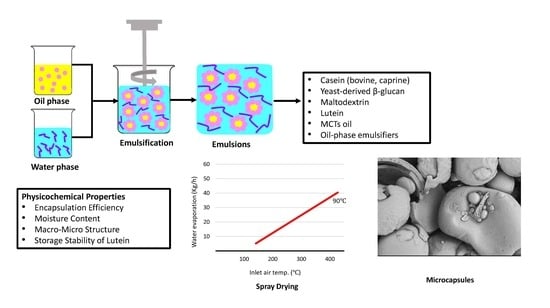
Mixed Biopolymer Systems Based on Bovine and Caprine Caseins, Yeast β-Glucan, and Maltodextrin for Microencapsulating Lutein Dispersed in Emulsified Lipid Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bovine and Caprine Caseins
2.3. Emulsion Preparation
2.4. Viscosity of the Emulsions
2.5. Droplet Size of the Emulsions
2.6. Encapsulation of the Emulsions by Spray Drying
2.7. Encapsulation Efficiency
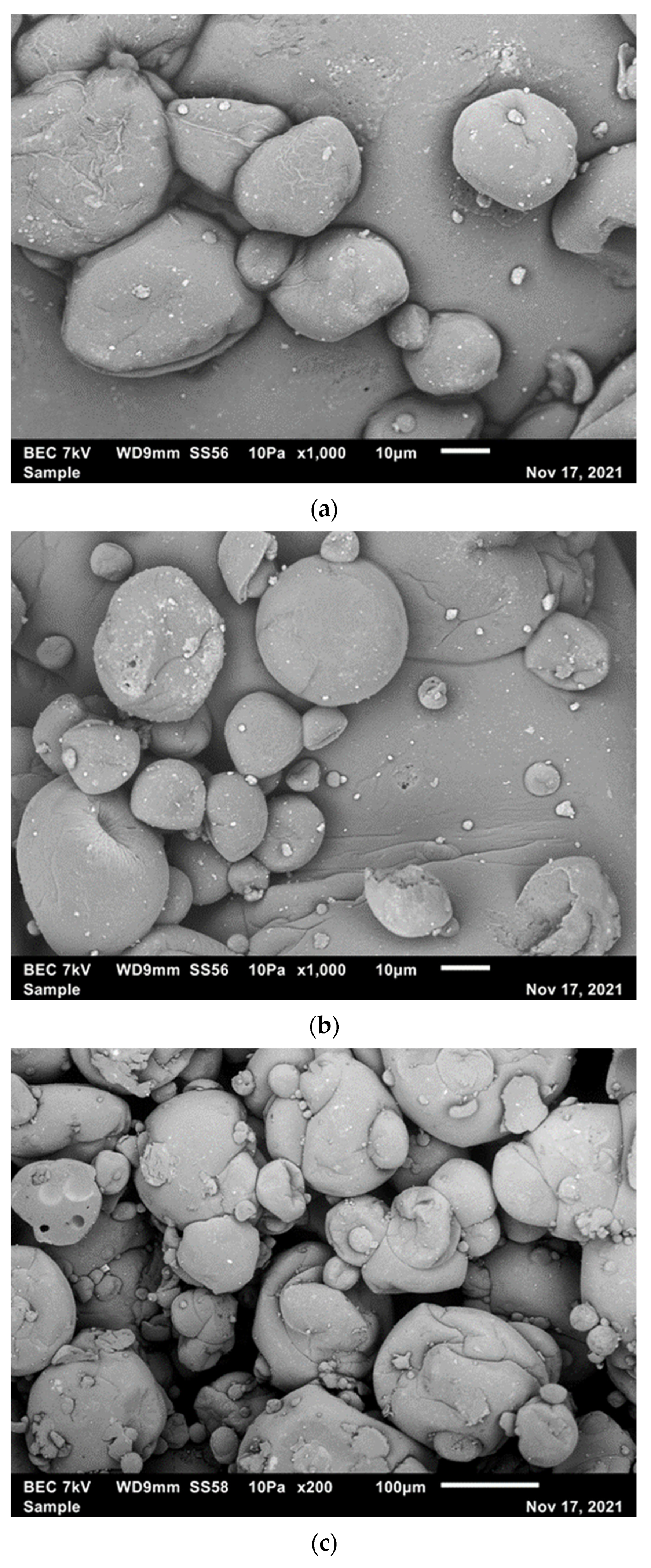
2.8. Scanning Electron Microscopy (SEM) Analysis of the Microcapsules
2.9. Structural Characterization of Lutein in the Microcapsules
2.10. Moisture Content of the Microcapsules
2.11. Storage Conditions of the Microcapsules
2.12. HPLC Analysis of Lutein in the Microcapsules
2.13. Antioxidant Activity of Lutein in the Microcapsules
2.14. Evaluation of Lipid Oxidation in the Microcapsules
2.15. Determination of Caprylic Acid and Capric Acid Fatty Acids in the Microcapsules
2.16. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Bovine and Caprine Caseins
3.2. Physical Properties of Emulsions
3.3. Physical Properties of Microcapsules
3.4. Morphology of Microcapsules
3.5. Structural Characterization of Lutein in the Microcapsules
3.6. Stability of Lutein in the Microcapsules
3.7. Antioxidant Activity of Lutein in the Microcapsules
3.8. Accelerated Shelf-Life Tests in the Microcapsules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenfield, M. Computer vision syndrome (aka digital eye strain). Optometry 2016, 17, 1–10. Available online: https://www.researchgate.net/publication/295902618 (accessed on 17 March 2022).
- Tosini, G.; Fergusson, I.; Tsubota, K. Effects of blue light on the circadian system and eye physiology. Mol. Vis. 2016, 22, 61–72. [Google Scholar] [PubMed]
- Ozkaya, E.K.; Anderson, G.; Dhillon, B.; Bagnaninchi, P.O. Blue-light induced breakdown of barrier function on human retinal epithelial cells is mediated by PKC-ζ over-activation and oxidative stress. Exp. Eye Res. 2019, 189, 107817. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.; Yathisha, U.G.; Karunasagar, I.; Mamatha, B.S. Nutraceutical approach to enhance lutein bioavailability via nanodelivery systems. Nutr. Rev. 2020, 78, 709–724. [Google Scholar] [CrossRef]
- Bhat, I.; Jose, N.M.; Mamatha, B.S. Oxidative stability of lutein on exposure to varied extrinsic factors. J. Food Sci. Technol. 2022, 1–9. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Peng, Y.; Wen, J.; Quek, S.Y. A comparison of microfluidic-jet spray drying, two-fluid nozzle spray drying, and freeze-drying for co-encapsulating β-carotene, lutein, zeaxanthin, and fish oil. Foods 2021, 10, 1522. [Google Scholar] [CrossRef]
- Kuang, P.; Zhang, H.; Bajaj, P.R.; Yuan, Q.; Tang, J.; Chen, S.; Sablani, S. Physicochemical properties and storage stability of lutein microcapsules prepared with maltodextrins and sucrose by spray drying. J. Food Sci. 2015, 80, E359–E369. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Bora, U. Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules 2008, 9, 2905–2912. [Google Scholar] [CrossRef]
- Sarantis, S.D.; Eren, N.M.; Kowalcyk, B.; Jimenez-Flores, R. Thermodynamic interactions of micellar casein and oat β-glucan in a model food system. Food Hyd. 2021, 115, 106559. [Google Scholar] [CrossRef]
- Atanase, L.I. Micellar drug delivery systems based on natural biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc, C.E.; Atanase, L.I.; Jérỏme, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-based complex particles’ protective role on the stability and bioactivity of immobilized curcumin. Int. J. Mol. Sci. 2021, 22, 3075. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Yin, M.; Wang, H.; Xiaolong, J. Recent progress in the research of Angelica sinensis (Oliv.) Diels polysaccharides: Extraction, purification, structure and bioactivities. Chem. Biol. Technol. Agric. 2021, 8, 13. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Barani, M.; Sadegh Amiri, M.; Yazdi, M.E.T.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of plant-based nanoparticles in nanomedicine: A review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [Green Version]
- Kharat, M.; Zhang, G.; McClements, D.J. Stability of curcumin in oil-in-water emulsions: Impact of emulsifier type and concentration on chemical degradation. Food Res. Inter. 2018, 111, 178–186. [Google Scholar] [CrossRef]
- Kharat, M.; Skzynski, M.; Decker, E.A.; McClements, D.J. Enhancement of chemical stability of curcumin-enriched oil-in-water emulsions: Impact of antioxidant type and concentration. Food Chem. 2020, 320, 126653. [Google Scholar] [CrossRef]
- Sethiya, A.; Agarwal, D.K.; Agarwal, S. Current trends in drug delivery system of curcumin and its therapeutic applications. Mini Rev. Med. Chem. 2020, 20, 1190–1232. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.-F. Preparation and characterization of 2-hydroxymethyl starch microparticles for co-delivery of multiple bioactive agents. Drug Deliv. 2021, 28, 1562–1568. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, G.; Zhou, J.; Li, H.; Xu, X.; Cheng, B.; Zhuang, X. Proton donor-regulated mechanically robust aramid nanofiber aerogel membranes for high-temperature thermal insulation. ACS Nano 2022, 16, 5984–5993. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-R.; Deng, L.; Liu, G.-C.; Wen, L.; Wang, J.G.; Huang, K.-B.; Tang, H.-T.; Pan, Y.-M. Porous organic polymer-derived nanopalladium catalysts for chemoselective synthesis of antitumor benzofuro [2,3-b] pyrazine from 2-bromophenol and isonitriles. Org. Lett. 2019, 21, 4929–4932. [Google Scholar] [CrossRef] [PubMed]
- Mora-Gutierrez, A.; Attaie, R.; Núñez de González, M.T.; Jung, Y.; Woldesenbet, S.; Marquez, S.A. Complexes of lutein with bovine and caprine caseins and their impact on lutein chemical stability in emulsion systems: Effect of arabinogalactan. J. Dairy Sci. 2018, 101, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Gutierrez, A.; Attaie, R.; Núñez de González, M.T.; Jung, Y.; Marquez, S.A. Interface compositions as determinants of resveratrol stability in nanoemulsion delivery systems. Foods 2020, 9, 1394. [Google Scholar] [CrossRef] [PubMed]
- Mora-Gutierrez, A.; Attaie, R.; Farrell, H.M., Jr. Lipid oxidation in algae oil-in-water emulsions stabilized by bovine and caprine caseins. J. Agric. Food Chem. 2010, 58, 5131–5139. [Google Scholar] [CrossRef] [PubMed]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-glucans and immunity: State of the art and future directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar] [CrossRef]
- Chang, C.; Nickerson, M.T. Encapsulation of omega 3-6-9 fatty acids rich oils using protein-based emulsions with spray drying. J. Food Sci. Technol. 2018, 55, 2850–2861. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Kumosinski, T.F.; Farrell, H.M., Jr. Quantification of αs1-casein in goat milk from French-Alpine and Anglo-Nubian breeds using reversed-phase high performance liquid chromatography. J. Dairy Sci. 1991, 74, 3303–3307. [Google Scholar] [CrossRef]
- Basch, J.J.; Farrell, H.M., Jr.; Walsh, R.A.; Konstance, R.P.; Kumosinski, T.F. Development of a quantitative model for enzyme-catalyzed time-dependent changes in protein composition of Cheddar cheese during storage. J. Dairy Sci. 1989, 72, 591–603. [Google Scholar] [CrossRef]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray-drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Marisiddaiah, R.; Zaripheh, S.; Wiener, D.; Rubin, L.P. Mitochondrial beta-carotene 9’,10’ oxygenase modulates prostate cancer growth via NF-kappaB inhibition: A lycopene independent function. Mol. Cancer Res. 2016, 14, 966–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, L.; Salgado, P.R.; Mauri, A.N. Encapsulation of fish oil in soybean protein particles by emulsification and spray-drying. Food Hyd. 2019, 87, 891–901. [Google Scholar] [CrossRef]
- Armah-Agyeman, G.; Gyamerath, M.P.; Biney, P.O.; Woldesenbet, S. Extraction and characterization of triglycerides from coffeeweed and switchgrass seeds as potential feedstocks for biodiesel production. J. Sci. Food Agric. 2016, 96, 4390–4397. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Attaie, R.; Kirven, J.M.; Farrell, H.M., Jr. Cross-linking of bovine and caprine caseins by microbial transglutaminase and their use as microencapsulating agents for n-3 fatty acids. Int. J. Food Sci. Technol. 2014, 49, 1530–1543. [Google Scholar] [CrossRef]
- Buitink, J.; van den Dries, L.J.; Hoesktra, F.A.; Alberda, M.; Hemminga, M.A. High critical temperature above Tg may contribute to the stability of biological systems. Biophys. J. 2000, 79, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Berton, C.; Ropers, M.-H.; Viau, M.; Genot, C. Contribution of the interfacial layer to the protection of emulsified lipids against oxidation. J. Agric. Food Chem. 2011, 59, 5052–5061. [Google Scholar] [CrossRef]
- Nakamura, A.; Takahashi, T.; Yoshida, R.; Maeda, M.; Corredig, M. Emulsifying properties of soybean soluble polysaccharides. Food Hydrocoll. 2004, 18, 795–803. [Google Scholar] [CrossRef]
- Nakamura, A.; Yoshida, R.; Maeda, M.; Furuta, H.; Corredig, M. Study of the role of the carbohydrate and protein moieties of soy soluble polysaccharides in their emulsifying properties. J. Agric. Food Chem. 2004, 52, 5506–5512. [Google Scholar] [CrossRef]
- Jansen-Alves, C.; Fernandes, K.F.; Crizel-Cardozo, M.M.; Krumreich, F.D.; Borges, C.D.; Zambiazi, R.C. Microencapsulation of propolis in protein matrix using spray-drying for applications in food systems. Food Bio. Technol. 2018, 11, 1422–1436. [Google Scholar] [CrossRef]
- Geller, A.; Shestha, R.; Yan, J. Yeast-derived β-glucan in cancer: Novel uses of a traditional therapeutic. Int. J. Mol. Sci. 2019, 20, 3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshminarayana, R.; Aruna, G.; Sangeetha, R.K.; Bhaskar, N.; Divakar, S.; Baskaran, V. Possible degradation/biotransformation of lutein in vitro and in vivo: Isolation and structural elucidation of lutein metabolites by HPLC and LC-MS (atmospheric pressure chemical ionization). Free Radic. Biol. Med. 2008, 45, 982–993. [Google Scholar] [CrossRef]
- Müller, L.; Frőhlich, K.; Bőhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing power (FRAP), ABTS bleaching assay (α TEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Li, R.-Y.; Shi, Y. Microencapsulation of borage oil with blends of milk protein, β-glucan and maltodextrin through spray drying: Physicochemical characteristics and stability of the microcapsules. J. Sci. Food Agric. 2018, 98, 896–904. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid oxidation in corn-oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef]
- Surh, J.; Decker, E.A.; McClements, D.J. Properties and stability of oil-in-water emulsions stabilized by fish gelatin. Food Hyd. 2006, 20, 596–606. [Google Scholar] [CrossRef]
- Anarjan, N.; Nehdi, I.A.; Shihi, H.M.; Al-Resayes, S.I.; Jafarizadeh Malmiri, H.; Tan, C.P. Preparation of astaxanthin using gelatin-based stabilizer systems. Molecules 2014, 19, 14257–14265. [Google Scholar] [CrossRef] [Green Version]
- Goula, A.M.; Adamopoulos, K.G. A new technique for spray-dried encapsulation of lycopene. Dry. Technol. 2012, 30, 641–652. [Google Scholar] [CrossRef]
- Wolf-Schnurrbusch, U.E.K.; Zinkernagel, M.S.; Munk, M.R.; Ebneter, A.; Wolf, S. Oral lutein supplementation enhances macular pigment density and contrast sensitivity but not in combination with polyunsaturated fatty acids. IOVS 2015, 56, 8069–8074. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Henao, M.V.; Saavedra, N.; Medina, S.; Jiménez Cartagena, C.; Alzate, L.M.; Londoño-Londoño, J. Microencapsulation of lutein by spray-drying: Characterization and stability analyses to promote its use as a functional ingredient. Food Chem. 2018, 256, 181–187. [Google Scholar] [CrossRef] [PubMed]

| Casein Fraction, % | |||||
|---|---|---|---|---|---|
| Sample | αs2 | αs1 * | αs1 * | β | κ |
| Caprine casein high in αs1-I | 9.2 | 4 | 21.1 | 51.6 | 13.8 |
| Caprine casein high in αs1-II | 5.3 | … | 25.6 | 60.6 | 9.6 |
| Bovine casein | 12.1 | … | 39.5 | 37.2 | 11.2 |
| Bovine CN + BG + MD 1 | Caprine (αs1-I) CN + BG + MD | Caprine (αs1-II) CN + BG + MD | |
|---|---|---|---|
| Emulsion (before drying) | |||
| Droplet size (nm) | 202.00 ± 1.53 | 197.33 ± 0.67 | 198.00 ± 1.16 |
| Viscosity (mPa.s) | 6.73 ± 0.03 | 6.82 ± 0.07 | 6.93 ± 0.05 |
| Microcapsule (after drying) | |||
| EE 2 (%) | 91.07 ± 0.56 b | 92.76 ± 1.71 b | 97.70 ± 1.35 a |
| Moisture content (g/Kg) | 12.81 ± 0.08 a | 11.71 ± 0.22 b | 11.52 ± 0.34 b |
| Treatment/Storage Time | Persistence of Lutein (%) 2 |
|---|---|
| Microcapsules1 | |
| Bovine CN-BG-MD | 56.01 ± 0.77 c |
| Caprine (αs1-I) CN-BG-MD | 60.82 ± 0.77 b |
| Caprine (αs1-II) CN-BG-MD | 64.57 ± 0.77 a |
| Storage (week) | |
| 0 | 91.40 ± 0.77 a |
| 3 | 69.26 ± 0.77 b |
| 6 | 20.73 ± 0.77 c |
| Treatment/Storage Time | ABTS (μmol/L TE/g) 2 |
|---|---|
| Microcapsules1 | |
| Bovine CN-BG-MD | 227.81 ± 28.94 c |
| Caprine (αs1-I) CN-BG-MD | 327.26 ± 34.22 b |
| Caprine (αs1-II) CN-BG-MD | 361.42 ± 38.54 a |
| Storage (week) | |
| 0 | 425.76 ± 25.47 a |
| 3 | 298.74 ± 19.00 b |
| 6 | 191.99 ± 16.25 c |
| Microcapsules 1 | PV (meqO2/Kg) 2 | TBA (mg MDA/Kg) 2 | ||
|---|---|---|---|---|
| Week 0 | Week 6 | Week 0 | Week 6 | |
| Bovine CN-BG-MD | 0.88 ± 0.04 a | 2.63 ± 0.23 b | 0.19 ± 0.03 a | 0.86 ± 0.01 b |
| Caprine (αs1-I) CN-BG-MD | 0.87 ± 0.02 a | 2.48 ± 0.11 b | 0.18 ± 0.01 a | 0.81 ± 0.02 b |
| Caprine (αs1-II) CN-BG-MD | 0.86 ± 0.02 a | 2.35 ± 0.30 b | 0.16 ± 0.01 a | 0.76 ± 0.04 b |
| Microcapsules 1 | Methyl Octanoate (C8:0) 2 | Methyl Decanoate (C10:0) 2 |
|---|---|---|
| Bovine CN-BG-MD | 217.77 ± 11.93 | 65.33 ± 2.31 |
| Caprine (αs1-I) CN-BG-MD | 233.52 ± 13.10 | 73.50 ± 4.69 |
| Caprine (αs1-II) CN-BG-MD | 280.96 ± 33.24 | 80.16 ± 6.86 |
| Treatment/Storage Time | Persistence of Lutein (%) 2 |
|---|---|
| Microcapsules 1 | |
| Bovine CN-BG-MD | 42.81 ± 0.427 c |
| Caprine (αs1-I) CN-BG-MD | 47.99 ± 0.427 b |
| Caprine (αs1-II) CN-BG-MD | 54.26 ± 0.427 a |
| Storage (week) | |
| 0 | 90.86 ± 0.427 a |
| 3 | 42.17± 0.427 b |
| 6 | 12.02 ± 0.427 c |
| Interaction of microcapsule type × storage | |
| Bovine CN-BG-MD × week 0 | 86.44 ± 0.740 c |
| Caprine (αs1-I) CN-BG-MD × week 0 | 91.17 ± 0.740 b |
| Caprine (αs1-II) CN-BG-MD × week 0 | 94.98 ± 0.740 a |
| Bovine CN-BG-MD × week 3 | 34.96 ± 0.740 f |
| Caprine (αs1-I) CN-BG-MD × week 3 | 40.76 ± 0.740 e |
| Caprine (αs1-II) CN-BG-MD × week 3 | 50.79 ± 0.740 d |
| Bovine CN-BG-MD × week 6 | 7.04 ± 0.740 i |
| Caprine (αs1-I) CN-BG-MD × week 6 | 12.02 ± 0.740 h |
| Caprine (αs1-II) CN-BG-MD × week 6 | 17.01 ± 0.740 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Gutierrez, A.; Marquez, S.A.; Attaie, R.; Núñez de González, M.T.; Jung, Y.; Woldesenbet, S.; Moussavi, M. Mixed Biopolymer Systems Based on Bovine and Caprine Caseins, Yeast β-Glucan, and Maltodextrin for Microencapsulating Lutein Dispersed in Emulsified Lipid Carriers. Polymers 2022, 14, 2600. https://doi.org/10.3390/polym14132600
Mora-Gutierrez A, Marquez SA, Attaie R, Núñez de González MT, Jung Y, Woldesenbet S, Moussavi M. Mixed Biopolymer Systems Based on Bovine and Caprine Caseins, Yeast β-Glucan, and Maltodextrin for Microencapsulating Lutein Dispersed in Emulsified Lipid Carriers. Polymers. 2022; 14(13):2600. https://doi.org/10.3390/polym14132600
Chicago/Turabian StyleMora-Gutierrez, Adela, Sixto A. Marquez, Rahmat Attaie, Maryuri T. Núñez de González, Yoonsung Jung, Selamawit Woldesenbet, and Mahta Moussavi. 2022. "Mixed Biopolymer Systems Based on Bovine and Caprine Caseins, Yeast β-Glucan, and Maltodextrin for Microencapsulating Lutein Dispersed in Emulsified Lipid Carriers" Polymers 14, no. 13: 2600. https://doi.org/10.3390/polym14132600
APA StyleMora-Gutierrez, A., Marquez, S. A., Attaie, R., Núñez de González, M. T., Jung, Y., Woldesenbet, S., & Moussavi, M. (2022). Mixed Biopolymer Systems Based on Bovine and Caprine Caseins, Yeast β-Glucan, and Maltodextrin for Microencapsulating Lutein Dispersed in Emulsified Lipid Carriers. Polymers, 14(13), 2600. https://doi.org/10.3390/polym14132600