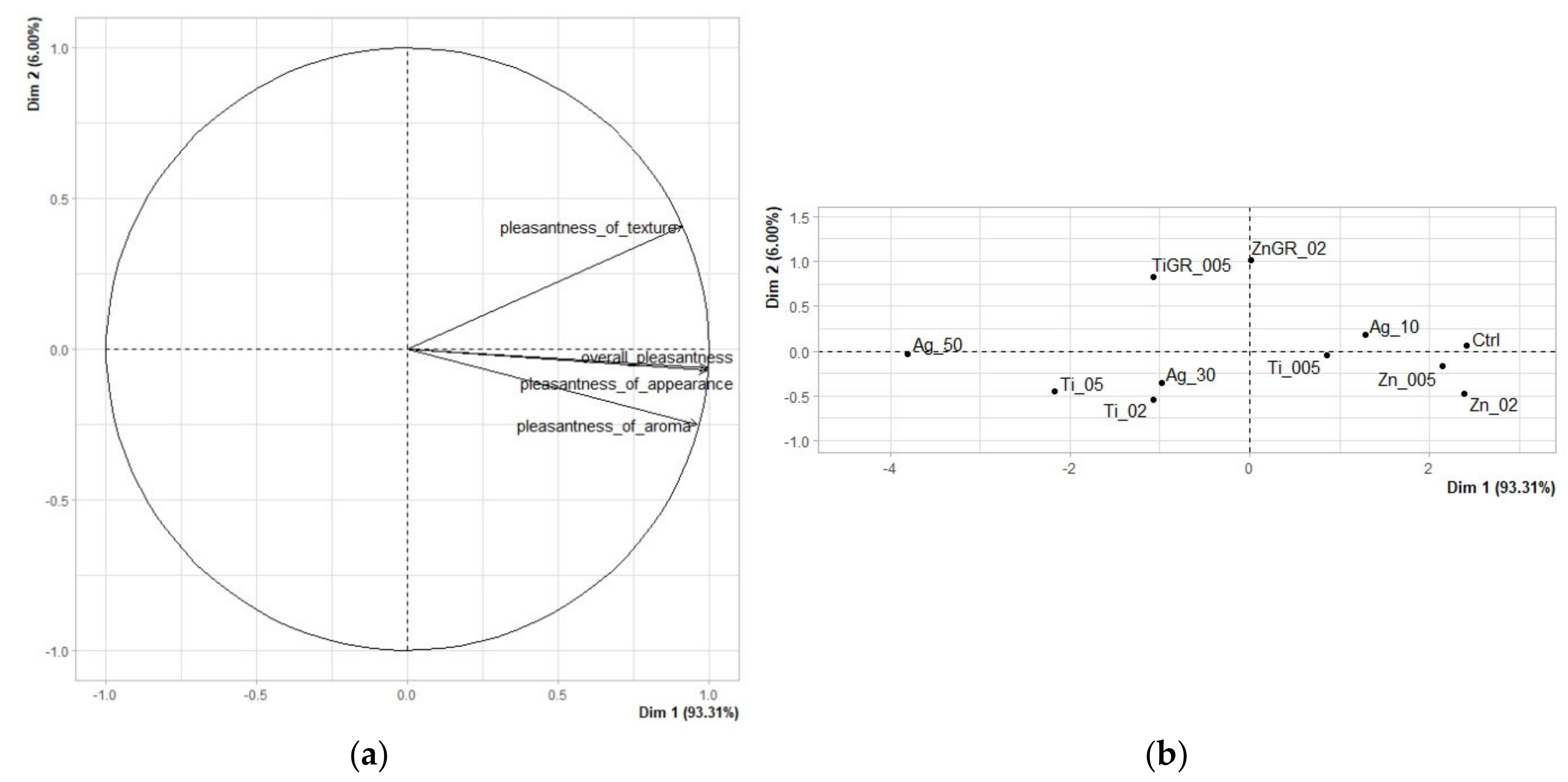
3.1. Results of Sensory Analysis of Edible Packaging
3.1.1. Quantitative Descriptive Analysis
3.1.2. Hedonic Analysis
3.1.3. Assessment of the Probability of Purchasing Food Commodities in Edible Packaging
3.2. Evaluation of Color and Color Properties of Packaging
3.2.1. Opacity
3.2.2. Whiteness Index; Yellowness Index
3.3. Evaluation of the Surface of Edible Packaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the Performance of Edible Food Packaging Films by Using Nanocellulose as an Additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Darmajana, D.A.; Afifah, N.; Solihah, E.; Indriyanti, N. Effects of Carrageenan Edible Coating on Fresh Cut Melon Quality in Cold Storage. Agritech 2017, 37, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent Advance in Edible Coating and Its Effect on Fresh/Fresh-Cut Fruits Quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- La, D.D.; Nguyen-Tri, P.; Le, K.H.; Nguyen, P.T.M.; Nguyen, M.D.-B.; Vo, A.T.K.; Nguyen, M.T.H.; Chang, S.W.; Tran, L.D.; Chung, W.J.; et al. Effects of Antibacterial ZnO Nanoparticles on the Performance of a Chitosan/Gum Arabic Edible Coating for Post-Harvest Banana Preservation. Prog. Org. Coat. 2021, 151, 106057. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nano-Food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J. Food Sci. 2015, 80, R910–R923. [Google Scholar] [CrossRef]
- Pushparaj, K.; Liu, W.-C.; Meyyazhagan, A.; Orlacchio, A.; Pappusamy, M.; Vadivalagan, C.; Robert, A.A.; Arumugam, V.A.; Kamyab, H.; Klemeš, J.J.; et al. Nano- from Nature to Nurture: A Comprehensive Review on Facets, Trends, Perspectives and Sustainability of Nanotechnology in the Food Sector. Energy 2022, 240, 122732. [Google Scholar] [CrossRef]
- Trajkovska Petkoska, A.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible Packaging: Sustainable Solutions and Novel Trends in Food Packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology Application in Food Packaging: A Plethora of Opportunities versus Pending Risks Assessment and Public Concerns. Food Res. Int. 2020, 137, 109664. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Sudhani, H.P.K.; Bajpai, V.K.; Chen, L.; Shukla, S.; Mukherjee, A. Plant Extract Mediated Silver Nanoparticles and Their Applications as Antimicrobials and in Sustainable Food Packaging: A State-of-the-Art Review. Trends Food Sci. Technol. 2021, 112, 651–666. [Google Scholar] [CrossRef]
- Bahrami, A.; Mokarram, R.R.; Khiabani, M.S.; Ghanbarzadeh, B.; Salehi, R. Physico-Mechanical and Antimicrobial Properties of Tragacanth/Hydroxypropyl Methylcellulose/Beeswax Edible Films Reinforced with Silver Nanoparticles. Int. J. Biol. Macromol. 2019, 129, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Oyom, W.; Zhang, Z.; Bi, Y.; Tahergorabi, R. Application of Starch-Based Coatings Incorporated with Antimicrobial Agents for Preservation of Fruits and Vegetables: A Review. Prog. Org. Coat. 2022, 166, 106800. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible Coatings to Incorporate Active Ingredients to Fresh-Cut Fruits: A Review. Trends Food Sci. Technol. 2009, 20, 438–447. [Google Scholar] [CrossRef]
- Dordevic Jancikova, S.; Dordević, D.; Sedlacek, P.; Kalina, M.; Těšíková, K.; Antonic, B.; Tremlová, B.; Treml, J.; Nejezchlebova, M.; Vapenka, L.; et al. Incorporation of Natural Blueberry, Red Grapes and Parsley Extract By-Products into the Production of Chitosan Edible Films. Polymers 2021, 13, 3388. [Google Scholar] [CrossRef] [PubMed]
- Tauferova, A.; Pospiech, M.; Javurkova, Z.; Tremlova, B.; Dordevic, D.; Jancikova, S.; Tesikova, K.; Zdarsky, M.; Vitez, T.; Vitezova, M. Plant Byproducts as Part of Edible Coatings: A Case Study with Parsley, Grape and Blueberry Pomace. Polymers 2021, 13, 2578. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.A.; Lopez, D.A.; Tapia, M.J.; Enrione, J.; Skurtys, O.; Pedreschi, F.; Brown, D.I.; Creixell, W.; Osorio, F. Using RGB Image Processing for Designing an Alginate Edible Film. Food Bioprocess Technol. 2012, 5, 1511–1520. [Google Scholar] [CrossRef]
- Sharma, G. Color Fundamentals for Digital Imaging, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9781315220086. [Google Scholar]
- Luo, M.R. The CIE 2000 Colour Difference Formula: CIEDE2000. In Proceedings of the AIC: 9th Congress of the International Colour Association, Rochester, NY, USA, 24–29 June 2001; Volume 4421, pp. 554–559. [Google Scholar]
- Navarro, R.; Arancibia, C.; Lidia Herrera, M.; Matiacevich, S. Effect of Type of Encapsulating Agent on Physical Properties of Edible Films Based on Alginate and Thyme Oil. Food Bioprod. Processing 2016, 97, 63–75. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Sun, Y.; Chen, B.; Wu, X.; Le, T. Improvement of Banana Postharvest Quality Using a Novel Soybean Protein Isolate/Cinnamaldehyde/Zinc Oxide Bionanocomposite Coating Strategy. Sci. Hortic. 2019, 258, 108786. [Google Scholar] [CrossRef]
- Saberi, B.; Thakur, R.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Optimization of Physical and Optical Properties of Biodegradable Edible Films Based on Pea Starch and Guar Gum. Ind. Crops Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Yu, W.; Zhao, L.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Study on the Volatile Composition of Table Grapes of Three Aroma Types. LWT 2019, 115, 108450. [Google Scholar] [CrossRef]
- Zifkin, M.; Jin, A.; Ozga, J.A.; Zaharia, L.I.; Schernthaner, J.P.; Gesell, A.; Abrams, S.R.; Kennedy, J.A.; Constabel, C.P. Gene Expression and Metabolite Profiling of Developing Highbush Blueberry Fruit Indicates Transcriptional Regulation of Flavonoid Metabolism and Activation of Abscisic Acid Metabolism. Plant Physiol. 2012, 158, 200–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marvizadeh, M.M.; Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Jokar, M. Preparation and Characterization of Bionanocomposite Film Based on Tapioca Starch/Bovine Gelatin/Nanorod Zinc Oxide. Int. J. Biol. Macromol. 2017, 99, 1–7. [Google Scholar] [CrossRef]
- Mantilla, N.; Castell-Perez, M.E.; Gomes, C.; Moreira, R.G. Multilayered Antimicrobial Edible Coating and Its Effect on Quality and Shelf-Life of Fresh-Cut Pineapple (Ananas Comosus). LWT Food Sci. Technol. 2013, 51, 37–43. [Google Scholar] [CrossRef]
- Zhao, Y.; McDaniel, M. 24—Sensory Quality of Foods Associated with Edible Film and Coating Systems and Shelf-Life Extension. In Innovations in Food Packaging; Han, J.H., Ed.; Food Science and Technology; Academic Press: London, UK, 2005; pp. 434–453. ISBN 978-0-12-311632-1. [Google Scholar]
- Marchiore, N.G.; Manso, I.J.; Kaufmann, K.C.; Lemes, G.F.; de Oliveira Pizolli, A.P.; Droval, A.A.; Bracht, L.; Gonçalves, O.H.; Leimann, F.V. Migration Evaluation of Silver Nanoparticles from Antimicrobial Edible Coating to Sausages. LWT Food Sci. Technol. 2017, 76, 203–208. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wang, L.F.; Hong, S.I. Preparation and Characterization of Agar/Silver Nanoparticles Composite Films with Antimicrobial Activity. Food Hydrocoll. 2013, 33, 327–335. [Google Scholar] [CrossRef]
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough Evaluation of Sweet Potato Starch and Lemon-Waste Pectin Based-Edible Films with Nano-Titania Inclusions for Food Packaging Applications. Int. J. Biol. Macromol. 2019, 139, 449–458. [Google Scholar] [CrossRef]
- Hou, X.; Xue, Z.; Liu, J.; Yan, M.; Xia, Y.; Ma, Z. Characterization and Property Investigation of Novel Eco-friendly Agar/Carrageenan/TiO2 Nanocomposite Films. J. Appl. Polym. Sci. 2019, 136, 47113. [Google Scholar] [CrossRef]
- Wardana, A.A.; Suyatma, N.E.; Muchtadi, T.R.; Yaliani, S. Influence of ZnO Nanoparticles and Stearic Acid on Physical, Mechanical and Structural Properties of Cassava Starch-Based Bionanocomposite Edible Films. Int. Food Res. J. 2018, 25, 1837–1844. [Google Scholar]
- Bakhy, E.A.; Zidan, N.S.; Aboul-Anean, H.E.D. The Effect of Nano Materials On Edible Coating and Films’ Improvement. Int. J. Pharm. Res. Allied Sci. 2018, 7, 20–41. [Google Scholar]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable Hybrid Nanocomposites of Chitosan/Gelatin and Silver Nanoparticles for Active Food Packaging Applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of Antioxidant and Antimicrobial Coating Based on Whey Protein Nanofibrils with TiO2 Nanotubes on the Quality and Shelf Life of Chilled Meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Jiang, Y.; Liu, F.; Ren, F.; Zhao, G.; Leng, X. Fabrication and Characterization of TiO2/Whey Protein Isolate Nanocomposite Film. Food Hydrocoll. 2011, 25, 1098–1104. [Google Scholar] [CrossRef]
- Gohargani, M.; Lashkari, H.; Shirazinejad, A. Study on Biodegradable Chitosan-Whey Protein-Based Film Containing Bionanocomposite TiO2and Zataria Multiflora Essential Oil. J. Food Qual. 2020, 2020, 8844167. [Google Scholar] [CrossRef]
- Le, K.H.; Dac-Binh Nguyen, M.; Dai Tran, L.; Phuong, H.; Thi, N.; Van Tran, C.; Van Tran, K.; Phuong, H.; Thi, N.D.; Yoon, Y.S.; et al. A Novel Antimicrobial ZnO Nanoparticles-Added Polysaccharide Edible Coating for the Preservation of Postharvest Avocado under Ambient Conditions. Prog. Org. Coat. 2021, 158, 106339. [Google Scholar] [CrossRef]
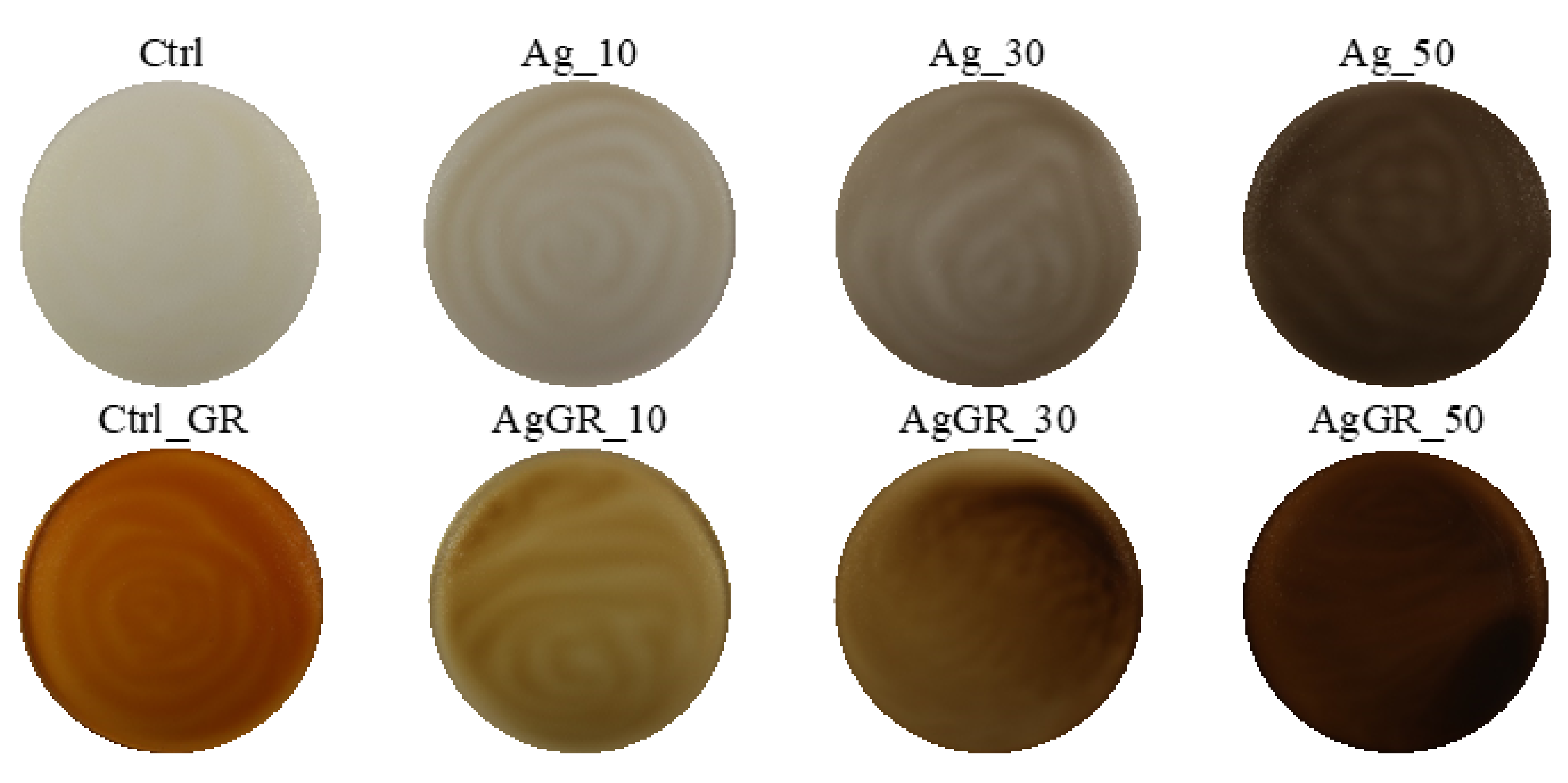
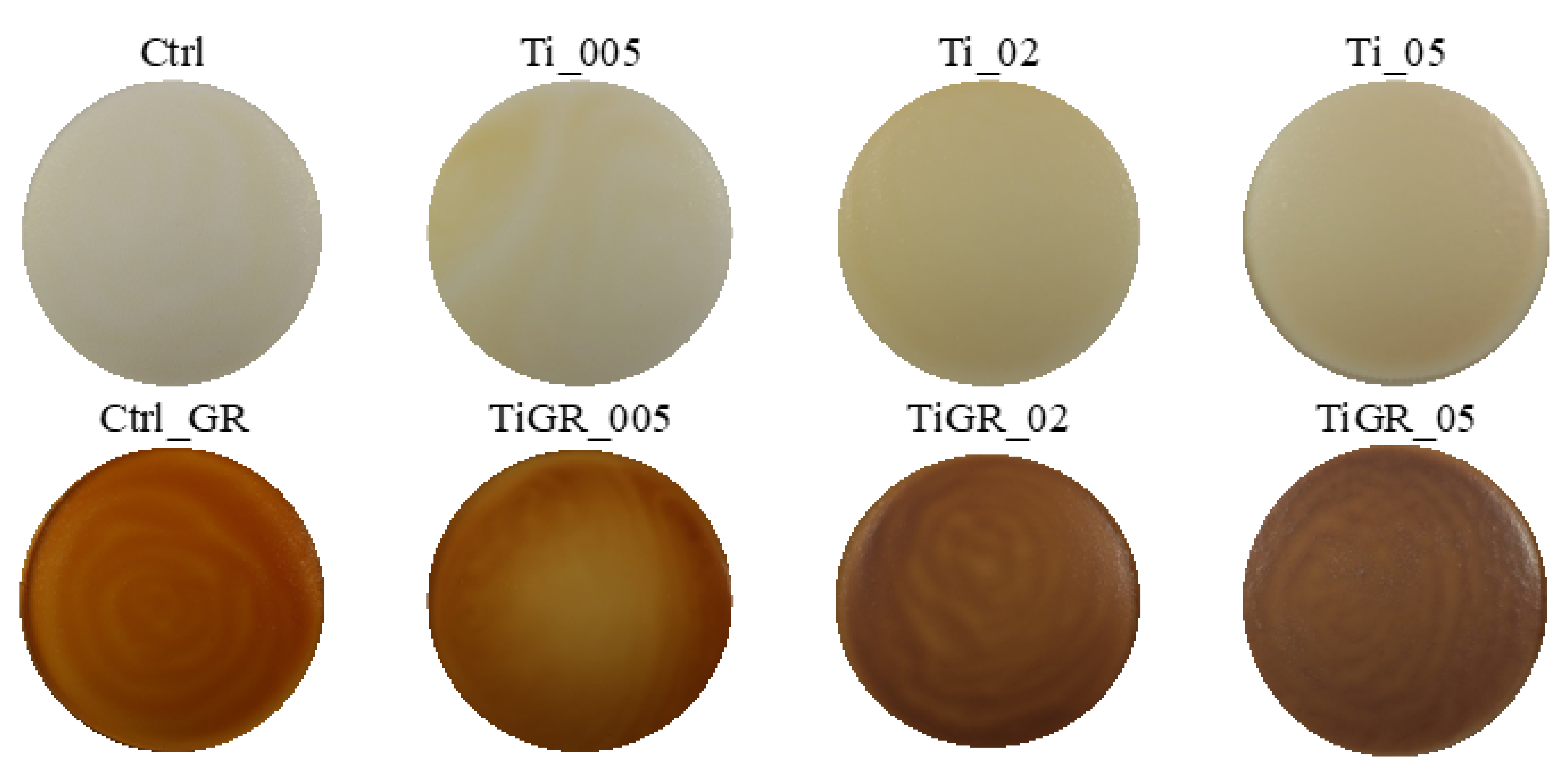








| Sample | Composition |
|---|---|
| Ctrl | Chitosan + 1% lactic acid + glycerol |
| CtrlGR | Chitosan + 1% lactic acid + 10% extract * + glycerol |
| Zn_005 | Chitosan + 1% lactic acid + 0.05% nano ZnO + glycerol |
| Zn_02 | Chitosan + 1% lactic acid + 0.2% nano ZnO + glycerol |
| Zn_05 | Chitosan + 1% lactic acid + 0.5% nano ZnO + glycerol |
| Ti_005 | Chitosan + 1% lactic acid + 0.05% nano TiO2 + glycerol |
| Ti_02 | Chitosan + 1% lactic acid + 0.2% nano TiO2 + glycerol |
| Ti_05 | Chitosan + 1% lactic acid + 0.5% nano TiO2 + glycerol |
| Ag_10 | Chitosan + 1% lactic acid + colloidal Ag 10 ppm + glycerol |
| Ag_30 | Chitosan + 1% lactic acid + colloidal Ag 30 ppm + glycerol |
| Ag_50 | Chitosan + 1% lactic acid + colloidal Ag 50 ppm + glycerol |
| ZnGR_005 | Chitosan + 1% lactic acid + 0.05% nano ZnO + 10% extract * + glycerol |
| ZnGR_02 | Chitosan + 1% lactic acid + 0.2% nano ZnO + 10% extract * + glycerol |
| ZnGR_05 | Chitosan + 1% lactic acid + 0.5% nano ZnO + 10% extract * + glycerol |
| TiGR_005 | Chitosan + 1% lactic acid + 0.05% nano TiO2 + 10% extract * + glycerol |
| TiGR_02 | Chitosan + 1% lactic acid + 0.2% nano TiO2 + 10% extract * + glycerol |
| TiGR_05 | Chitosan + 1% lactic acid + 0.5% nano TiO2 + 10% extract * + glycerol |
| AgGR_10 | Chitosan + 1% lactic acid + colloidal Ag 10 ppm + 10% extract * + glycerol |
| AgGR_30 | Chitosan + 1% lactic acid + colloidal Ag 30 ppm + 10% extract * + glycerol |
| AgGR_50 | Chitosan + 1% lactic acid + colloidal Ag 50 ppm + 10% extract * + glycerol |
| Flexibility | Stickiness | Aroma Intensity | Color Intensity | Surface Roughness | |
|---|---|---|---|---|---|
| Ctrl | 8.041 | 4.625 | 3.636 | 2.618 | 2.986 |
| Ag_10 | 8.005 | 3.630 | 4.216 | 3.158 | 2.592 |
| Ti_005 | 7.605 | 3.830 | 3.416 | 3.658 | 3.592 |
| Ag_30 | 8.205 | 4.330 | 5.216 | 4.458 | 3.092 |
| Zn_005 | 6.705 | 3.330 | 3.016 | 1.858 | 3.592 |
| Ag_50 | 8.705 | 4.330 | 4.616 | 5.858 | 4.092 |
| Zn_02 | 7.705 | 2.830 | 3.916 | 2.058 | 4.492 |
| Ti_02 | 7.905 | 3.13 | 3.716 | 6.358 | 3.492 |
| Ti_05 | 7.605 | 3.13 | 4.116 | 6.758 | 3.592 |
| ZnGR_02 | 7.705 | 2.73 | 4.616 | 6.558 | 3.992 |
| TiGR_005 | 4.405 | 3.43 | 4.616 | 7.958 | 1.892 |
| AgGR_50 | 5.205 | 3.33 | 4.816 | 8.958 | 3.192 |
| ZnGR_005 | 4.905 | 3.13 | 4.916 | 7.658 | 3.492 |
| TiGR_02 | 6.605 | 2.93 | 4.416 | 8.558 | 5.192 |
| AgGR_30 | 4.305 | 3.33 | 4.616 | 8.658 | 3.892 |
| TiGR_05 | 6.305 | 2.73 | 5.116 | 8.558 | 4.792 |
| Zn_05 | 4.005 | 2.03 | 2.516 | 2.858 | 8.392 |
| CtrlGR | 3.405 | 3.13 | 4.816 | 7.758 | 4.292 |
| AgGR_10 | 4.605 | 3.23 | 5.416 | 8.058 | 4.692 |
| ZnGR_05 | 1.705 | 1.63 | 5.316 | 7.358 | 8.492 |
| Texture Pleasantness | Aroma Pleasantness | Appearance Pleasantness | Overall Pleasantness | |
|---|---|---|---|---|
| Ag_50 | 5.800 | 4.708 | 3.031 | 4.569 |
| Ti_05 | 6.000 | 5.123 | 4.185 | 5.262 |
| TiGR_005 | 6.677 | 5.031 | 4.785 | 5.708 |
| Ti_02 | 6.215 | 5.385 | 4.815 | 5.723 |
| Ag_30 | 6.308 | 5.369 | 4.785 | 5.769 |
| ZnGR_02 | 7.031 | 5.308 | 5.215 | 6.000 |
| Ti_005 | 6.754 | 5.538 | 6.262 | 6.723 |
| Ag_10 | 6.954 | 5.631 | 6.415 | 6.769 |
| Zn_005 | 6.985 | 5.815 | 7.138 | 7.262 |
| Zn_02 | 6.938 | 5.954 | 7.323 | 7.323 |
| Ctrl | 7.169 | 5.908 | 7.031 | 7.246 |
| Meat Products | Milk Products (Cheese) | Bakery Products | Fruit | Vegetables | |
|---|---|---|---|---|---|
| Ag_50 | 1.865 | 1.676 | 1.703 | 1.595 | 1.595 |
| ZnGR_02 | 3.081 | 2.027 | 2.270 | 2.189 | 2.162 |
| Ti_05 | 2.351 | 3.351 | 2.081 | 2.162 | 2.162 |
| TiGR_005 | 3.459 | 1.946 | 2.162 | 2.243 | 2.081 |
| Ag_30 | 2.676 | 2.324 | 2.514 | 2.432 | 2.405 |
| Ti_02 | 2.270 | 3.378 | 2.351 | 2.514 | 2.459 |
| Ag_10 | 2.946 | 2.784 | 2.649 | 2.919 | 3.000 |
| Ti_005 | 2.973 | 3.405 | 2.973 | 3.216 | 3.270 |
| Ctrl | 3.000 | 3.405 | 2.649 | 3.676 | 3.459 |
| Zn_02 | 3.703 | 3.595 | 3.378 | 4.000 | 3.838 |
| Zn_005 | 3.730 | 3.811 | 3.432 | 4.324 | 4.297 |
| L* | a* | b* | ΔE | Opacity | Whiteness Index | Yellowness Index | |
|---|---|---|---|---|---|---|---|
| Ag_10 | 56.15 ± 0.21 | 0.56 ± 0.04 | 11.90 ± 0.08 | 9.63 ± 0.31 l | 17.57 ± 0.37 | 54.56 ± 0.20 | 30.28 ± 0.21 |
| Ag_30 | 41.36 ± 0.18 | 2.66 ± 0.02 | 13.44 ± 0.09 | 25.11 ± 0.36 | 26.11 ± 0.47 | 39.78 ± 0.16 | 46.43 ± 0.16 v |
| Ag_50 | 17.19 ± 0.24 | 5.43 ± 0.05 e | 12.76 ± 0.09 | 45.88 ± 0.41 | 66.86 ± 1.67 | 16.04 ± 0.22 t | 106.09 ± 0.87 |
| AgGR_10 | 42.26 ± 0.28 | 5.33 ± 0.12 e | 35.58 ± 0.15 | 15.57 ± 0.17 | 12.95 ± 0.13 o | 31.97 ± 0.17 | 120.29 ± 0.45 |
| AgGR_30 | 24.11 ± 0.45 | 10.32 ± 0.24 f | 26.30 ± 0.16 | 12.43 ± 0.23 | 21.23 ± 1.03 | 19.02 ± 0.42 | 155.93 ± 2.38 |
| AgGR_50 | 9.06 ± 0.20 | 10.45 ± 0.14 f | 11.32 ± 0.30 g | 23.04 ± 0.40 | 45.35 ± 1.27 | 7.76 ± 0.14 | 178.45 ± 0.99 |
| Ti_005 | 64.66 ± 0.25 a | −2.20 ± 0.12 d | 17.12 ± 0.24 | 3.91 ± 0.13 i | 32.42 ± 0.32 | 60.67 ± 0.26 | 37.82 ± 0.56 |
| Ti_02 | 63.41 ± 0.28 | −1.76 ± 0.05 | 24.51 ± 0.09 | 7.75 ± 0.18 k | 70.54 ± 0.49 | 55.92 ± 0.26 | 55.23 ± 0.40 |
| Ti_05 | 64.27 ± 0.36 a | −0.51 ± 0.02 | 20.96 ± 0.09 h | 6.05 ± 0.20 j | 86.48 ± 0.85 | 58.57 ± 0.35 | 46.60 ± 0.44 v |
| TiGR_005 | 35.16 ± 0.30 | 17.93 ± 0.08 | 40.17 ± 0.23 | 5.37 ± 0.07 | 16.03 ± 0.35 s | 21.64 ± 0.13 u | 163.22 ± 0.47 |
| TiGR_02 | 30.62 ± 0.26 | 16.42 ± 0.05 | 26.91 ± 0.11 | 7.54 ± 0.27 k | 62.91 ± 1.01 | 23.80 ± 0.20 | 125.55 ± 0.59 |
| TiGR_05 | 33.41 ± 0.31 b | 13.46 ± 0.07 | 21.08 ± 0.13 h | 9.85 ± 0.11 l | 81.05 ± 0.96 | 28.87 ± 0.26 u | 90.14 ± 0.60 |
| Zn_005 | 68.42 ± 0.36 | −1.07 ± 0.05 | 8.03 ± 0.05 | 2.75 ± 0.25 | 14.65 ± 0.22 qr | 67.39 ± 0.34 | 16.78 ± 0.16 |
| Zn_02 | 68.66 ± 0.39 c | −0.82 ± 0.08 | 6.52 ± 0.09 | 3.88 ± 0.17 i | 15.01 ± 0.29 prs | 67.98 ± 0.40 | 13.56 ± 0.25 |
| Zn_05 | 70.33 ± 0.42 c | −0.28 ± 0.03 | 4.38 ± 0.10 | 6.05 ± 0.22 j | 24.06 ± 0.76 | 70.01 ± 0.42 | 8.90 ± 0.23 |
| ZnGR_005 | 47.00 ± 0.35 | 10.09 ± 0.15 | 46.77 ± 0.20 | 16.70 ± 0.45 | 8.38 ± 0.18 n | 28.60 ± 0.20 | 142.17 ± 0.70 |
| ZnGR_02 | 57.89 ± 0.42 | 0.16 ± 0.10 | 28.16 ± 0.09 | 29.09 ± 0.49 m | 10.45 ± 0.20 | 49.34 ± 0.32 | 69.50 ± 0.42 |
| ZnGR_05 | 52.11 ± 0.35 | −2.08 ± 0.04 d | 7.70 ± 0.06 | 28.67 ± 0.29 m | 89.04 ± 0.82 | 51.45 ± 0.35 | 21.11 ± 0.25 |
| Ctrl | 66.91 ± 0.37 | −1.41 ± 0.03 | 11.54 ± 0.10 g | - | 14.13 ± 0.55 opq | 64.93 ± 0.40 | 24.63 ± 0.32 |
| Ctrl_GR | 33.89 ± 0.43 a | 27.49 ± 0.18 | 43.91 ± 0.34 | - | 7.59 ± 0.20 n | 16.01 ± 0.16 t | 185.12 ± 0.96 |
| Sample | Nearest Object Distance (nm) | Sample | Nearest Object Distance (nm) |
|---|---|---|---|
| Ctrl | 38.25 ± 23.12 a | Ctrl | 43.95 ± 23.77 a |
| Ag_10 | 68.49 ± 25.32 b | AgGR_10 | 61.42 ± 23.95 b |
| Ag_30 | 54.27 ± 24.63 c | AgGR_30 | 57.56 ± 24.26 c |
| Ag_50 | 62.95 ± 24.77 d | AgGR_50 | 62.86 ± 23.42 d |
| Ti_005 | 46.11 ± 19.93 e | TiGR_005 | 50.18 ± 23.86 e |
| Ti_02 | 55.7 ± 24.56 f | TiGR_02 | 49.46 ± 23.84 f |
| Ti_05 | 63.26 ± 25.09 g | TiGR_05 | 45.84 ± 23.76 g |
| Zn_005 | 46.49 ± 24.07 h | ZnGR_005 | 43.48 ± 23.2 a |
| Zn_02 | 33.54 ± 27.62 e | ZnGR_02 | 40.36 ± 21.63 h |
| Zn_05 | 43.48 ± 23.2 i | ZnGR_05 | 93.06 ± 31.52 i |
| Sample | Area (µm2) | Sample | Area (µm2) |
|---|---|---|---|
| Ctrl | 0.52 ± 1.53 a | Ctrl | 1.04 ± 3.72 a |
| Ag_10 | 1.97 ± 3.47 b | AgGR_10 | 1.12 ± 1.65 b |
| Ag_30 | 1.5 ± 2.89 c | AgGR_30 | 1.55 ± 3.08 c |
| Ag_50 | 1.82 ± 3.87 d | AgGR_50 | 1.54 ± 2.94 d |
| Ti_005 | 1.77 ± 14.22 e | TiGR_005 | 1.71 ± 4.13 e |
| Ti_02 | 1.67 ± 3.95 e | TiGR_02 | 1.97 ± 5.78 e |
| Ti_05 | 0.97 ± 1.47 f | TiGR_05 | 1.41 ± 4 f |
| Zn_005 | 1.66 ± 6.02 g | ZnGR_005 | 1.31 ± 3.69 g |
| Zn_02 | 0.99 ± 1.93 g | ZnGR_02 | 0.6 ± 6 a |
| Zn_05 | 0.35 ± 3.65 h | ZnGR_05 | 6.67 ± 6.59 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauferová, A.; Javůrková, Z.; Pospiech, M.; Koudelková Mikulášková, H.; Těšíková, K.; Dordevic, D.; Dordevic, S.; Tremlová, B. Nanoparticles and Plant By-Products for Edible Coatings Production: A Case Study with Zinc, Titanium, and Silver. Polymers 2022, 14, 2837. https://doi.org/10.3390/polym14142837
Tauferová A, Javůrková Z, Pospiech M, Koudelková Mikulášková H, Těšíková K, Dordevic D, Dordevic S, Tremlová B. Nanoparticles and Plant By-Products for Edible Coatings Production: A Case Study with Zinc, Titanium, and Silver. Polymers. 2022; 14(14):2837. https://doi.org/10.3390/polym14142837
Chicago/Turabian StyleTauferová, Alexandra, Zdeňka Javůrková, Matej Pospiech, Hana Koudelková Mikulášková, Karolína Těšíková, Dani Dordevic, Simona Dordevic, and Bohuslava Tremlová. 2022. "Nanoparticles and Plant By-Products for Edible Coatings Production: A Case Study with Zinc, Titanium, and Silver" Polymers 14, no. 14: 2837. https://doi.org/10.3390/polym14142837







