Biophysical Characterization and Cytocompatibility of Cellulose Cryogels Reinforced with Chitin Nanowhiskers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Preparation and Characterization of Cellulose Cryogels
3.2. Crystallinity of Cellulose and Cellulose Cryogels
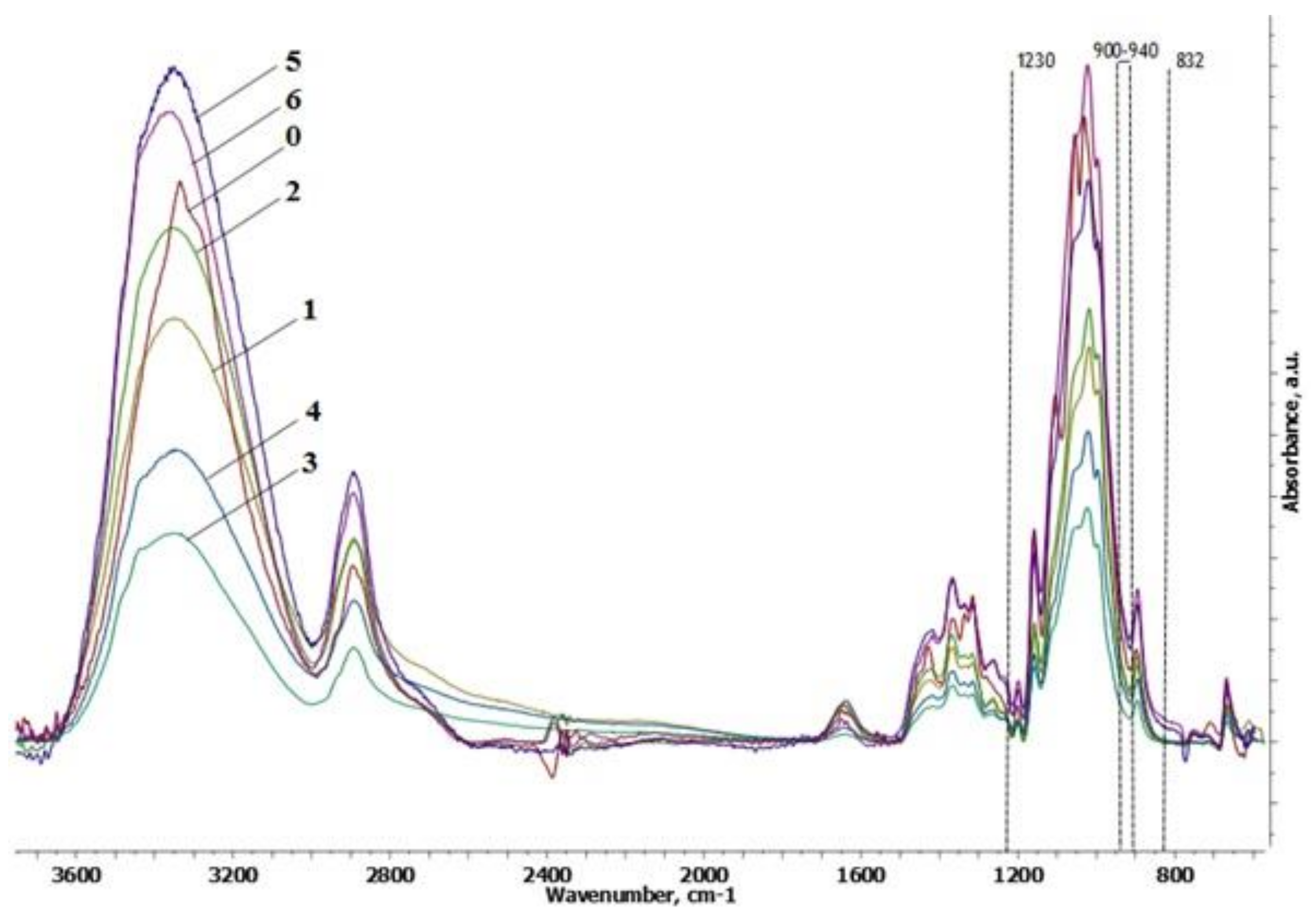
3.3. FTIR Spectroscopy
3.4. Mechanical Properties
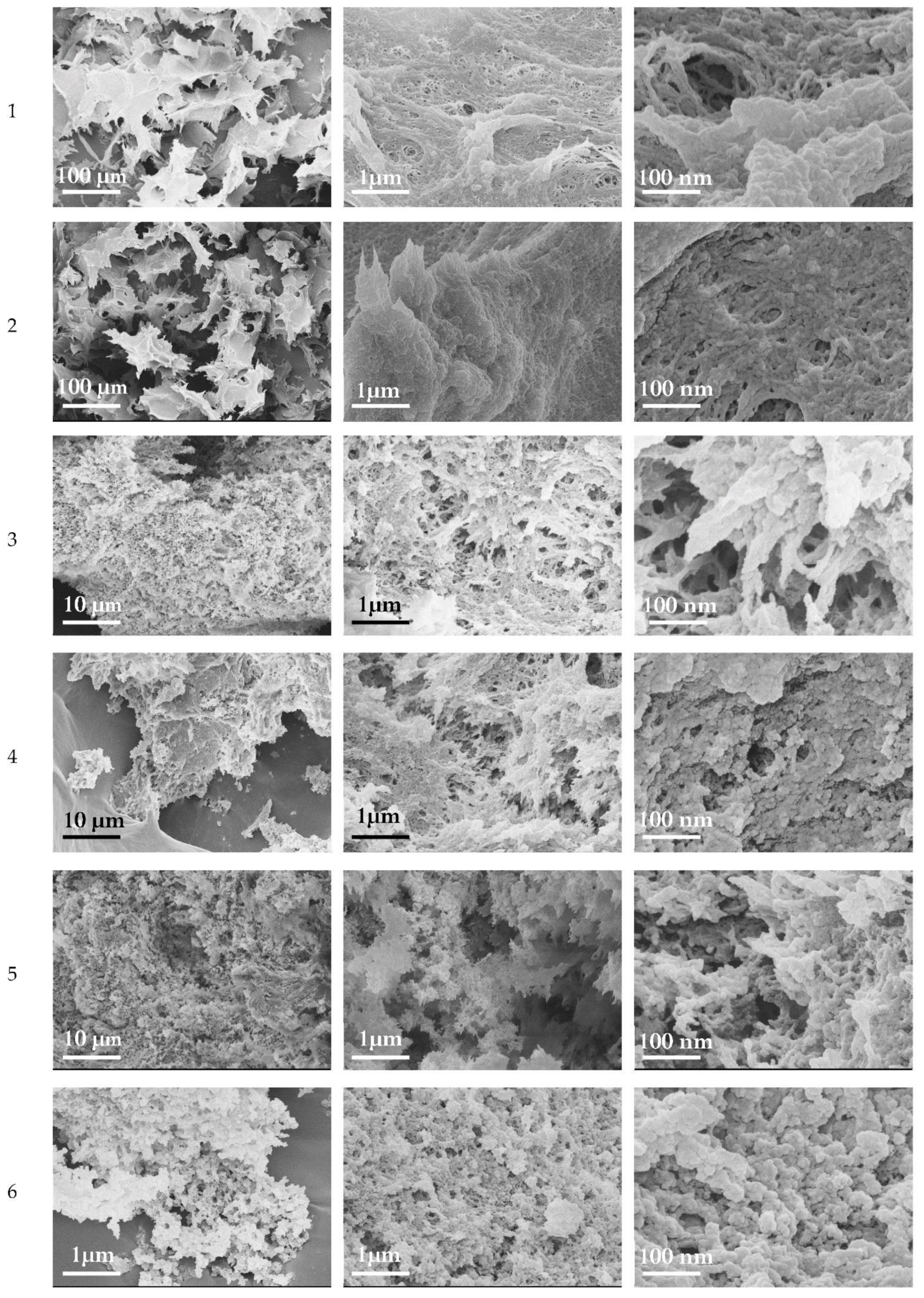
3.5. Morphology of Cellulose Cryogels
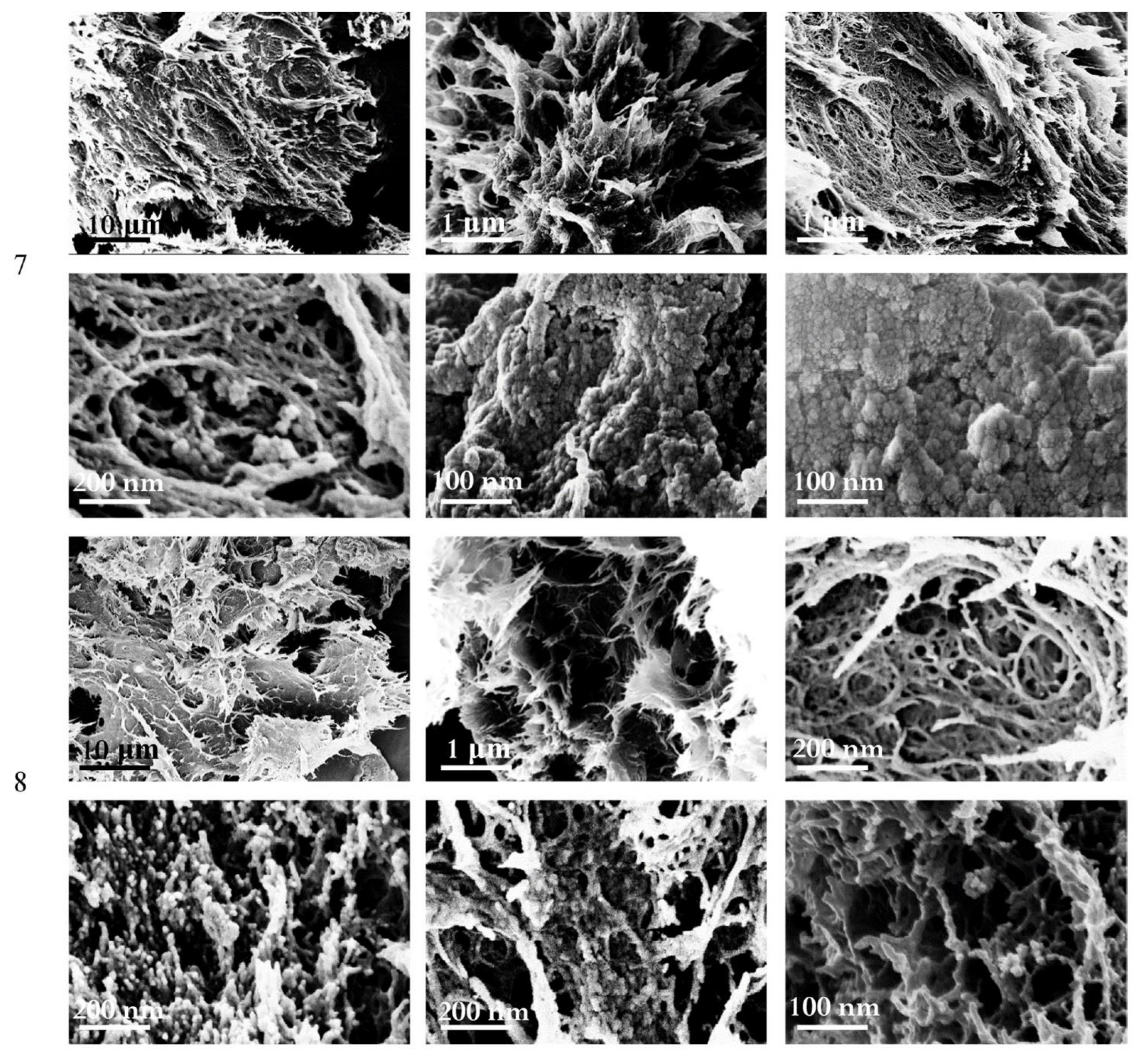
3.6. Effects of Freezing Time on the Properties of Cryogels
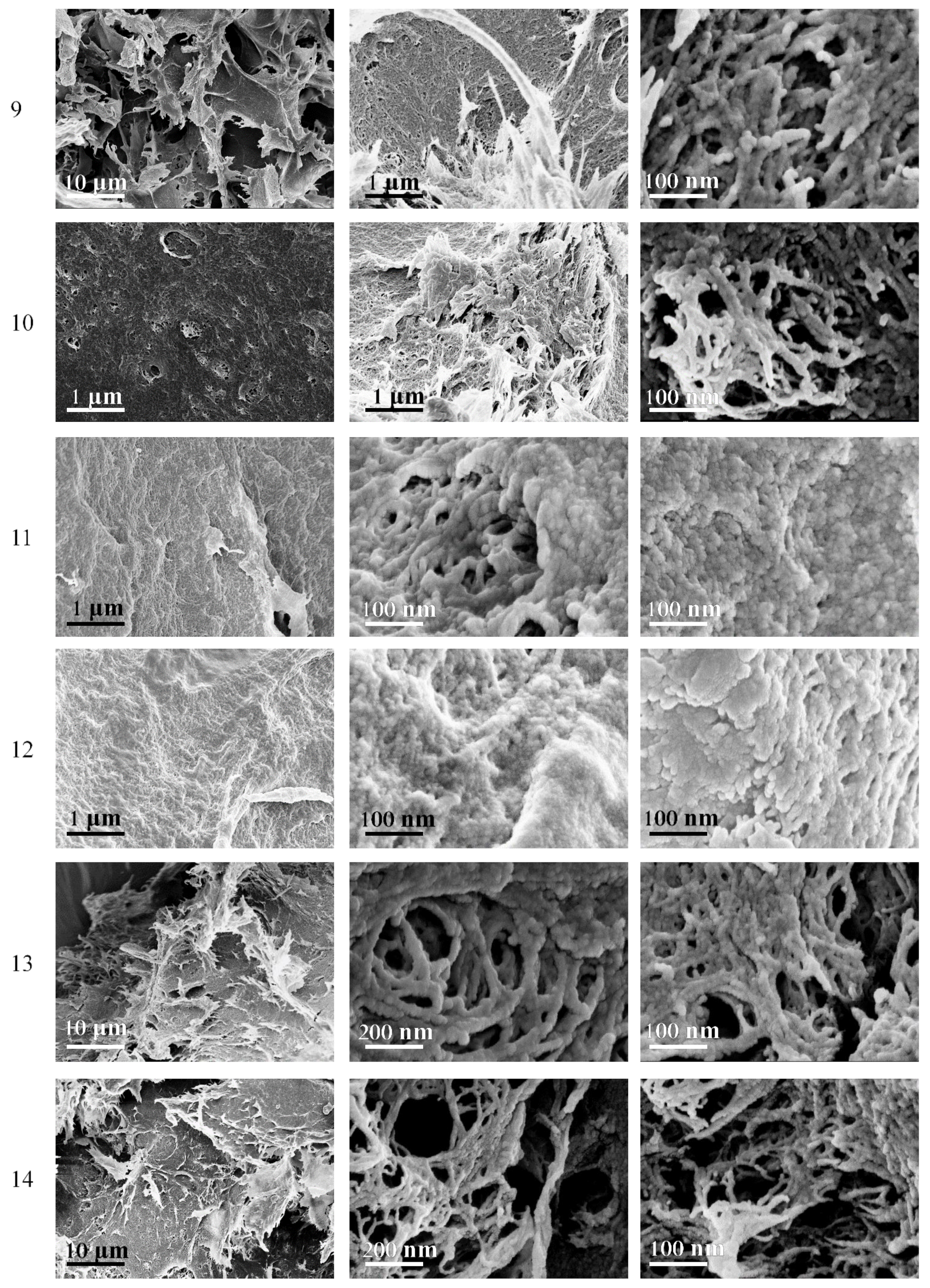
3.7. Composite Cryogels
3.8. Biocompatibility of Cryogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, J.; Bakhshpour, M.; Idil, N.; Perçin, I.; Denizli, A. Biomedical applications of polymeric cryogels. Appl. Sci. 2019, 9, 553. [Google Scholar]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Ozmen, M.M.; Dragan, E.S.; Okay, O. Freezing as a path to build macroporous structures: Superfast responsive polyacrylamide hydrogels. Polymer 2007, 48, 195–204. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, R.; Reinwald, Y.; Bhat, S. Cryogels: Freezing unveiled by thawing. Mater. Today 2010, 13, 42–44. [Google Scholar] [CrossRef]
- Liu, P.; Chen, W.; Bai, S.; Wang, Q.; Duan, W. Facile Preparation of Poly (Vinyl Alcohol)/Graphene Oxide Nanocomposites and Their Foaming Behavior in Supercritical Carbon Dioxide; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Chirani, N.; Gritsch, L.; Motta, F.L.; Fare, S. History and applications of hydrogels. J. Biomed. Sci. 2015, 4, 2. [Google Scholar]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The potential of polymeric cryogels in bioseparation. Bioseparation 2001, 10, 163–188. [Google Scholar] [CrossRef]
- Hu, T.; Shi, M.; Zhao, X.; Liang, Y.; Bi, L.; Zhang, Z.; Liu, S.; Chen, B.; Duan, X.; Guo, B. Biomimetic 3d aligned conductive tubular cryogel scaffolds with mechanical anisotropy for 3d cell alignment, differentiation and in vivo skeletal muscle regeneration. Chem. Eng. J. 2022, 428, 131017. [Google Scholar] [CrossRef]
- Olov, N.; Mirzadeh, H.; Moradi, R.; Rajabi, S.; Bagheri-Khoulenjani, S. Shape memory injectable cryogel based on carboxymethyl chitosan/gelatin for minimally invasive tissue engineering: In vitro and in vivo assays. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 1–14. [Google Scholar] [CrossRef]
- Tyshkunova, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose cryogels as promising materials for biomedical applications. Int. J. Mol. Sci. 2022, 23, 2037. [Google Scholar] [CrossRef]
- Lozinsky, V.; Vainerman, E.; Ivanova, S.; Titova, E.; Shtil’man, M.; Belavtseva, E.; Rogozhin, S. Study of cryostructurization of polymer systems. Vi. The influence of the process temperature on the dynamics of formation and structure of cross-linked polyacrylamide cryogels. Acta Polym. 1986, 37, 142–146. [Google Scholar] [CrossRef]
- Muslumova, S.; Yetiskin, B.; Okay, O. Highly stretchable and rapid self-recoverable cryogels based on butyl rubber as reusable sorbent. Gels 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.; Gao, H.; Lin, Z.; Dai, Q.; Zhu, S.; Li, S.; Liu, C.; Feng, Q.; Li, Q.; Wang, G.; et al. A shape memory and antibacterial cryogel with rapid hemostasis for noncompressible hemorrhage and wound healing. Chem. Eng. J. 2022, 428, 131005. [Google Scholar] [CrossRef]
- Hwang, Y.; Zhang, C.; Varghese, S. Poly (ethylene glycol) cryogels as potential cell scaffolds: Effect of polymerization conditions on cryogel microstructure and properties. J. Mater. Chem. 2010, 20, 345–351. [Google Scholar] [CrossRef]
- Gang, E.J.; Jeong, J.A.; Hong, S.H.; Hwang, S.H.; Kim, S.W.; Yang, I.H.; Ahn, C.; Han, H.; Kim, H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells 2004, 22, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Ozmen, M.M.; Dinu, M.V.; Dragan, E.S.; Okay, O. Preparation of macroporous acrylamide-based hydrogels: Cryogelation under isothermal conditions. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 1195–1202. [Google Scholar] [CrossRef]
- He, X.; Yao, K.; Shen, S.; Yun, J. Freezing characteristics of acrylamide-based aqueous solution used for the preparation of supermacroporous cryogels via cryo-copolymerization. Chem. Eng. Sci. 2007, 62, 1334–1342. [Google Scholar] [CrossRef]
- Buchtova, N.; Budtova, T. Cellulose aero-, cryo-and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Tyshkunova, I.V.; Chukhchin, D.G.; Gofman, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose cryogels prepared by regeneration from phosphoric acid solutions. Cellulose 2021, 28, 4975–4989. [Google Scholar] [CrossRef]
- Ivanov, R.V.; Lozinsky, V.I.; Noh, S.K.; Lee, Y.R.; Han, S.S.; Lyoo, W.S. Preparation and characterization of polyacrylamide cryogels produced from a high-molecular-weight precursor. Ii. The influence of the molecular weight of the polymeric precursor. J. Appl. Polym. Sci. 2008, 107, 382–390. [Google Scholar] [CrossRef]
- Tripathi, A.; Kathuria, N.; Kumar, A. Elastic and macroporous agarose–gelatin cryogels with isotropic and anisotropic porosity for tissue engineering. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 90, 680–694. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Lippens, E.; Cornelissen, M.; Schacht, E. Correlation between cryogenic parameters and physico-chemical properties of porous gelatin cryogels. J. Biomater. Sci. Polym. Ed. 2009, 20, 1417–1438. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Zhang, H.; Lyu, S.; Zhou, X.; Gu, H.; Ma, C.; Wang, C.; Ding, T.; Shao, Q.; Liu, H.; Guo, Z. Super light 3d hierarchical nanocellulose aerogel foam with superior oil adsorption. J. Colloid Interface Sci. 2019, 536, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, J.; Saito, T.; Isogai, A. Simple freeze-drying procedure for producing nanocellulose aerogel-containing, high-performance air filters. ACS Appl. Mater. Interfaces 2015, 7, 19809–19815. [Google Scholar] [CrossRef]
- Nordli, H.R.; Chinga-Carrasco, G.; Rokstad, A.M.; Pukstad, B. Producing ultrapure wood cellulose nanofibrils and evaluating the cytotoxicity using human skin cells. Carbohydr. Polym. 2016, 150, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The uninvited guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, S.; Shen, J.; Chen, Y.; Xiao, Y. A composite hydrogel based on pectin/cellulose via chemical cross-linking for hemorrhage. Front. Bioeng. Biotechnol. 2020, 8, 627351. [Google Scholar] [CrossRef]
- Păduraru, O.M.; Ciolacu, D.; Darie, R.N.; Vasile, C. Synthesis and characterization of polyvinyl alcohol/cellulose cryogels and their testing as carriers for a bioactive component. Mater. Sci. Eng. C 2012, 32, 2508–2515. [Google Scholar] [CrossRef]
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Moreira, A.P.D.; Thiré, R.M.d.S.M.; Quilty, B.; Passos, T.M.; Simon, P.; Mancini, M.C.; McGuinness, G.B. Absorbent polyvinyl alcohol–sodium carboxymethyl cellulose hydrogels for propolis delivery in wound healing applications. Polym. Eng. Sci. 2017, 57, 1224–1233. [Google Scholar] [CrossRef]
- Béduer, A.; Braschler, T.; Peric, O.; Fantner, G.E.; Mosser, S.; Fraering, P.C.; Benchérif, S.; Mooney, D.J.; Renaud, P. A compressible scaffold for minimally invasive delivery of large intact neuronal networks. Adv. Healthc. Mater. 2015, 4, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, B.; Agarwal, R.; Alam, M.S. Antimicrobial and release study of drug loaded pva/peo/cmc wound dressings. J. Mater. Sci. Mater. Med. 2014, 25, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Souza, L.P.; Martins, T.M.M.; Lopes, J.H.; Mattos, B.D.; Mariano, M.; Pinheiro, I.F.; Valverde, T.M.; Livi, S.; Camilli, J.A.; et al. Nanocellulose/bioactive glass cryogels as scaffolds for bone regeneration. Nanoscale 2019, 11, 19842–19849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odabas, S. Collagen–carboxymethyl cellulose–tricalcium phosphate multi-lamellar cryogels for tissue engineering applications: Production and characterization. J. Bioact. Compat. Polym. 2016, 31, 411–422. [Google Scholar] [CrossRef]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.M.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef] [PubMed]
- Chukhchin, D.G.; Malkov, A.V.; Tyshkunova, I.V.; Mayer, L.V.; Novozhilov, E.V. Diffractometric method for determining the degree of crystallinity of materials. Crystallogr. Rep. 2016, 61, 371–375. [Google Scholar] [CrossRef]
- Chukhchin, D.G.; Bolotova, K.S. Program for Calculating the Degree of Crystallinity of Various Substances According to X-ray Diffractometric Analysis. R.U. Patent 2018661836, 2018. [Google Scholar]
- Tyshkunova, I.V.; Chukhchin, D.G.; Gofman, I.V.; Pavlova, E.N.; Ushakov, V.A.; Vlasova, E.N.; Poshina, D.N.; Skorik, Y.A. Chitin cryogels prepared by regeneration from phosphoric acid solutions. Materials 2021, 14, 5191. [Google Scholar] [CrossRef]
- Kameda, T.; Miyazawa, M.; Ono, H.; Yoshida, M. Hydrogen bonding structure and stability of α-chitin studied by 13c solid-state nmr. Macromol. Biosci. 2005, 5, 103–106. [Google Scholar] [CrossRef]
- Pereira, A.G.; Muniz, E.C.; Hsieh, Y.-L. Chitosan-sheath and chitin-core nanowhiskers. Carbohydr. Polym. 2014, 107, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razali, N.; Hossain, M.S.; Taiwo, O.A.; Ibrahim, M.; Nadzri, N.W.M.; Razak, N.; Rawi, N.F.M.; Mahadar, M.M.; Kassim, M.H.M. Influence of acid hydrolysis reaction time on the isolation of cellulose nanowhiskers from oil palm empty fruit bunch microcrystalline cellulose. BioResources 2017, 12, 6773–6788. [Google Scholar] [CrossRef] [Green Version]
- Satish, N.; Patkar, P.D.P. Development and validation of method for molecular weight determination of cellulose using gpc column in hplc. Int. J. Adv. Res. 2016, 4, 516–530. [Google Scholar]
- Petrova, V.A.; Khripunov, A.K.; Golovkin, A.S.; Mishanin, A.I.; Gofman, I.V.; Romanov, D.P.; Migunova, A.V.; Arkharova, N.A.; Klechkovskaya, V.V.; Skorik, Y.A. Bacterial cellulose (komagataeibacter rhaeticus) biocomposites and their cytocompatibility. Materials 2020, 13, 4558. [Google Scholar] [CrossRef] [PubMed]
- Hoepfner, S.; Ratke, L.; Milow, B. Synthesis and characterisation of nanofibrillar cellulose aerogels. Cellulose 2008, 15, 121–129. [Google Scholar] [CrossRef]
- Sehaqui, H.; Salajková, M.; Zhou, Q.; Berglund, L.A. Mechanical performance tailoring of tough ultra-high porosity foams prepared from cellulose i nanofiber suspensions. Soft Matter 2010, 6, 1824–1832. [Google Scholar] [CrossRef]
- Buchtová, N.; Pradille, C.; Bouvard, J.-L.; Budtova, T. Mechanical properties of cellulose aerogels and cryogels. Soft Matter 2019, 15, 7901–7908. [Google Scholar] [CrossRef]
- Mansikkamäki, P.; Lahtinen, M.; Rissanen, K. The conversion from cellulose i to cellulose ii in naoh mercerization performed in alcohol–water systems: An x-ray powder diffraction study. Carbohydr. Polym. 2007, 68, 35–43. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Lin, L.; Chen, T.; Zhang, J.; Liu, S.; Li, Z.; Ouyang, P. Dissolution of microcrystalline cellulose in phosphoric acid—molecular changes and kinetics. Molecules 2009, 14, 5027–5041. [Google Scholar] [CrossRef] [Green Version]
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wågberg, L. Phosphorylated cellulose nanofibrils: A renewable nanomaterial for the preparation of intrinsically flame-retardant materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef]
- Ram, B.; Chauhan, G.S. New spherical nanocellulose and thiol-based adsorbent for rapid and selective removal of mercuric ions. Chem. Eng. J. 2018, 331, 587–596. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Deng, X.-Y.; Shen, W.-H.; Jia, M.-Y. Preparation and characterization of the spherical nanosized cellulose by the enzymatic hydrolysis of pulp fibers. Carbohydr. Polym. 2018, 181, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.A.; Wang, J.; Guan, A.Q.; Naguib, H.E. Chitin nano-whiskers (cnws) as a bio-based bio-degradable reinforcement for epoxy: Evaluation of the impact of cnws on the morphological, fracture, mechanical, dynamic mechanical, and thermal characteristics of dgeba epoxy resin. RSC Adv. 2019, 9, 11063–11076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Huang, J.; Luo, B.; Zhou, C. Tough and highly stretchable polyacrylamide nanocomposite hydrogels with chitin nanocrystals. Int. J. Biol. Macromol. 2015, 78, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Kadla, J.F. Effect of nanofillers on carboxymethyl cellulose/hydroxyethyl cellulose hydrogels. J. Appl. Polym. Sci. 2009, 114, 1664–1669. [Google Scholar] [CrossRef]
- Yang, X.; Bakaic, E.; Hoare, T.; Cranston, E.D. Injectable polysaccharide hydrogels reinforced with cellulose nanocrystals: Morphology, rheology, degradation, and cytotoxicity. Biomacromolecules 2013, 14, 4447–4455. [Google Scholar] [CrossRef]
- João, C.F.C.; Silva, J.C.; Borges, J.P. Chitin-based nanocomposites: Biomedical applications. In Eco-Friendly Polymer Nanocomposites Advanced Structured Materials; Springer India: New Delhi, India, 2015; Volume 74, pp. 439–457. [Google Scholar]
- Baraniak, P.R.; Cooke, M.T.; Saeed, R.; Kinney, M.A.; Fridley, K.M.; McDevitt, T.C. Stiffening of human mesenchymal stem cell spheroid microenvironments induced by incorporation of gelatin microparticles. J. Mech. Behav. Biomed. Mater. 2012, 11, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Cesarz, Z.; Tamama, K. Spheroid culture of mesenchymal stem cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.S.; Dai, L.G.; Yen, B.L.; Hsu, S.H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Liu, B.H.; Sieber, M.; Hsu, S.H. Substrate-dependent gene regulation of self-assembled human msc spheroids on chitosan membranes. BMC Genom. 2014, 15, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartosh, T.J.; Ylostalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (mscs) into 3d spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [Green Version]
- Jaukovic, A.; Abadjieva, D.; Trivanovic, D.; Stoyanova, E.; Kostadinova, M.; Pashova, S.; Kestendjieva, S.; Kukolj, T.; Jeseta, M.; Kistanova, E.; et al. Specificity of 3d msc spheroids microenvironment: Impact on msc behavior and properties. Stem Cell Rev. Rep. 2020, 16, 853–875. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.W.; Lu, Y.J.; Lin, Y.J.; Huang, Y.T.; Hsieh, L.H.; Wu, B.H.; Lin, Y.C.; Chen, L.C.; Wang, H.W.; Chuang, J.C.; et al. Transplantation of 3d msc/huvec spheroids with neuroprotective and proangiogenic potentials ameliorates ischemic stroke brain injury. Biomaterials 2021, 272, 120765. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, N.; Zhu, Y.; Jin, R.; Wu, F. Msc spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through pi3k-akt signaling pathway. Biomaterials 2021, 265, 120448. [Google Scholar] [CrossRef] [PubMed]



| Sample | Cellulose Concentration, % | Dissolution Time, h | Temperature, °C | Sample Shape | Yield, % | ∆ V, % | % | Mw (Ð *) | Crystallinity, % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 24 | 20 ± 2 | Intact cylindrical | 93.6 | 60 | 3319 | 53,770 (3.8) | 28.2 |
| 2 | 5 | 46 | 86.2 | 60 | 3647 | 29,620 (2.6) | 28.4 | ||
| 3 | 5 | 24 | 29 ± 1 | Partially cylindrical and powder | 88.4 | −45 | 1189 | 18,110 (2.7) | 28.0 |
| 4 | 10 | 24 | Intact cylindrical | 90.9 | 17.5 | 1210 | 24,000 (3.0) | 27.0 | |
| 5 | 5 | 24 | 39 ± 1 | Partially cylindrical and powder | 90.3 | −34 | 1388 | 19,390 (2.3) | 27.7 |
| 6 | 5 | 48 | 86.1 | −62.5 | 803 | 13,190 (2.4) | 27.9 |
| Sample | Cryogel Volume, cm3 | Volume Shrinkage, % | ρ, g/cm3 | Porosity, % | SR, g/g | Specific Surface Area, m2/g |
|---|---|---|---|---|---|---|
| 1 | 4.5 | 10.7 | 0.052 | 96.2 | 8.7 | 38.1 |
| 2 | 4.7 | 6.9 | 0.046 | 96.7 | 4.4 | 4.6 |
| 4 | 12.6 | 37.3 | 0.144 | 89.6 | Sample broken | 6.0 |
| Sample | E, kPa | σy, kPa | σmax, kPa | εmax, % |
|---|---|---|---|---|
| 1 | 1231 ± 132 | 79 ± 26 | 366 ± 45 | 70 |
| 2 | 126 ± 25 | 43 ± 11 | 268 ± 30 | 70 |
| 4 | Broken under load | |||
| Sample | Freezing Time, Days | Yield, % | Cryogel Volume, cm3 | Volume Shrinkage, % | ρ, g/cm3 | Porosity, % | ∆ V, % | % | SR, g/g | E, kPa | Crystallinity, % | Specific Surface Area, m2/g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 93.6 | 4.5 | 10.7 | 0.052 | 96.2 | 60 | 3319 | 8.7 | 1231 ± 132 | 28.2 | 38.062 |
| 7 | 10 | 90.0 | 1.43 | 71.4 | 0.157 | 88.6 | 66 | 3566 | 7.1 | 1099 ± 121 | 27.4 | 4.647 |
| 8 | 28 | 91.2 | 1.40 | 71.9 | 0.162 | 88.2 | 60 | 3336 | 5.1 | 2872 ± 478 | 26.8 | 16.765 |
| Sample | CNW, % | Freezing Time, Days | Yield, % | Cryogel Volume, cm3 | Volume Shrinkage, % | ∆ V, % | ρ, g/cm3 | Porosity, % | % | SR, g/g | Specific Surface Area, m2/g | E, kPa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 3 | 93.6 | 4.50 | 10.7 | 60 | 0.052 | 96.2 | 3319 | 8.7 | 38.062 | 1231 ± 132 |
| 9 | 1 | 3 | 89.2 | 1.84 | 63.2 | 40 | 0.121 | 91.2 | 3010 | 4.9 | 0.981 | 1238 |
| 10 | 2.5 | 3 | 90.8 | 2.76 | 44.9 | 40 | 0.082 | 94.0 | 2946 | 7.7 | 2.629 | 1570 |
| 11 | 5 | 3 | 92.6 | 3.10 | 37.8 | 124 | 0.078 | 94.3 | 4529 | 6.6 | 1.012 | 273 |
| 12 | 10 | 3 | 83.3 | 2.49 | 50.2 | 104 | 0.092 | 93.3 | 4349 | 6.8 | 0.530 | 383 ± 150 |
| 13 | 1 | 10 | 75.6 | 1.80 | 63.8 | 60 | 0.106 | 92.3 | 4144 | 4.6 | 9.737 | 3120 ± 120 |
| 14 | 2.5 | 10 | 90.5 | 2.51 | 49.8 | 64 | 0.092 | 93.3 | 3567 | 4.9 | 0.935 | 3050 ± 160 |
| Sample | Number of Spheroids/mm2 | Spheroid Size, µm | Depth of Spheroids Location, µm |
|---|---|---|---|
| 7 | 7.3 ± 0.9 | 41.0 ± 1.4 | 223 ± 5 |
| 8 | 6.6 ± 1.8 | 44 ± 2 | 230 ± 5 |
| 13 | 8.6 ± 0.7 | 57 ± 3 * | 230 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyshkunova, I.V.; Gofman, I.V.; Chukhchin, D.G.; Malkov, A.V.; Mishanin, A.I.; Golovkin, A.S.; Pavlova, E.N.; Poshina, D.N.; Skorik, Y.A. Biophysical Characterization and Cytocompatibility of Cellulose Cryogels Reinforced with Chitin Nanowhiskers. Polymers 2022, 14, 2694. https://doi.org/10.3390/polym14132694
Tyshkunova IV, Gofman IV, Chukhchin DG, Malkov AV, Mishanin AI, Golovkin AS, Pavlova EN, Poshina DN, Skorik YA. Biophysical Characterization and Cytocompatibility of Cellulose Cryogels Reinforced with Chitin Nanowhiskers. Polymers. 2022; 14(13):2694. https://doi.org/10.3390/polym14132694
Chicago/Turabian StyleTyshkunova, Irina V., Iosif V. Gofman, Dmitry G. Chukhchin, Alexey V. Malkov, Alexander I. Mishanin, Alexey S. Golovkin, Ekaterina N. Pavlova, Daria N. Poshina, and Yury A. Skorik. 2022. "Biophysical Characterization and Cytocompatibility of Cellulose Cryogels Reinforced with Chitin Nanowhiskers" Polymers 14, no. 13: 2694. https://doi.org/10.3390/polym14132694
APA StyleTyshkunova, I. V., Gofman, I. V., Chukhchin, D. G., Malkov, A. V., Mishanin, A. I., Golovkin, A. S., Pavlova, E. N., Poshina, D. N., & Skorik, Y. A. (2022). Biophysical Characterization and Cytocompatibility of Cellulose Cryogels Reinforced with Chitin Nanowhiskers. Polymers, 14(13), 2694. https://doi.org/10.3390/polym14132694







