Hemp Stem Epidermis and Cuticle: From Waste to Starter in Bio-Based Material Development
Abstract
:1. Introduction
2. Materials and Methods
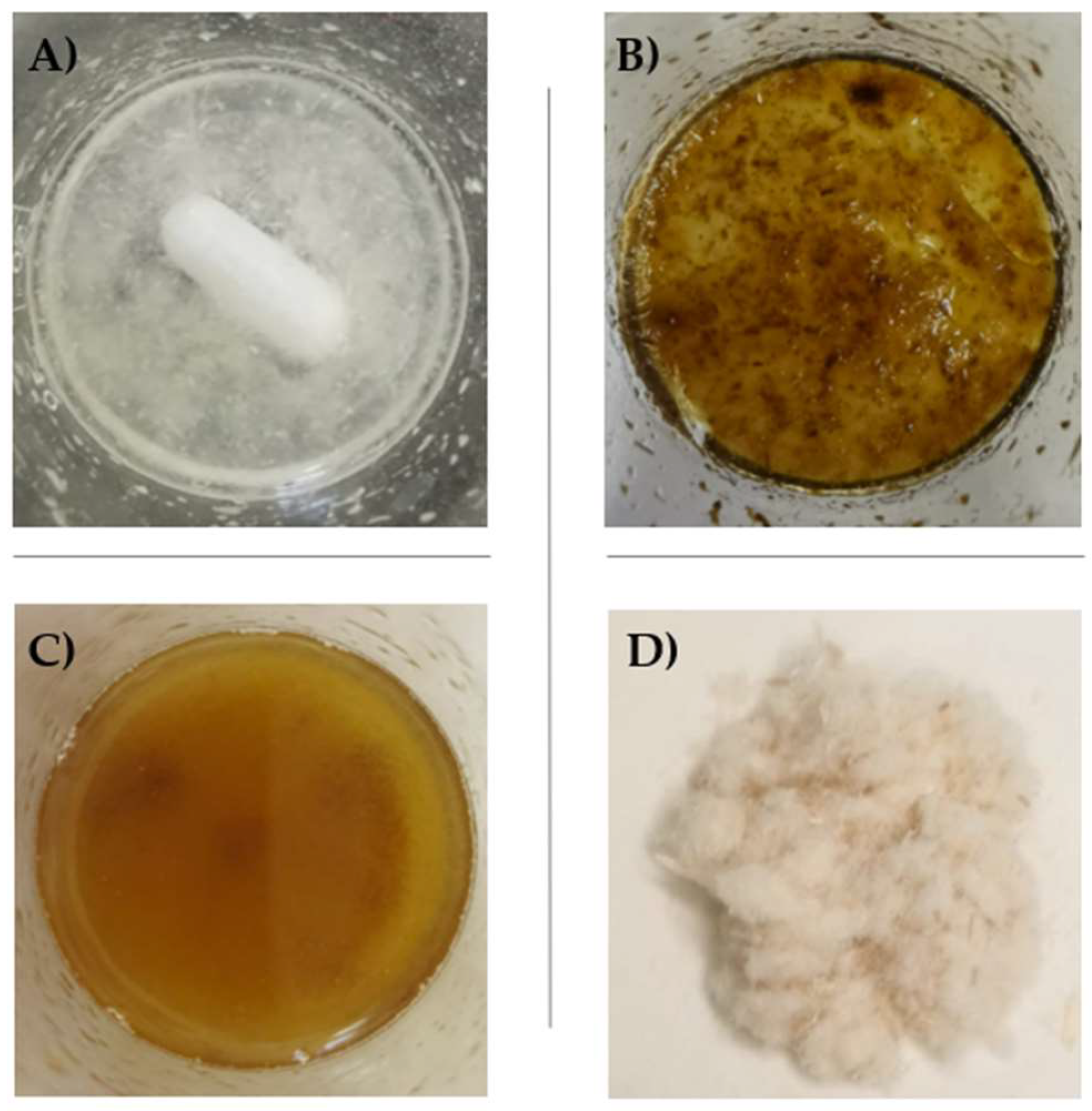
2.1. Hemp Cellulose (HC) Extraction
2.2. Hemp Cellulose Functionalization
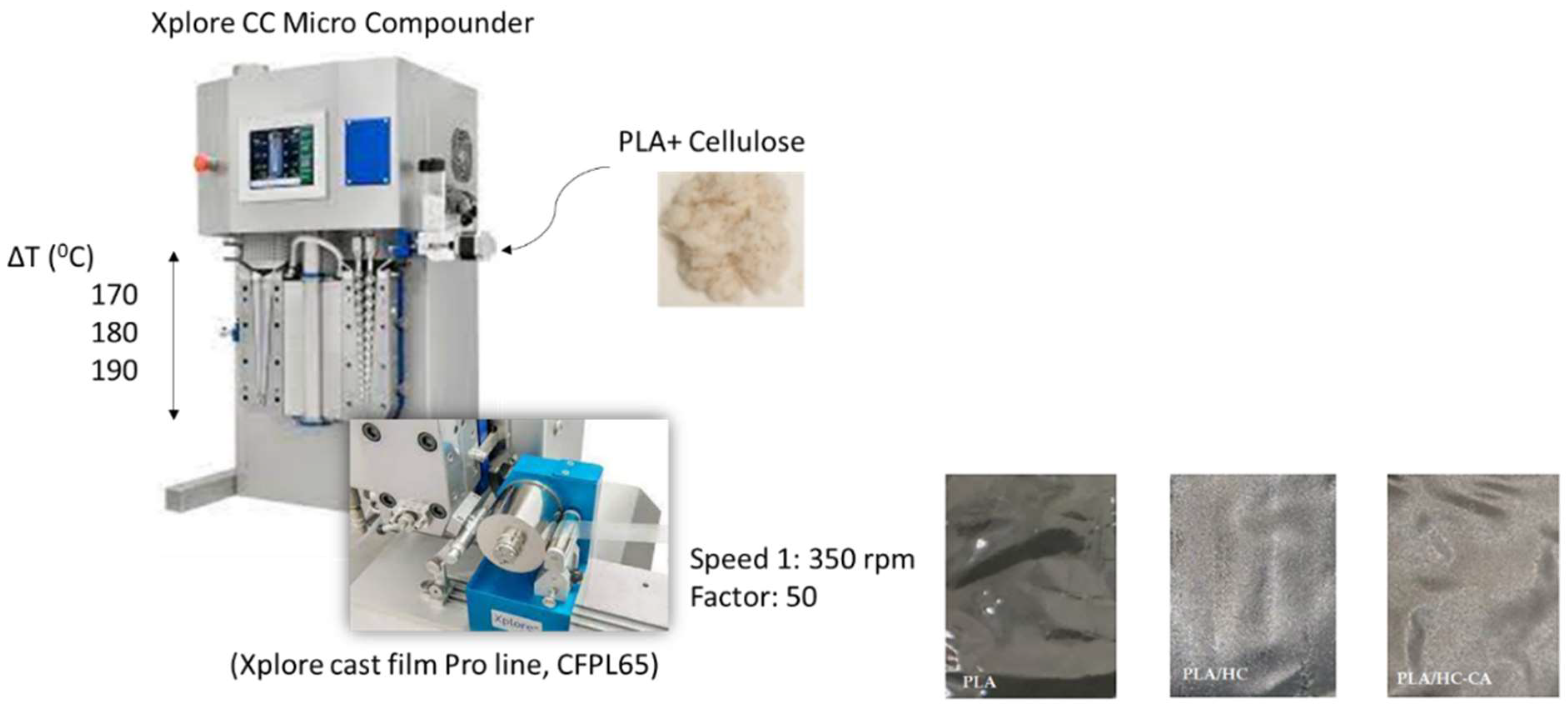
2.3. PLA-Based Biocomposite Films Preparation
2.4. Characterization
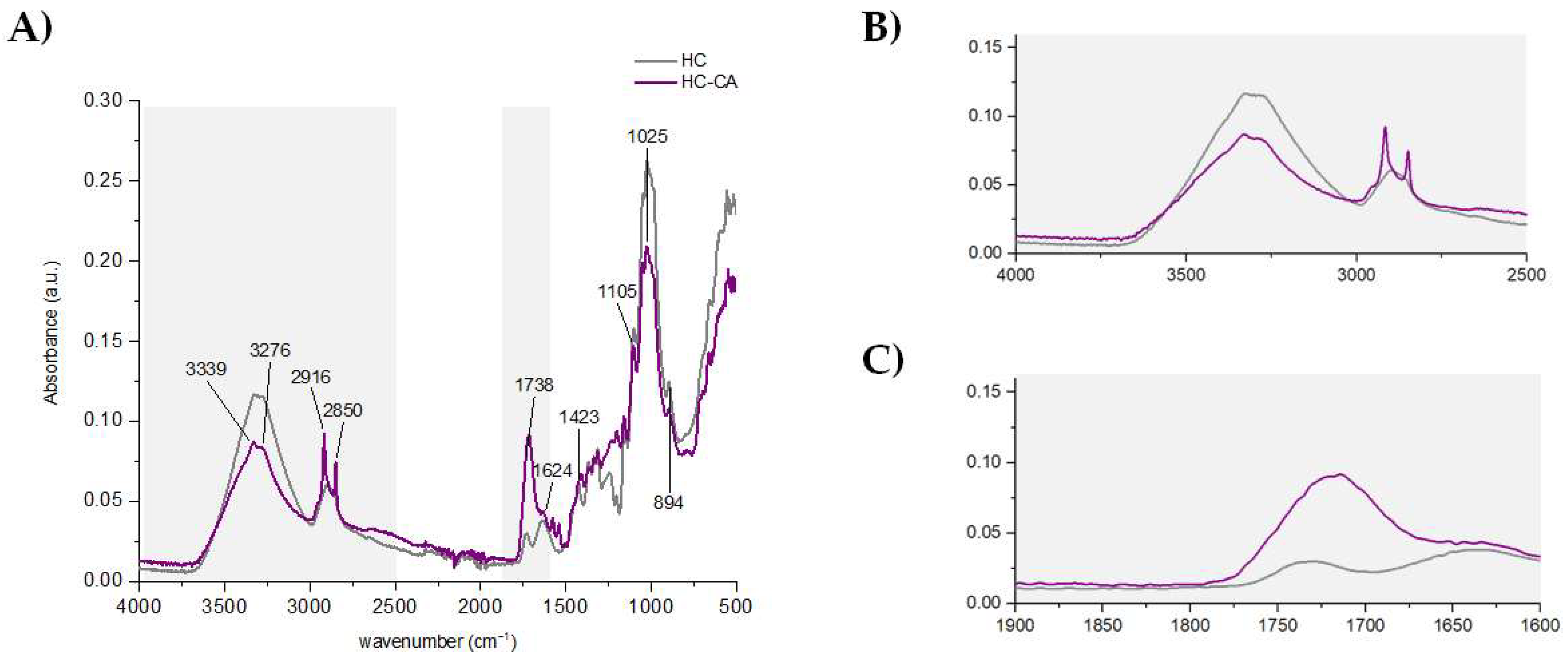
2.4.1. ATR-FTIR
2.4.2. X-ray Diffraction (XRD)
2.4.3. Transmission Electron Microscopy (TEM)
2.4.4. Field Emission Scanning Electron Microscopy (FE-SEM)
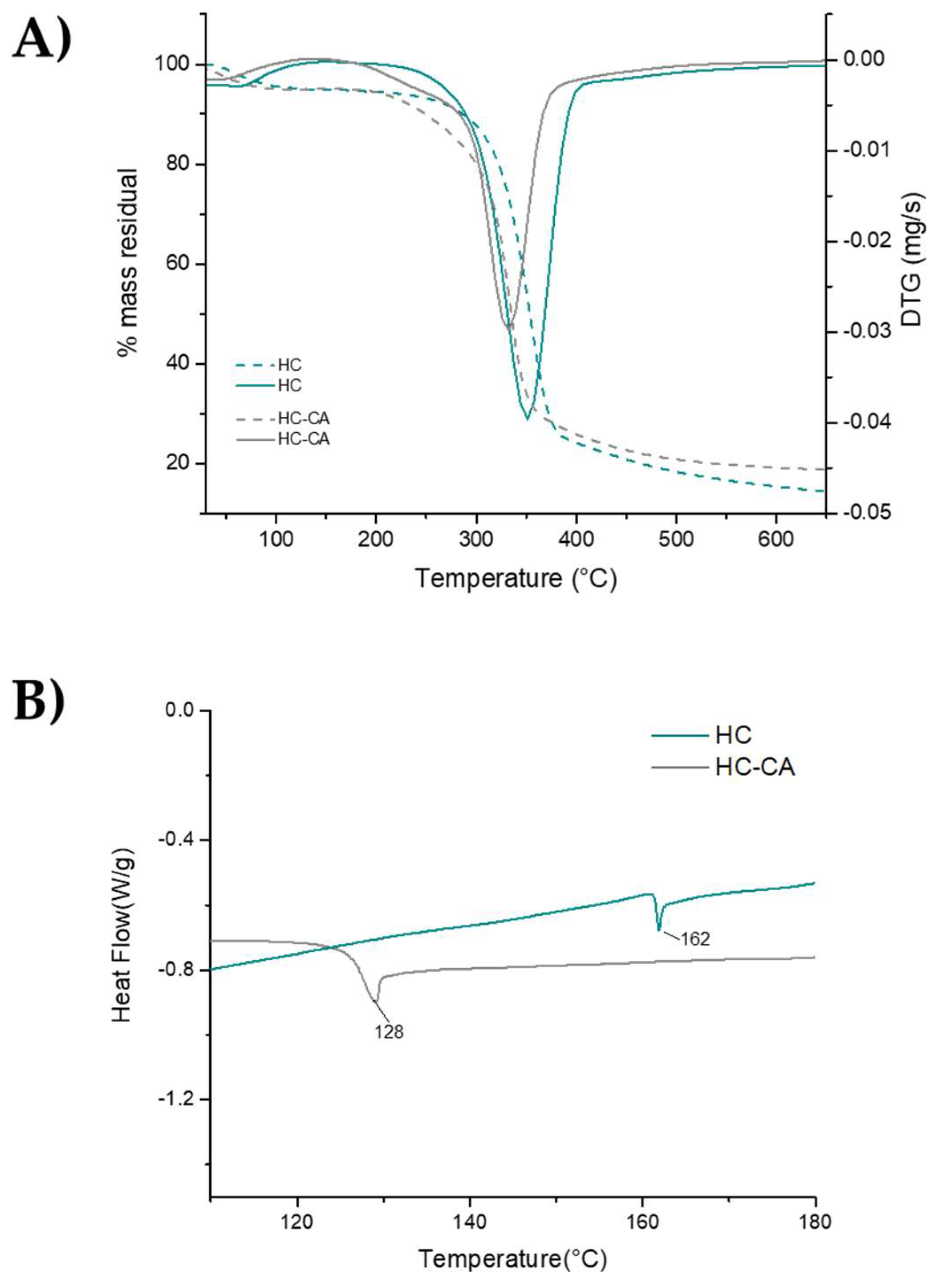
2.4.5. Thermal Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Hemp Cellulose Extraction
3.2. Chemical and Thermal Characterization of Hemp Fiber Cellulose Functionalized with Citric Acid (HC-CA)
3.3. PLA-Based Biocomposite Films: Morphological, Structural, and Thermal Characterization
3.3.1. Morphological Analysis by FE-SEM
3.3.2. ATR-FTIR and XRD Analyses
3.3.3. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paini, J.; Benedetti, V.; Ail, S.S.; Castaldi, M.J.; Baratieri, M.; Patuzzi, F. Valorization of Wastes from the Food Production Industry: A Review towards an Integrated Agri-Food Processing Biorefinery. Waste Biomass Valorization 2021, 13, 31–50. [Google Scholar] [CrossRef]
- Europe Moving towards a Sustainable Future. Available online: https://ec.europa.eu/info/sites/default/files/sdg_multi-stakeholder_platform_input_to_reflection_paper_sustainable_europe2030.pdf (accessed on 22 February 2022).
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food Waste Biorefinery: Pathway towards Circular Bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- LEGGE 2 Dicembre 2016, n. 242 Disposizioni per la Promozione Della Coltivazione e Della Filiera Agroindustriale Della Canapa. (16G00258) (GU Serie Generale n.304 del 30-12-2016). Available online: https://www.gazzettaufficiale.it/eli/id/2016/12/30/16G00258/sg (accessed on 22 February 2022).
- Piccolella, S.; Crescente, G.; Formato, M.; Pacifico, S. A Cup of Hemp Coffee by Moka Pot from Southern Italy: An UHPLC-HRMS Investigation. Foods 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Formato, M.; Crescente, G.; Scognamiglio, M.; Fiorentino, A.; Pecoraro, M.T.; Piccolella, S.; Catauro, M.; Pacifico, S. (−)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020, 25, 2638. [Google Scholar] [CrossRef]
- Nigro, E.; Crescente, G.; Formato, M.; Pecoraro, M.T.; Mallardo, M.; Piccolella, S.; Daniele, A.; Pacifico, S. Hempseed Lignanamides Rich-Fraction: Chemical Investigation and Cytotoxicity towards U-87 Glioblastoma Cells. Molecules 2020, 25, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigro, E.; Pecoraro, M.T.; Formato, M.; Piccolella, S.; Ragucci, S.; Mallardo, M.; Russo, R.; Di Maro, A.; Daniele, A.; Pacifico, S. Cannabidiolic acid in Hemp Seed Oil Table Spoon and Beyond. Molecules 2022, 27, 2566. [Google Scholar] [CrossRef] [PubMed]
- Salentijn, E.M.J.; Petit, J.; Trindade, L.M. The Complex Interactions Between Flowering Behavior and Fiber Quality in Hemp. Front. Plant Sci. 2019, 10, 614. [Google Scholar] [CrossRef]
- Musio, S.; Müssig, J.; Amaducci, S. Optimizing Hemp Fiber Production for High Performance Composite Applications. Front. Plant Sci. 2018, 9, 1702. [Google Scholar] [CrossRef]
- Ahmed, A.T.M.F.; Islam, M.Z.; Mahmud, M.S.; Sarker, M.E.; Islam, M.R. Hemp as a potential raw material toward a sustainable world: A review. Heliyon 2022, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Lefeuvre, A.; Pontoire, B.; Bourmaud, A.; Baley, C. Analysis of the hemp fiber mechanical properties and their scattering (Fedora 17). Ind. Crops Prod. 2013, 51, 317–327. [Google Scholar] [CrossRef]
- Dhakal, H.N.; Zhang, Z. The use of hemp fibres as reinforcements in composites. In Biofiber Reinforcements in Composite Materials; Faruk, O., Sain, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3, pp. 86–103. [Google Scholar] [CrossRef]
- Shahzad, A. Hemp fiber and its composites—A review. J. Compos. Mater. 2011, 46, 973–986. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Stelea, L.; Filip, I.; Lisa, G.; Ichim, M.; Drobotă, M.; Sava, C.; Mureșan, A. Characterisation of Hemp Fibres Reinforced Composites Using Thermoplastic Polymers as Matrices. Polymers 2022, 14, 481. [Google Scholar] [CrossRef] [PubMed]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geffert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties Characterization of Chemically Modified Hemp Hurds. Materials 2014, 7, 8131–8150. [Google Scholar] [CrossRef]
- Cui, X.; Honda, T.; Asoh, T.A.; Uyama, H. Cellulose Modified by Citric Acid Reinforced Polypropylene Resin as Fillers. Carbohydr. Polym. 2020, 230, 115662. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Jimenez, A.; Kenny, J.M.; Garrigós, M.C. Characterization and disintegrability under composting conditions of PLA-based nanocomposite films with thymol and silver nanoparticles. Polym. Degrad. Stab. 2016, 132, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Manaia, J.P.; Manaia, A.T.; Rodriges, L. Industrial Hemp Fibers: An Overview. Fibers 2019, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Panaitescu, D.M.; Vuluga, D.M.; Paven, H.; Iorga, M.D.; Ghiurea, M.; Matasaru, I.; Nechita, P. Properties of polymer composites with cellulose microfibrils. Mol. Cryst. Liq. Cryst. 2008, 484, 86–452. [Google Scholar] [CrossRef]
- Bokhari, S.M.Q.; Chi, K.; Catchmark, J.M. Structural and physico-chemical characterization of industrial hemp hurd: Impacts of chemical pretreatments and mechanical refining. Ind. Crops Prod. 2021, 171, 113818. [Google Scholar] [CrossRef]
- Momeni, S.; Safder, M.; Khondoker, M.A.H.; Elias, A.L. Valorization of Hemp Hurds as Bio-Sourced Additives in PLA-Based Biocomposites. Polymers 2021, 13, 3786. [Google Scholar] [CrossRef]
- Kassab, Z.; Abdellaoui, Y.; Salim, M.H.; Bouhfid, R.; Qaiss, A.E.K.; El Achaby, M. Micro- and nano-celluloses derived from hemp stalks and their effect as polymer reinforcing materials. Carbohydr. Polym. 2020, 245, 116506. [Google Scholar] [CrossRef]
- Ouajai, S.; Shanks, R.A. Morphology and Structure of Bioscouring Hemp Fibre. Macromol. Biosci. 2005, 5, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Kusmono; Listyanda, R.F.; Wildan, M.W.; Ilman, M.N. Preparation and characterization of cellulose nanocrystal extracted from ramie fibers by sulfuric acid hydrolysis. Heliyon 2020, 11, 05486. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, Y.; Guo, Y.; Yue, J. Isolation and Characterization of Nanocellulose with a Novel Shape from Walnut (Juglans Regia L.) Shell Agricultural Waste. Polymers 2019, 11, 1130. [Google Scholar] [CrossRef] [Green Version]
- Mascheroni, E.; Rampazzo, R.; Ortenzi, M.A.; Piva, G.; Bonetti, S.; Piergiovanni, L. Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials. Cellulose 2016, 23, 779–793. [Google Scholar] [CrossRef] [Green Version]
- García-García, D.; Balart, R.; Lopez-Martinez, J.; Ek, M.; Moriana, R. Optimizing the yield and physico-chemical properties of pine cone cellulose nanocrystals by different hydrolysis time. Cellulose 2018, 25, 2925–2938. [Google Scholar] [CrossRef] [Green Version]
- Jordan, J.H.; Easson, M.W.; Dien, B.; Thompson, S.; Condon, B.D. Extraction and characterization of nanocellulose crystals from cotton gin motes and cotton gin waste. Cellulose 2019, 26, 5959–5979. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Puglia, D.; Lavorgna, M.; Santulli, C.; Kenny, J.M.; Torre, L. Optimized extraction of cellulose nanocrystals from pristine and carded hemp fibres. Ind. Crops Prod. 2014, 56, 175–186. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Ouajai, S.; Shanks, R.A. Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Polym. Degrad. Stab. 2005, 89, 327–335. [Google Scholar] [CrossRef]
- Dalle Vacche, S.; Karunakaran, V.; Patrucco, A.; Zoccola, M.; Douard, L.; Ronchetti, S.; Gallo, M.; Schreier, A.; Leterrier, Y.; Bras, J.; et al. Valorization of Byproducts of Hemp Multipurpose Crop: Short Non-Aligned Bast Fibers as a Source of Nanocellulose. Molecules 2021, 26, 4723. [Google Scholar] [CrossRef] [PubMed]
- Sair, S.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; Oudrhiri Hassani, F.; Laachachi, A.; El Bouari, A. Effect of surface modification on morphological, mechanical and thermal conductivity of hemp fiber: Characterization of the interface of hemp –Polyurethane composite. Case Stud. Therm. Eng. 2017, 10, 550–559. [Google Scholar] [CrossRef]
- Cui, X.; Ozaki, A.; Asoh, T.A.; Uyama, H. Cellulose modified by citric acid reinforced Poly(lactic acid) resin as fillers. Polym. Degrad. Stab. 2020, 175, 109118. [Google Scholar] [CrossRef]
- Dhar, P.; Bhasney, S.M.; Kumar, A.; Katiyar, V. Acid functionalized cellulose nanocrystals and its effect on mechanical, thermal, crystallization and surfaces properties of poly (lactic acid) bionanocomposites films: A comprehensive study. Polymer 2016, 101, 75–92. [Google Scholar] [CrossRef]
- Gil Giraldo, G.A.; Mantovan, J.; Marim, B.M.; Kishima, J.O.F.; Mali, S. Surface Modification of Cellulose from Oat Hull with Citric Acid Using Ultrasonication and Reactive Extrusion Assisted Processes. Polysaccharides 2021, 2, 218–233. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Vârban, R.; Crișan, I.; Vârban, D.; Ona, A.; Olar, L.; Stoie, A.; Ștefan, R. Comparative FT-IR Prospecting for Cellulose in Stems of Some Fiber Plants: Flax, Velvet Leaf, Hemp and Jute. Appl. Sci. 2021, 11, 8570. [Google Scholar] [CrossRef]
- Troedec, M.L.; Sedan, D.; Peyratout, C.S.; Bonnet, J.P.; Smith, A.; Guinebretière, R.; Gloaguen, V.; Krausz, P. Influence of various chemical treatments on the composition and structure of hemp fibres. Compos. Part A Appl. Sci. Manuf. 2008, 39, 514–522. [Google Scholar] [CrossRef]
- Ji, H.; Xiang, Z.; Qi, H.; Pranovich, A.; Song, T. Strategy towards one-step preparation of carboxylic cellulose nanocrystals and nanofibrils with high yield, carboxylation and highly stable dispersibility using innocuous citric acid. Curr. Green Chem. 2019, 21, 1956–1964. [Google Scholar] [CrossRef]
- Hachaichi, A.; Kouini, B.; Kian, L.K.; Asim, M.; Jawaid, M. Extraction and characterization of microcrystalline cellulose from date palm fibers using successive chemical treatments. J. Polym. Environ. 2021, 29, 1990–1999. [Google Scholar] [CrossRef]
- Sangeetha, V.H.; Deka, H.; Varghese, T.O.; Nayak, S.K. State of the art and future prospectives of poly (lactic acid) based blends and composites. Polym. Compos. 2016, 39, 81–101. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos. Sci. Technol. 2010, 70, 1742. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, C.; Sun, J.; Zhu, Q.; Liu, J. Structure and properties of Polylactic acid biocomposite films reinforced with cellulose Nanofibrils. Molecules 2020, 25, 3306. [Google Scholar] [CrossRef] [PubMed]
- Aumnate, C.; Soatthiyanon, N.; Makmoon, T. Polylactic acid/kenaf cellulose biocomposite filaments for melt extrusion based-3D printing. Cellulose 2021, 28, 8509–8525. [Google Scholar] [CrossRef]
- Yang, W.; Dominici, F.; Fortunati, E.; Kenny, J.M.; Puglia, D. Melt free radical grafting of glycidyl methacrylate (GMA) onto fully biodegradable poly(lactic) acid films: Effect of cellulose nanocrystals and a masterbatch process. RSC Adv. 2015, 5, 32350–32357. [Google Scholar] [CrossRef]
- Nim, B.; Sreearunothai, P.; Opaprakasit, P.; Petchsuk, A. Preparation and properties of electrospun fibers of titanium dioxide-loaded polylactide/polyvinylpyrrolidone blends. Appl. Sci. Eng. Prog. 2019, 12, 52–58. [Google Scholar] [CrossRef]
- Wan Ishak, W.H.; Rosli, N.A.; Ahmad, I. Influence of amorphous cellulose on mechanical, thermal, and hydrolytic degradation of poly (lactic acid) biocomposites. Sci. Rep. 2020, 10, 11342. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and Characterization of Plasticized Pla/Phb Blends for Biodegradable Multiphase Systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Lu, F.; Yu, H.; Yan, C.; Yao, J. Polylactic acid nanocomposite films with spherical nanocelluloses as efficient nucleation agents: Effects on crystallization, mechanical and thermal properties. RSC Adv. 2016, 6, 46008–46018. [Google Scholar] [CrossRef]



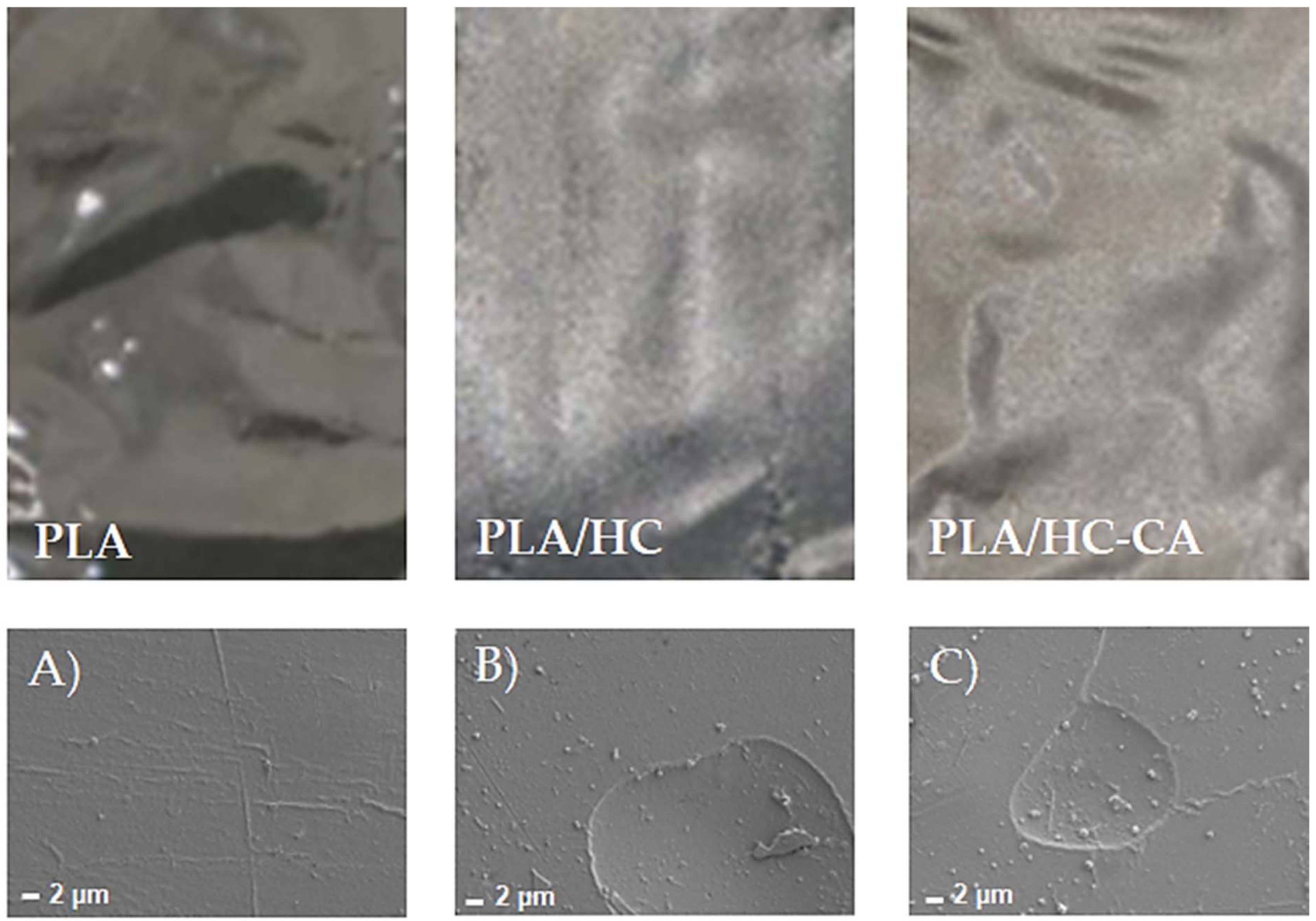
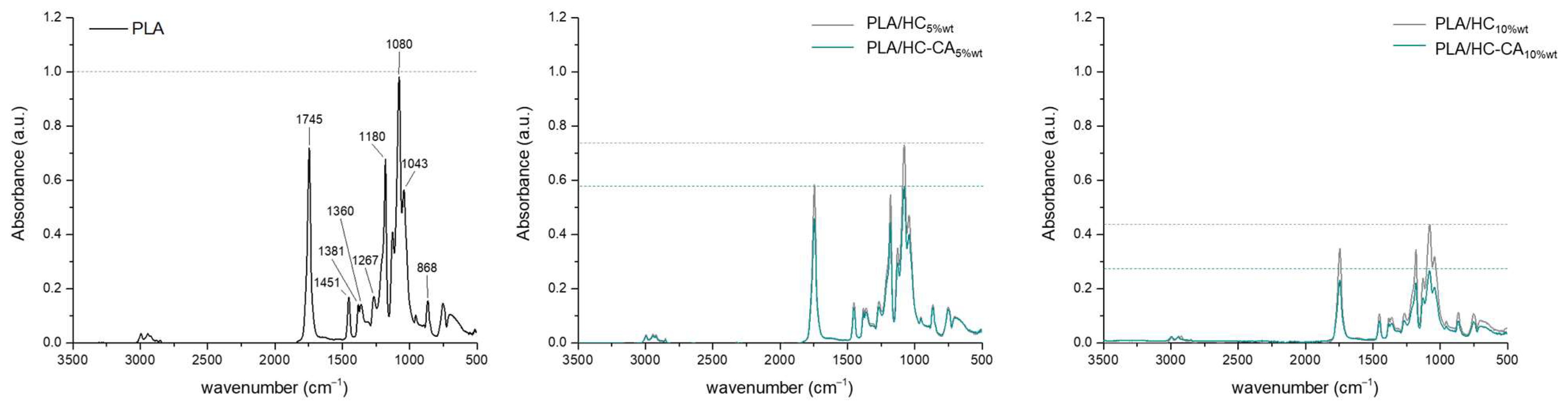
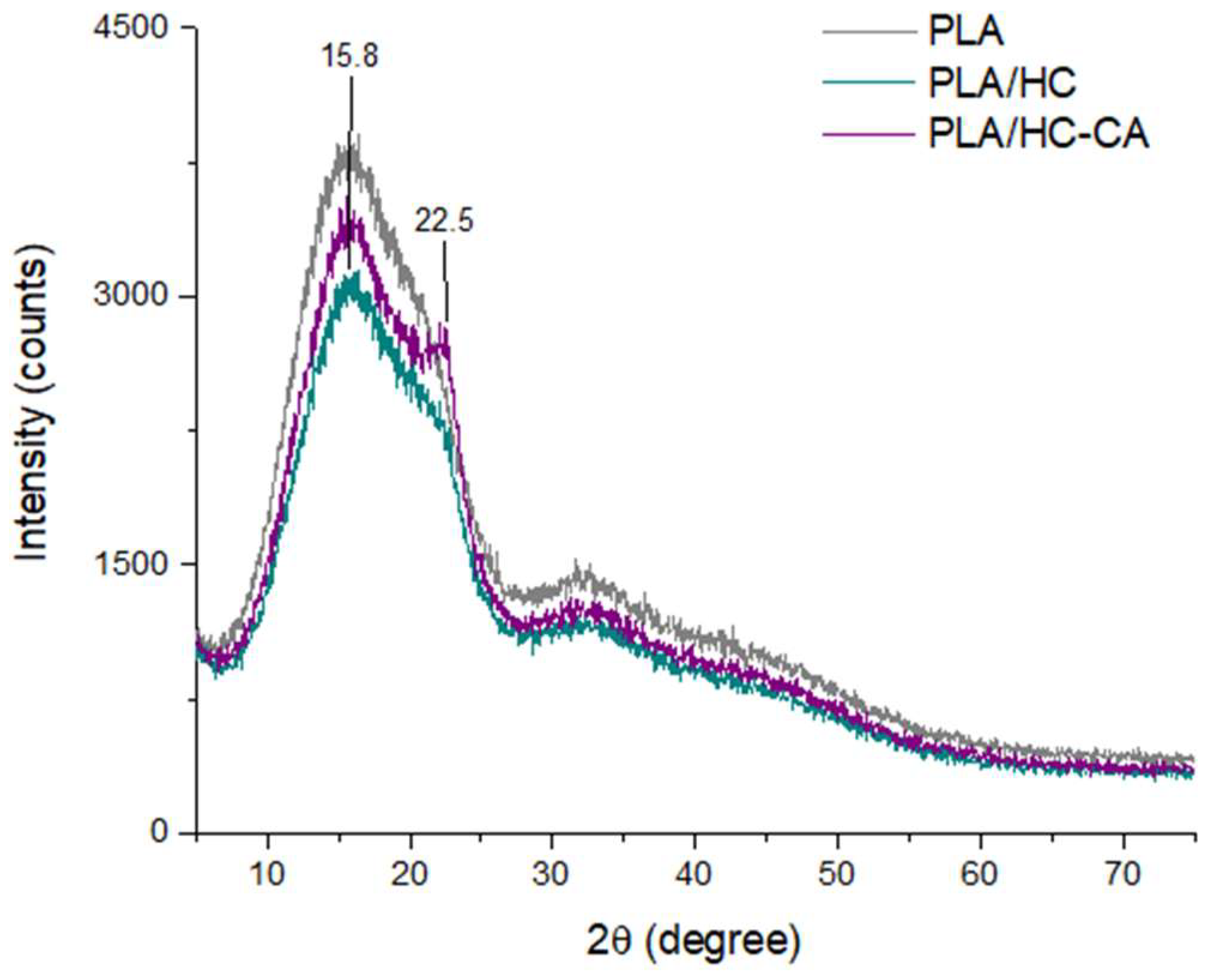

| Acronym | Biocomposite Formulation |
|---|---|
| PLA | Neat PLA |
| PLA/HC5%wt | PLA + extracted hemp cellulose (5%wt) |
| PLA/HC-CA5%wt | PLA + hemp cellulose esterified with citric acid (5%wt) |
| PLA/HC10%wt | PLA + extracted hemp cellulose (10%wt) |
| PLA/HC-CA10%wt | PLA + hemp cellulose esterified with citric acid (10%wt) |
| Sample | Tini5 (°C) | Tmax (°C) | Tfinal (°C) | % Residual |
|---|---|---|---|---|
| HC | 101 ± 4 a | 355 ± 4 a | 399 ± 1 a | 14 ± 1 a |
| HC-CA | 87 ± 3 b | 336 ± 4 b | 387 ± 1 b | 19 ± 1 b |
| Sample | TGA | DSC | ||
|---|---|---|---|---|
| Tini5 (°C) | Tmax (°C) | Tfinal (°C) | Tg (°C) | |
| PLA | 314 ± 5 a | 363 ± 5 a | 405 ± 1 a | 57.9 ± 1.0 a |
| PLA/HC5%wt | 322 ± 5 b | 352 ± 1 a | 402 ± 3 a | 57.4 ± 0.7 a |
| PLA/HC-CA5%wt | 298 ± 1 a | 354 ± 6 a | 406 ± 1 a | 56.5 ± 0.1 a |
| PLA/HC10%wt | 309 ± 1 a | 358 ± 1 a | 406 ± 1 a | 57.2 ± 0.2 a |
| PLA/HC-CA10%wt | 320 ± 1 a | 360 ± 1 a | 408 ± 1 a | 56.7 ± 0.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecoraro, M.T.; Mellinas, C.; Piccolella, S.; Garrigos, M.C.; Pacifico, S. Hemp Stem Epidermis and Cuticle: From Waste to Starter in Bio-Based Material Development. Polymers 2022, 14, 2816. https://doi.org/10.3390/polym14142816
Pecoraro MT, Mellinas C, Piccolella S, Garrigos MC, Pacifico S. Hemp Stem Epidermis and Cuticle: From Waste to Starter in Bio-Based Material Development. Polymers. 2022; 14(14):2816. https://doi.org/10.3390/polym14142816
Chicago/Turabian StylePecoraro, Maria Tommasina, Cristina Mellinas, Simona Piccolella, Maria Carmen Garrigos, and Severina Pacifico. 2022. "Hemp Stem Epidermis and Cuticle: From Waste to Starter in Bio-Based Material Development" Polymers 14, no. 14: 2816. https://doi.org/10.3390/polym14142816
APA StylePecoraro, M. T., Mellinas, C., Piccolella, S., Garrigos, M. C., & Pacifico, S. (2022). Hemp Stem Epidermis and Cuticle: From Waste to Starter in Bio-Based Material Development. Polymers, 14(14), 2816. https://doi.org/10.3390/polym14142816










