Chiroptical Characteristics of Nanosegregated Phases in Binary Mixture Consisting of Achiral Bent-Core Molecule and Bent-Core Base Main-Chain Polymer
Abstract
:1. Introduction
2. Experimental Section
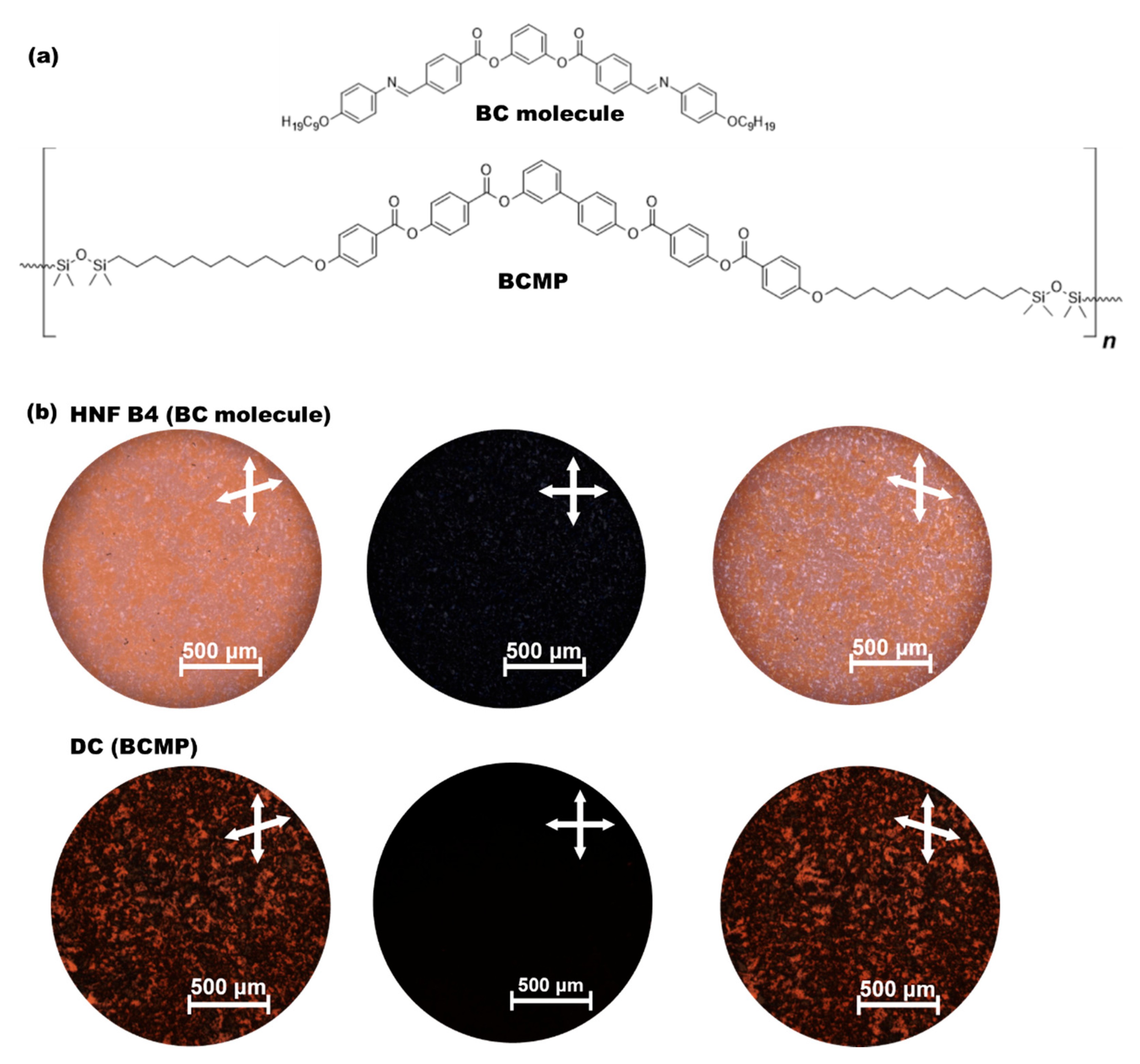
2.1. Materials
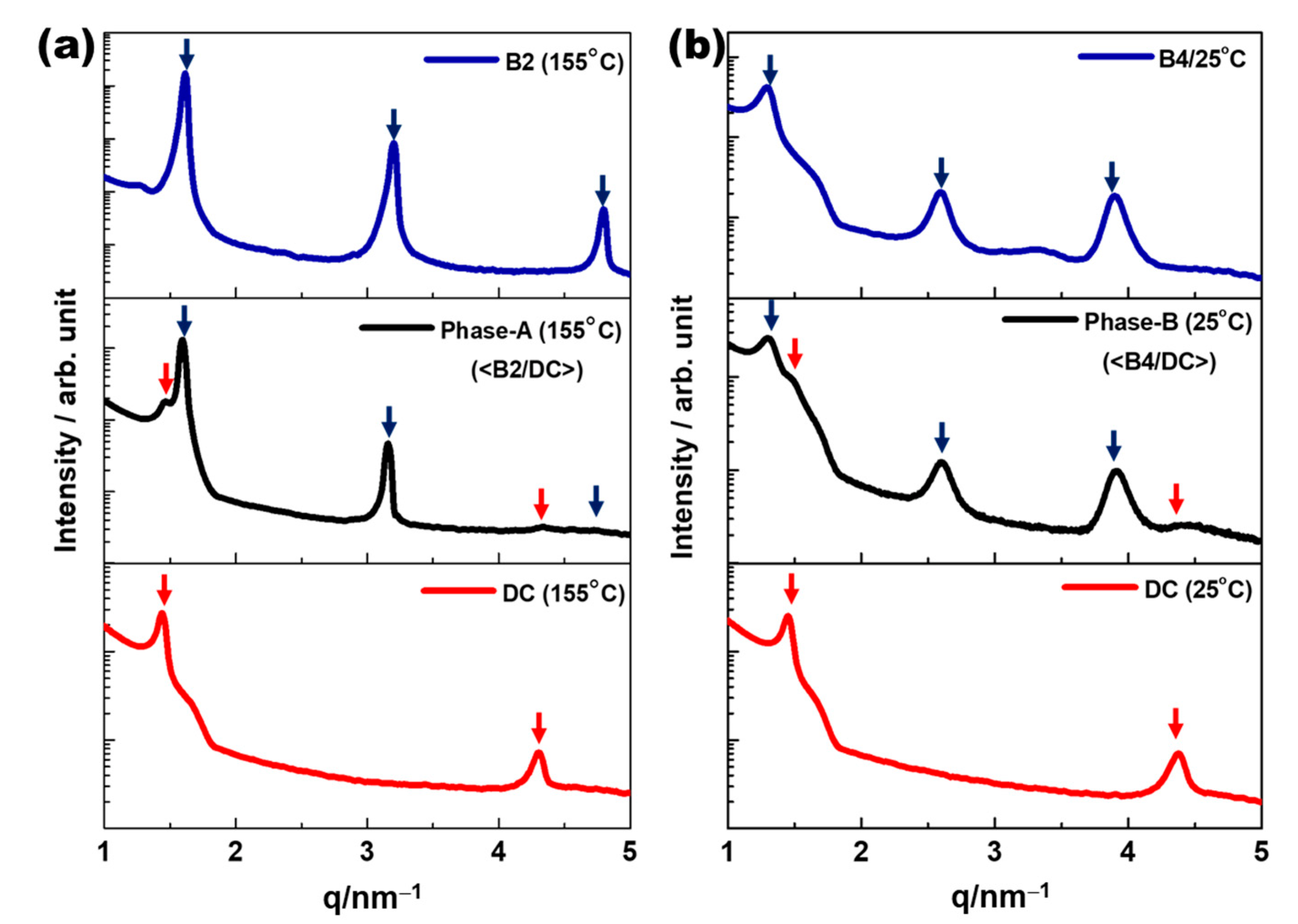
2.2. XRD Analysis
2.3. CD Measurements
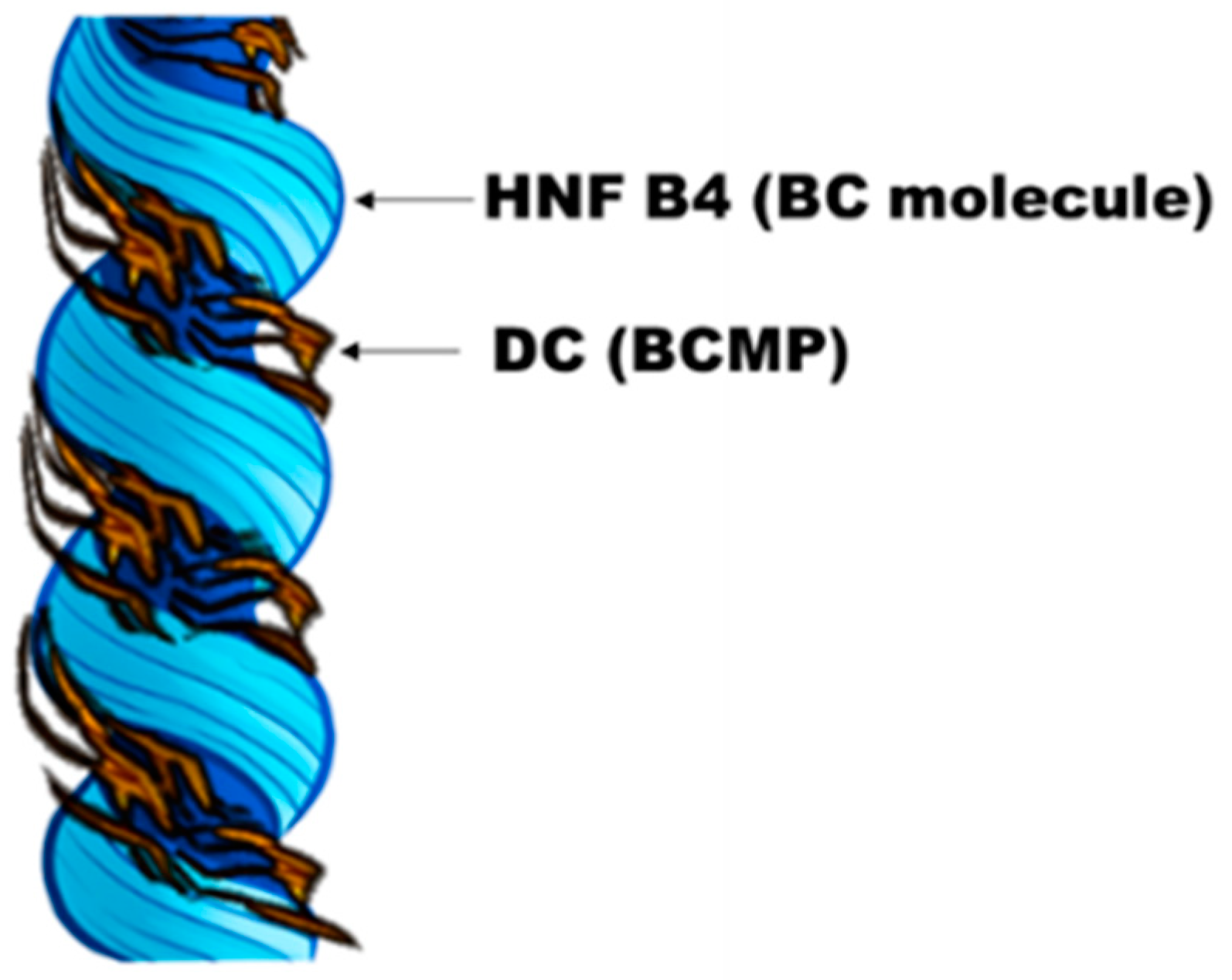
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sekine, T.; Niori, T.; Watanabe, J.; Furukawa, T.; Choi, S.W.; Takezoe, H. Spontaneous helix formation in smectic liquid crystals comprising achiral molecules. J. Mater. Chem. 1997, 7, 1307–1309. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Jo, S.Y.; Araoka, F.; Yoon, D.K.; Choi, S.W. Photomodulated supramolecular chirality in achiral photoresponsive rodlike compounds nanosegregated from the helical nanofilaments of achiral bent-core molecules. ACS Appl. Mater. Interfaces 2015, 7, 22686–22691. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Choi, H.J.; Lee, J.J.; Araoka, F.; Choi, S.W. Circularly polarized luminescence induced by chiral super nanospaces. Adv. Funct. Mater. 2019, 29, 1903246. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, B.C.; Choi, H.J.; Bae, S.; Araoka, F.; Choi, S.W. Inverse helical nanofilament networks serving as a chiral nanotemplate. ACS Nano 2020, 14, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Hough, L.E.; Spannuth, M.; Nakata, M.; Coleman, D.A.; Jones, C.D.; Dantlgraber, G.; Tschierske, C.; Watanabe, J.; Körblova, E.; Walba, D.M.; et al. Chiral isotropic liquids from achiral molecules. Science 2009, 325, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Hough, L.E.; Jung, H.T.; Krüerke, D.; Heberling, M.S.; Nakata, M.; Jones, C.D.; Chen, D.; Link, D.R.; Zasadzinski, J.; Heppke, G.; et al. Helical nanofilament phases. Science 2009, 325, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Maclennan, J.E.; Shao, R.; Yoon, D.K.; Wang, H.; Korblova, E.; Walba, D.M.; Glaser, M.A.; Clark, N.A. Chirality-preserving growth of helical filaments in the B4 phase of bent-core liquid crystals. J. Am. Chem. Soc. 2011, 133, 12656–12663. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Yoon, D.K. Orientation control of helical nanofilament phase and its chiroptical applications. Crystals 2020, 10, 675. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, D.; Shen, Y.; Jones, C.D.; Glaser, M.A.; Maclennan, J.E.; Clark, N.A. Nanophase segregation in binary mixtures of a bent-core and a rodlike liquid-crystal molecule. Phys. Rev. E 2010, 81, 011704. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, C. Mirror symmetry breaking in liquids and liquid crystals. Liq. Cryst. 2018, 45, 2221–2252. [Google Scholar] [CrossRef]
- Gimeno, N.; Sánchez-Ferrer, A.; Sebastián, N.; Mezzenga, R.; Ros, M.B. Bent-core based main-chain polymers showing the dark conglomerate liquid crystal phase. Macromolecules 2011, 44, 9586–9594. [Google Scholar] [CrossRef]
- Takezoe, H.; Takanishi, Y. Bent-core liquid crystals: Their mysterious and attractive world. Jpn. J. Appl. Phys. 2006, 45, 597–625. [Google Scholar] [CrossRef]
- Reddy, R.A.; Tschierske, C. Bent-core liquid crystals: Polar order, superstructural chirality and spontaneous desymmetrisation in soft matter systems. J. Mater. Chem. 2006, 16, 907–961. [Google Scholar] [CrossRef]
- Le, K.V.; Takezoe, H.; Araoka, F. Chiral superstructure mesophases of achiral bent-shaped molecules—Hierarchical chirality amplification and physical properties. Adv. Mater. 2017, 29, 27966798. [Google Scholar] [CrossRef] [PubMed]
- Walba, D.M.; Eshdat, L.; Körblova, E.; Shoemaker, R.K. On the nature of the B4 banana phase: crystal or not a crystal? Cryst. Growth Des. 2005, 5, 2091–2099. [Google Scholar] [CrossRef]
- Takanishi, Y.; Shin, G.J.; Jung, J.C.; Choi, S.W.; Ishikawa, K.; Watanabe, J.; Takezoe, H.; Toledano, P. Observation of very large chiral domains in a liquid crystal phase formed by mixtures of achiral bent-core and rod molecules. J. Mater. Chem. 2005, 15, 4020–4024. [Google Scholar] [CrossRef]
- Otani, T.; Araoka, F.; Ishikawa, K.; Takezoe, H. Enhanced optical activity by achiral rod-like molecules nanosegregated in the B4 structure of achiral bent-core molecules. J. Am. Chem. Soc. 2009, 131, 12368–12372. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Kim, D.Y.; Araoka, F.; Jeong, K.U.; Choi, S.W. Nanosegregated chiral materials with self-assembled hierarchical mesophases: Effect of thermotropic and photoinduced polymorphism in rodlike molecules. Chem. Eur. J. 2017, 23, 17794–17799. [Google Scholar] [CrossRef] [PubMed]
- Akutakawa, T.; Matsunaga, Y.; Yashuhara, K. Mesomorphic behaviour of 1,3-phenylene bis[4-(4-alkoxyphenyliminomethyl) benzoates] and related compounds. Liq. Cryst. 1994, 17, 659–666. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Lee, J.-J.; Choi, S.-W. Chiroptical Characteristics of Nanosegregated Phases in Binary Mixture Consisting of Achiral Bent-Core Molecule and Bent-Core Base Main-Chain Polymer. Polymers 2022, 14, 2823. https://doi.org/10.3390/polym14142823
Kim J-Y, Lee J-J, Choi S-W. Chiroptical Characteristics of Nanosegregated Phases in Binary Mixture Consisting of Achiral Bent-Core Molecule and Bent-Core Base Main-Chain Polymer. Polymers. 2022; 14(14):2823. https://doi.org/10.3390/polym14142823
Chicago/Turabian StyleKim, Ju-Yong, Jae-Jin Lee, and Suk-Won Choi. 2022. "Chiroptical Characteristics of Nanosegregated Phases in Binary Mixture Consisting of Achiral Bent-Core Molecule and Bent-Core Base Main-Chain Polymer" Polymers 14, no. 14: 2823. https://doi.org/10.3390/polym14142823
APA StyleKim, J.-Y., Lee, J.-J., & Choi, S.-W. (2022). Chiroptical Characteristics of Nanosegregated Phases in Binary Mixture Consisting of Achiral Bent-Core Molecule and Bent-Core Base Main-Chain Polymer. Polymers, 14(14), 2823. https://doi.org/10.3390/polym14142823






