Morphological Dependence of Breast Cancer Cell Responses to Doxorubicin on Micropatterned Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Micropatterned Surfaces
2.2. Characterization of Micropatterned Surfaces
2.3. Coating of Fibronectin and Immunological Staining of Coated Fibronectin
2.4. Cell Culture
2.5. TUNEL Assay
2.6. Staining of Actin Filaments
2.7. Analysis of DNA Synthesis Activity by BrdU Staining
2.8. Immunofluorescent Staining of P Glycoprotein
2.9. Statistical Analysis
3. Results and Discussions
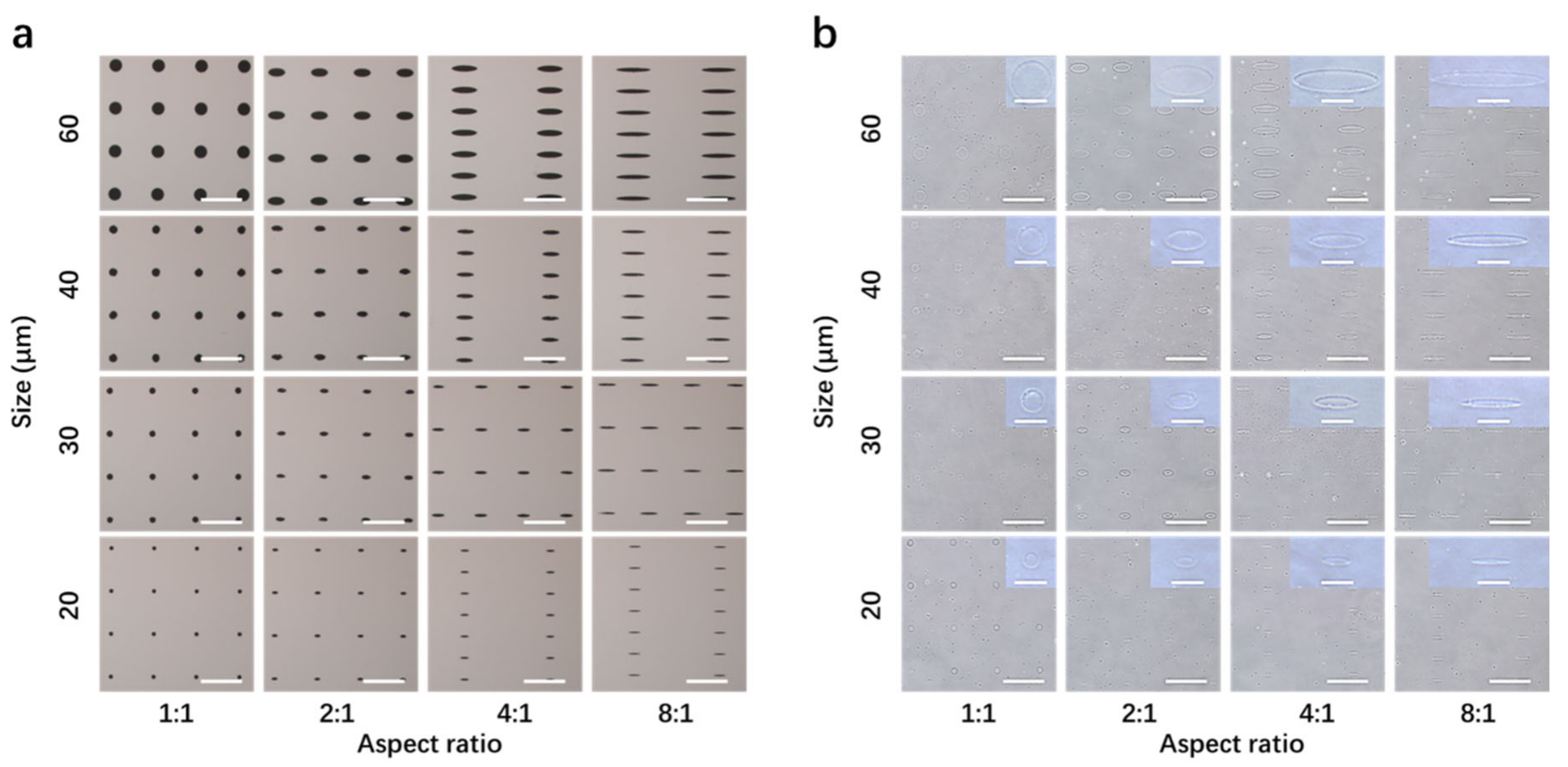
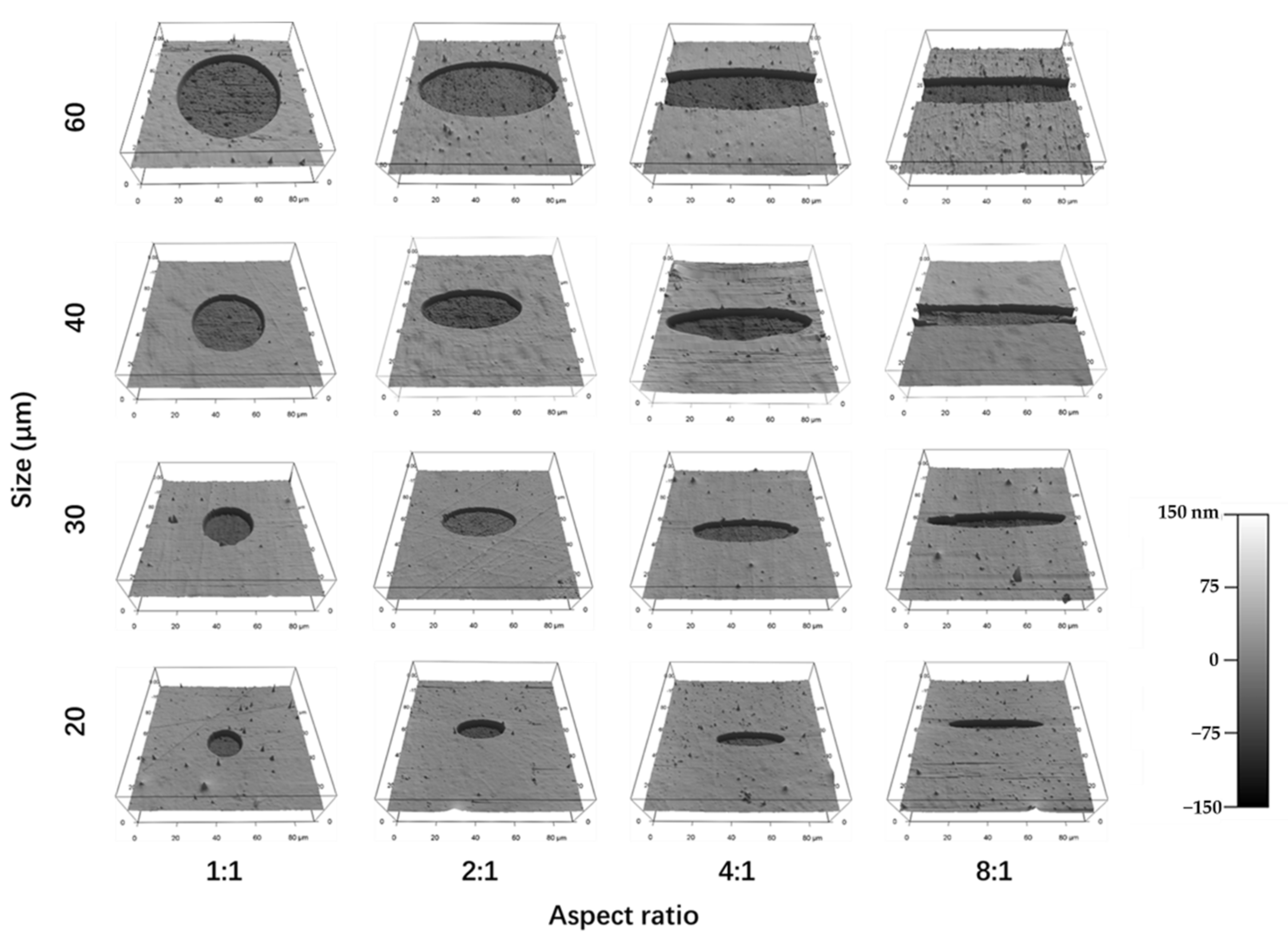
3.1. Preparation and Characterization of Micropatterned Surfaces
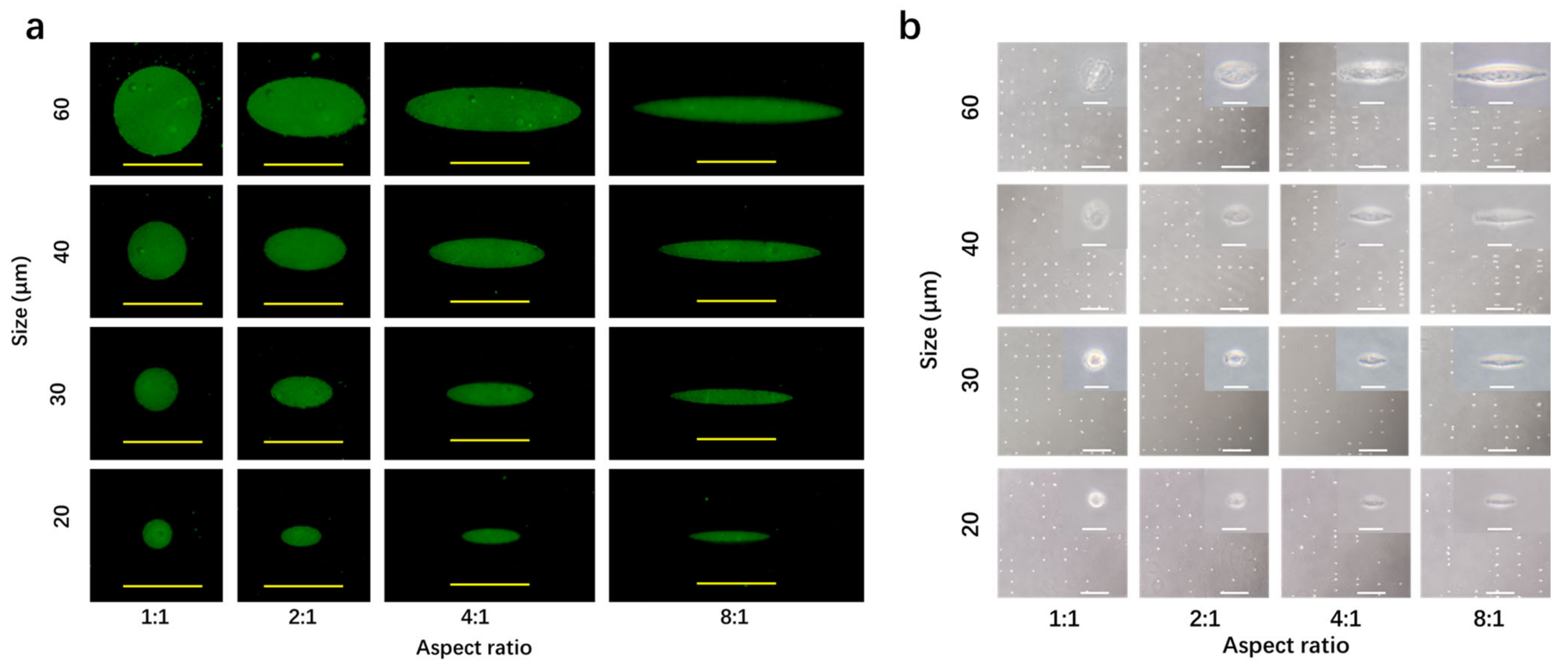
3.2. Coating of Fibronectin and Cell Culture on Micropatterned Surfaces
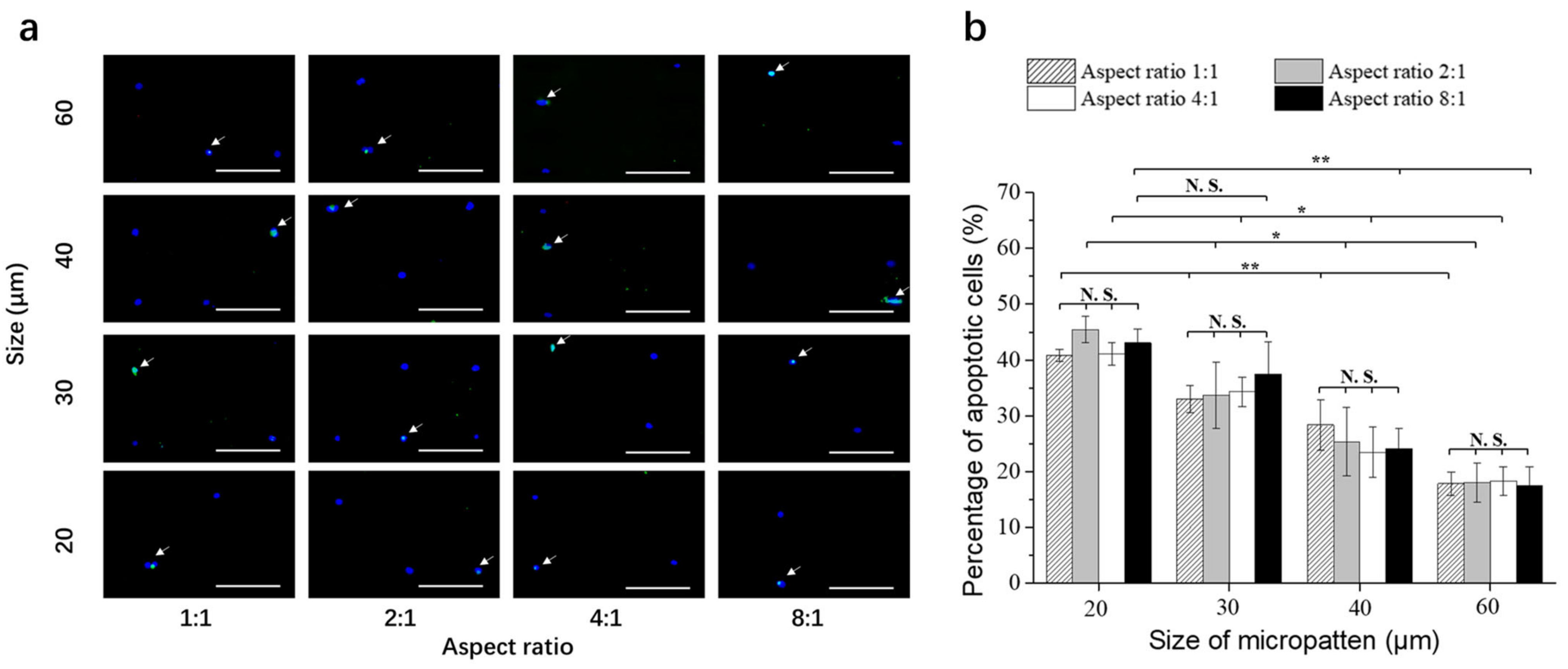
3.3. Doxorubicin-Induced Apoptosis of Breast Cancer Cells on Micropatterned Surfaces
3.4. Cytoskeletal Organization of Breast Cancer Cells on Micropatterned Surfaces
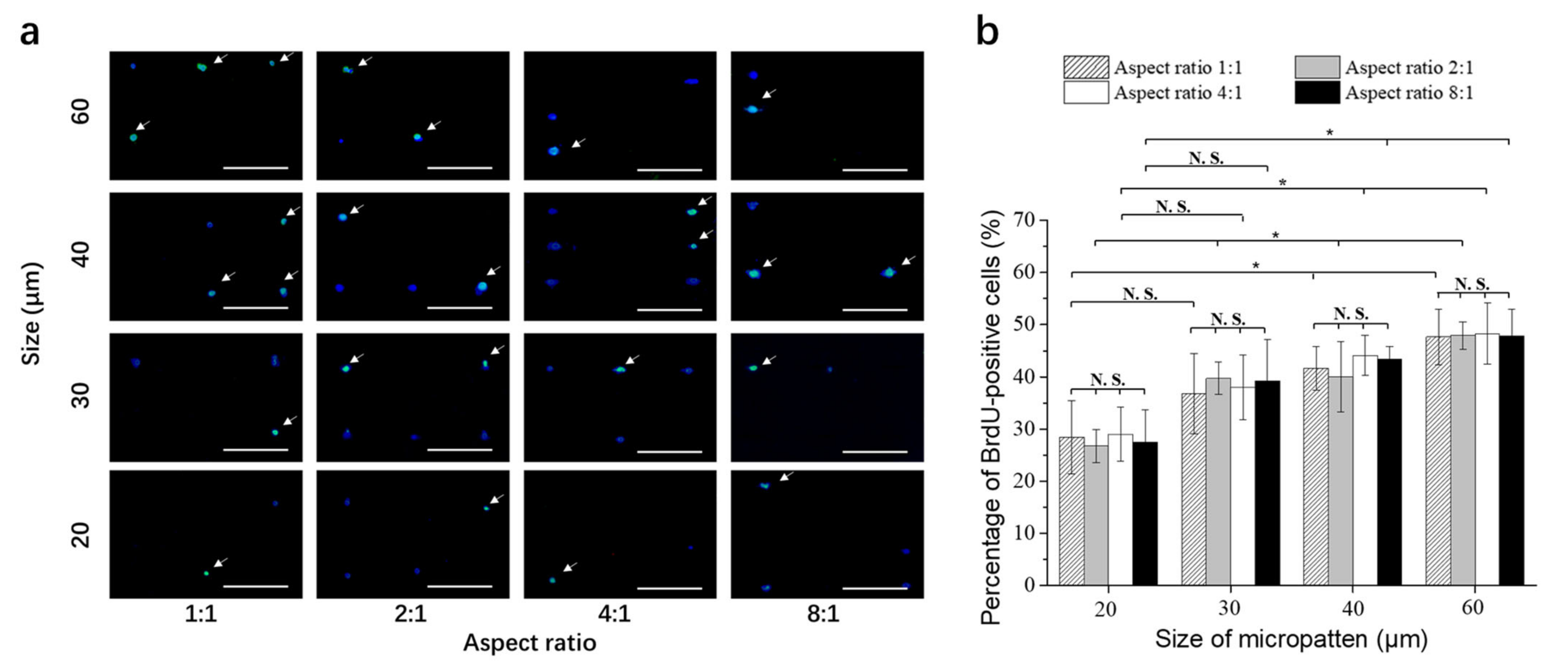
3.5. DNA Synthesis Activity of Breast Cancer Cells on Micropatterned Surfaces
3.6. Staining of P-Glycoprotein of Breast Cancer Cells on Micropatterned Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Assaraf, Y.G.; Brozovic, A.; Goncalves, A.C.; Jurkovicova, D.; Line, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Update 2019, 46, 100645. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Faheem, M.; Ismail, H.; Farhat, S.M.; Ali, M.; Younis, S.; Asghar, M.N. Breast Cancer Resistance Likelihood and Personalized Treatment Through Integrated Multiomics. Front. Mol. Biosci. 2022, 9, 783494. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, N.A.; Krchniakova, M.; Stacy, A.E.; Skoda, J.; Jansson, P.J. Tumour Microenvironment Stress Promotes the Development of Drug Resistance. Antioxidants 2021, 10, 1801. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Mooney, D.J. Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proc. Natl. Acad. Sci. USA 2016, 113, 12126–12131. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Correia, A.L.; Bissell, M.J. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist. Update 2012, 15, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Runa, F.; Hamalian, S.; Meade, K.; Shisgal, P.; Gray, P.; Kelber, J. Tumor microenvironment heterogeneity: Challenges and opportunities. Curr. Mol. Biol. Rep. 2017, 3, 218–229. [Google Scholar] [CrossRef]
- Song, W.; Lu, H.; Kawazoe, N.; Chen, G. Gradient patterning and differentiation of mesenchymal stem cells on micropatterned polymer surface. J. Bioact. Compat. Polym. 2011, 26, 242–256. [Google Scholar] [CrossRef]
- Mao, A.S.; Shin, J.-W.; Mooney, D.J. Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation. Biomaterials 2016, 98, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Dean, D.; Mikos, A.G.; Fisher, J.P. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly (propylene fumarate) disks. Biomacromolecules 2009, 10, 1810–1817. [Google Scholar] [CrossRef] [Green Version]
- Hui, T.; Cheung, K.; Cheung, W.; Chan, D.; Chan, B. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: Influence of cell seeding density and collagen concentration. Biomaterials 2008, 29, 3201–3212. [Google Scholar] [CrossRef]
- Takagi, M.; Umetsu, Y.; Fujiwara, M.; Wakitani, S. High inoculation cell density could accelerate the differentiation of human bone marrow mesenchymal stem cells to chondrocyte cells. J. Biosci. Bioeng. 2007, 103, 98–100. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Micropattern-Controlled Cell Density and Its Effect on Gene Transfection of Mesenchymal Stem Cells. Adv. Mater. Interfaces 2022, 9, 2101978. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, X.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Micropattern-controlled chirality of focal adhesions regulates the cytoskeletal arrangement and gene transfection of mesenchymal stem cells. Biomaterials 2021, 271, 120751. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, X.; Kawazoe, N.; Yang, Y.; Chen, G. The varied influences of cell adhesion and spreading on gene transfection of mesenchymal stem cells on a micropatterned substrate. Acta Biomater. 2021, 125, 100–111. [Google Scholar] [CrossRef]
- Winston, T.S.; Chen, C.; Suddhapas, K.; Tarris, B.A.; Elattar, S.; Sun, S.; Zhang, T.; Ma, Z. Controlling Mesenchyme Tissue Remodeling via Spatial Arrangement of Mechanical Constraints. Front. Bioeng. Biotechnol. 2022, 10, 833595. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Regulation of gene transfection by cell size, shape and elongation on micropatterned surfaces. J. Mat. Chem. B 2021, 9, 4329–4339. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Li, J.; Russe, A.C.; Kawazoe, N.; Yang, Y.; Chen, G. Influence of cell size on cellular uptake of gold nanoparticles. Biomater. Sci. 2016, 4, 970–978. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Dulinska-Molak, I.; Kawazoe, N.; Yang, Y.; Chen, G. Discriminating the Independent Influence of Cell Adhesion and Spreading Area on Stem Cell Fate Determination Using Micropatterned Surfaces. Sci. Rep. 2016, 6, 28708. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric control of cell life and death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef] [Green Version]
- Jiao, F.; Zhao, Y.; Sun, Q.; Huo, B. Spreading area and shape regulate the apoptosis and osteogenesis of mesenchymal stem cells on circular and branched micropatterned islands. J. Biomed. Mater. Res. Part A 2020, 108, 2080–2089. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Kawazoe, N.; Yang, Y.; Chen, G. Manipulating cell nanomechanics using micropatterns. Adv. Funct. Mater. 2016, 26, 7634–7643. [Google Scholar] [CrossRef]
- Al-Nakashli, R.; Oh, H.; Chapman, R.; Stenzel, M.H.; Lu, H.X. Regulating the uptake of poly(N-(2-hydroxypropyl) methacrylamide)-based micelles in cells cultured on micropatterned surfaces. Biointerphases 2021, 16, 041002. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Kawazoe, N.; Yang, Y.; Chen, G. Influences of viscosity on the osteogenic and adipogenic differentiation of mesenchymal stem cells with controlled morphology. J. Mat. Chem. B 2022, 10, 3989–4001. [Google Scholar] [CrossRef]
- Yamada, K.M.; Olden, K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature 1978, 275, 179–184. [Google Scholar] [CrossRef]
- Nuttelman, C.R.; Mortisen, D.J.; Henry, S.M.; Anseth, K.S. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J. Biomed. Mater. Res. 2001, 57, 217–223. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Hu, X.; Kawazoe, N.; Yang, Y.; Chen, G. Morphological and Mechanical Properties of Osteosarcoma Microenvironment Cells Explored by Atomic Force Microscopy. Anal. Sci. 2016, 32, 1177–1182. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, C.; Martins-Neves, S.R.; Paiva-Oliveira, D.; Oliveira, V.E.B.; Fontes-Ribeiro, C.; Gomes, C.M.F. Sensitizing osteosarcoma stem cells to doxorubicin-induced apoptosis through retention of doxorubicin and modulation of apoptotic-related proteins. Life Sci. 2015, 130, 47–56. [Google Scholar] [CrossRef]
- Xie, Y.; Lee, K.; Wang, X.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Interconnected collagen porous scaffolds prepared with sacrificial PLGA sponge templates for cartilage tissue engineering. J. Mat. Chem. B 2021, 9, 8491–8500. [Google Scholar] [CrossRef]
- Lee, K.; Chen, Y.; Li, X.; Wang, Y.; Kawazoe, N.; Yang, Y.; Chen, G. Solution viscosity regulates chondrocyte proliferation and phenotype during 3D culture. J. Mater. Chem. B 2019, 7, 7713–7722. [Google Scholar] [CrossRef]
- Higuchi, A.; Ling, Q.D.; Kumar, S.S.; Chang, Y.; Alarfaj, A.A.; Munusamy, M.A.; Murugan, K.; Hsu, S.T.; Umezawa, A. Physical cues of cell culture materials lead the direction of differentiation lineages of pluripotent stem cells. J. Mat. Chem. B 2015, 3, 8032–8058. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Nakamoto, T.; Kawazoe, N.; Chen, G. Influence of stepwise chondrogenesis-mimicking 3D extracellular matrix on chondrogenic differentiation of mesenchymal stem cells. Biomaterials 2015, 52, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Ling, Q.D.; Chang, Y.; Hsu, S.T.; Umezawa, A. Physical Cues of Biomaterials Guide Stem Cell Differentiation Fate. Chem. Rev. 2013, 113, 3297–3328. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Kawazoe, N.; Chen, G. The balance of osteogenic and adipogenic differentiation in human mesenchymal stem cells by matrices that mimic stepwise tissue development. Biomaterials 2012, 33, 2025–2031. [Google Scholar] [CrossRef]
- Shang, M.; Lim, S.; Jiang, K.; Yap, Y.; Khoo, B.; Han, J.; Lim, C. Microfluidic studies of hydrostatic pressure-enhanced doxorubicin resistance in human breast cancer cells. Lab Chip 2021, 21, 746–754. [Google Scholar] [CrossRef]
- Wang, X.; Song, W.; Kawazoe, N.; Chen, G. Influence of cell protrusion and spreading on adipogenic differentiation of mesenchymal stem cells on micropatterned surfaces. Soft Matter 2013, 9, 4160–4166. [Google Scholar] [CrossRef]
- Sodani, K.; Patel, A.; Kathawala, R.J.; Chen, Z.-S. Multidrug resistance associated proteins in multidrug resistance. Chin. J. Cancer Res. 2012, 31, 58. [Google Scholar] [CrossRef] [Green Version]
- Mao, Q.C.; Unadkat, J.D. Role of the Breast Cancer Resistance Protein (BCRP/ABCG2) in Drug Transport-an Update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed. Pharmacother. 2019, 114, 9. [Google Scholar] [CrossRef]
- Thakar, R.G.; Cheng, Q.; Patel, S.; Chu, J.; Nasir, M.; Liepmann, D.; Komvopoulos, K.; Li, S. Cell-Shape Regulation of Smooth Muscle Cell Proliferation. Biophys. J. 2009, 96, 3423–3432. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Coulombe, P.A. OPINION Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat. Rev. Mol. Cell Biol. 2010, 11, 75–81. [Google Scholar] [CrossRef]
- Fu, D.; Roufogalis, B.D. Actin disruption inhibits endosomal traffic of P-glycoprotein-EGFP and resistance to daunorubicin accumulation. Am. J. Physiol. Cell Physiol. 2007, 292, C1543–C1552. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Sun, R.; Chen, H.; Zeng, T.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Morphological Dependence of Breast Cancer Cell Responses to Doxorubicin on Micropatterned Surfaces. Polymers 2022, 14, 2761. https://doi.org/10.3390/polym14142761
Zheng J, Sun R, Chen H, Zeng T, Yoshitomi T, Kawazoe N, Yang Y, Chen G. Morphological Dependence of Breast Cancer Cell Responses to Doxorubicin on Micropatterned Surfaces. Polymers. 2022; 14(14):2761. https://doi.org/10.3390/polym14142761
Chicago/Turabian StyleZheng, Jing, Rui Sun, Huajian Chen, Tianjiao Zeng, Toru Yoshitomi, Naoki Kawazoe, Yingnan Yang, and Guoping Chen. 2022. "Morphological Dependence of Breast Cancer Cell Responses to Doxorubicin on Micropatterned Surfaces" Polymers 14, no. 14: 2761. https://doi.org/10.3390/polym14142761
APA StyleZheng, J., Sun, R., Chen, H., Zeng, T., Yoshitomi, T., Kawazoe, N., Yang, Y., & Chen, G. (2022). Morphological Dependence of Breast Cancer Cell Responses to Doxorubicin on Micropatterned Surfaces. Polymers, 14(14), 2761. https://doi.org/10.3390/polym14142761






