Preparation and Characterization of Strongly Sulfonated Acid Block and Random Copolymer Membranes for Acetic Acid Esterification with 2-Propanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Block and Random Copolymers
2.2.1. Synthesis of PAMPS-b-PMMA
2.2.2. Synthesis of PAMPS-co-PMMA
2.3. Preparation of Dense Membranes
2.4. Characterization
2.5. Esterification Reaction Performance of Copolymer Membranes
3. Results and Discussion
3.1. Synthesis and Characterization of PAMPS-b-PMMA
3.2. Synthesis and Characterization of PAMPS-co-PMMA
3.3. Ion Exchange Capacity (IEC) and Swelling Degree
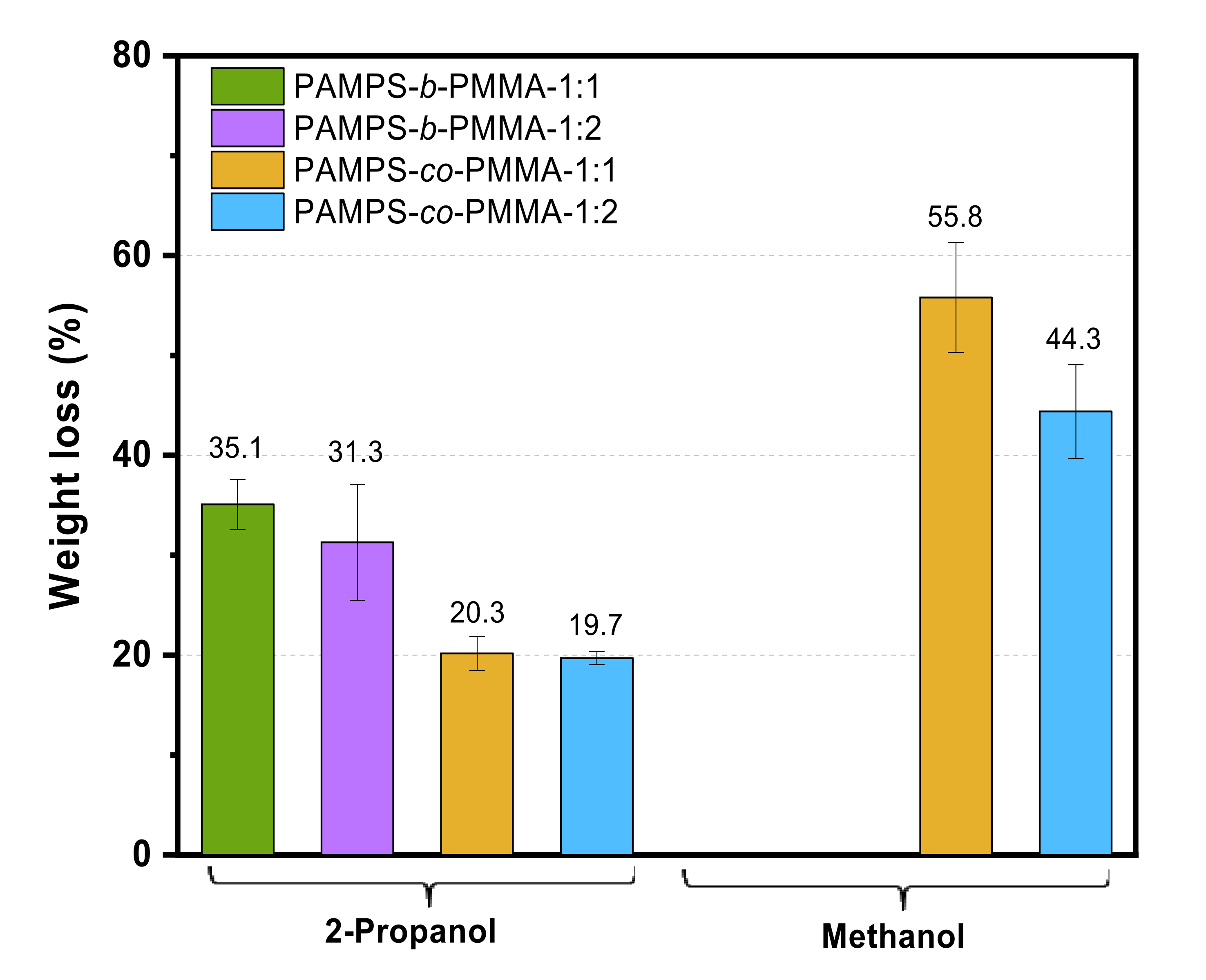
3.4. Catalytic Performance in the Esterification Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, S.; Zhang, Q.; Zhu, L.; Lin, H.; Kazanowska, B.; Doherty, C.; Hill, A.; Gao, P.; Guo, R. Highly selective and permeable microporous polymer membranes for hydrogen purification and CO2 removal from natural gas. Chem. Mater. 2018, 30, 5322–5332. [Google Scholar] [CrossRef]
- Aguilar-Vega, M.; Perez-Padilla, Y.; Loria-Bastarrachea, M.I. Sulfonated membranes from random aramide copolyisophtalamides with increasing sulfonation degree: Characterization for possible use as solid electrolyte in fuel cell. Polym. Plast. Technol. Eng. 2015, 54, 711–718. [Google Scholar] [CrossRef]
- Sequeira, R.S.; Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Ferreira, A.F.; Correia, I.J. Development of a poly(vinyl alcohol)/lysine electrospun membrane-based drug delivery system for improved skin regeneration. Int. J. Pharm. 2019, 570, 118640. [Google Scholar] [CrossRef] [PubMed]
- Shelepova, E.V.; Vedyagin, A.A. Intensification of the dehydrogenation process of different hydrocarbons in a catalytic membrane reactor. Chem. Eng. Process. Process Intensif. 2020, 155, 108072. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhang, W.; Li, W.; Xing, W. Optimization of dual-functional membrane and application for esterification enhancement. Chem. Eng. Process. Process Intensif. 2019, 139, 103–112. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Song, Y.; Ji, Y.; Cui, Z.; Li, J.; Younas, M. Biodiesel production through heterogeneous catalysis using a novel poly(phenylene sulfide) catalytic membrane. Energy Fuels 2020, 34, 7422–7429. [Google Scholar] [CrossRef]
- Heijnen, J.H.M.; De Bruijn, V.G.; Van den Broeke, L.J.P.; Keurentjes, J.T.F. Micellar catalysis for selective epoxidations of linear alkenes. Chem. Eng. Process. 2003, 42, 223–230. [Google Scholar] [CrossRef]
- Abdallah, H. A review on catalytic membranes production and applications. Bull. Chem. React. Eng. Catal. 2017, 12, 136–156. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ding, J.; Qiu, Y.; Zhao, Z. Kinetics of esterification of acidified oil with different alcohols by a cation ion-exchange resin/polyethersulfone hybrid catalytic membrane. Bioresour. Technol. 2012, 112, 28–33. [Google Scholar] [CrossRef]
- Shi, W.; He, B.; Cao, Y.; Li, J.; Yan, F.; Cui, Z.; Zou, Z.; Guo, S.; Qian, X. Continuous esterification to produce biodiesel by SPES/PES/NWF composite catalytic membrane in flow-through membrane reactor: Experimental and kinetic studies. Bioresour. Technol. 2013, 129, 100–107. [Google Scholar] [CrossRef]
- Zhu, M.; He, B.; Shi, W.; Feng, Y.; Ding, J.; Li, J.; Zeng, F. Preparation and characterization of PSSA/PVA catalytic membrane for biodiesel production. Fuel 2010, 89, 2299–2304. [Google Scholar] [CrossRef]
- Guerreiro, L.; Pereira, P.M.; Fonseca, I.M.; Martin-Aranda, R.M.; Ramos, A.M.; Diaz, J.M.L.; Oliveira, R.; Vital, J. PVA embedded hydrotalcite membranes as basic catalysts for biodiesel synthesis by soybean oil methanolysis. Catal. Today 2010, 156, 191–197. [Google Scholar] [CrossRef]
- Casimiro, M.H.; Silva, A.G.; Alvarez, R.; Ferreira, L.M.; Ramos, A.M.; Vital, J. PVA supported catalytic membranes obtained by γ-irradiation for biodiesel production. Radiat. Phys. Chem. 2014, 94, 171–175. [Google Scholar] [CrossRef]
- Shi, W.; He, B.; Li, J. Esterification of acidified oil with methanol by SPES/PES catalytic membrane. Bioresour. Technol. 2011, 102, 5389–5393. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, H.; Zhou, R.; Qin, X.; Zhang, H.; Su, Y.; Du, Q. Preparation and characterization of phosphotungstic acid/PVA nanofiber composite catalytyc membranes via electrospinning for biodiesel production. Fuel 2016, 180, 759–766. [Google Scholar] [CrossRef]
- Gómez-Trejo-López, E.; González-Díaz, M.O.; Aguilar-Vega, M. Waste cooking oil transesterification by sulfonated polyphenylsulfone catalytic membrane: Characterization and biodiesel production yield. Renew. Energy 2022, 182, 1219–1227. [Google Scholar] [CrossRef]
- Shi, W.; Yang, M.; Li, H.; Zhou, R.; Zhang, H. Preparation and characterization of sulfonated poly(ether sulfone) (SPES)/phosphotungstic acid (PWA) hybrid membrane for biodiesel production. Catal. Lett. 2015, 145, 1581–1590. [Google Scholar] [CrossRef]
- Corzo-González, Z.; Loria-Bastarrachea, M.I.; Hernández-Nuñez, E.; Aguilar-Vega, M.; González-Díaz, M.O. Preparation and characterization of crosslinked PVA/PAMPS blends catalytic membranes for biodiesel production. Polym. Bull. 2017, 74, 2741–2754. [Google Scholar] [CrossRef]
- Kuan, L.; Shih-Bo, H.; Wan-Jen, H.; Cheng-Ching, Y.; Ming-Jer, L.; Hsiao-Ping, H. Design and control of reactive distillation for ethyl and isopropyl acetates Production with Azeotropic Feeds. Chem. Eng. 2007, 62, 878. [Google Scholar]
- Qi, W.; Malone, M.F. Semibatch reactive distillation for isopropyl acetate symthesis. Ind. Eng. Chem. Res. 2011, 50, 1272–1277. [Google Scholar] [CrossRef]
- Ohyama, K.O.; Shimada, G.I.; Tokumoto, Y.; Sakamoto, K.Z. Process for the Preparation of Isopropyl Acetate. U.S. Patent 5384426, 7 December 1993. [Google Scholar]
- Zhang, W.; Qing, W.; Chen, N.; Ren, Z.; Chen, J.; Sun, W. Enhancement of esterification conversion using novel composite catalytically active pervaporation membranes. J. Memb. Sci. 2014, 451, 285–292. [Google Scholar] [CrossRef]
- Caetano, C.S.; Guerreiro, L.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Esterification of fatty acids to biodiesel over polymers with sulfonic acid groups. App. Catal. A-Gen. 2009, 359, 41–46. [Google Scholar] [CrossRef]
- Wang, T.; Shi, J.; Liang, Y.; Han, J.; Tong, Y.; Li, W. Novel SPVA/g-C3N4-SA/PAN Pervaporation Membranes with Porous Catalytic Layers for Esterification Enhancement. Ind. Eng. Chem. Res. 2021, 60, 6089–6100. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Wang, T.; Dong, W.; Li, W.; Xing, W. A novel catalytic composite membrane with anti-swelling for enhancing esterification of acetic acid with ethanol. Chem. Eng. J. Adv. 2021, 6, 100088. [Google Scholar] [CrossRef]
- Ryabov, D.A.; Le Lagadec, R.; Estevez, H.; Toscano, A.R.; Hernandez, S.; Alexandrova, L.; Kurova, S.V.; Fischer, A.; Sirlin, C.; Pfeffer, M. Synthesis, characterization, and electrochemistry of biorelevant photosensitive low potential orthometalated ruthenium complexes. Inorg. Chem. 2005, 44, 222–230. [Google Scholar] [CrossRef]
- Martínez-Cornejo, V.; Velázquez-Roblero, J.; Rosiles-González, V.; Correa-Duran, M.; Avila-Ortega, A.; Hernández-Núñez, E.; Le Lagadec, R.; González-Díaz, M.O. Synthesis of poly(2-acrylamido-2-methylpropane sulfonic acid) and its block copolymers with methyl methacrylate and 2-hydroxyethyl methacrylate by quasiliving radical polymerization catalyzed by a cyclometalated ruthenium (II) complex. Polymers 2020, 12, 1663. [Google Scholar] [CrossRef]
- Shen, Y.; Xi, J.; Qiu, X.; Zhu, W. A new proton conducting membrane based on copolymer of methyl methacrylate and 2-acrylamido-2-methyl-1-propanesulfonic acid for direct methanol fuel cells. Electrochim. Acta 2007, 52, 6956–6961. [Google Scholar] [CrossRef]
- Bagnell, L.; Cavell, K.; Hodges, A.M.; Mau, A.W.H.; Seen, A.J. The use of catalytically active pervaporation membranes in esterification reactions to simultaneously increase product yield, membrane permselectivity and flux. J. Memb. Sci. 1993, 85, 291–299. [Google Scholar] [CrossRef]
- Nadim, E.; Bouhendi, H.; Ziaee, F.; Nouri, A. Kinetic study of the aqueous free-radical polymerization of 2-acrylamido-2-methyl-1-propanesulfonic acid via an online proton nuclear magnetic resonance technique. J. Appl. Polym. Sci. 2012, 126, 156–161. [Google Scholar] [CrossRef]
- Vargas, N.A.; Espinosa, J.N.; Lopez, M.S.; Ryabov, D.A.; Le Lagadec, R.; Alexandrova, L. Light-driven living/controlled radical polymerization of hydrophobic monomers catalyzed by ruthenium (II) metalacycles. Macromolecules 2012, 45, 8135–8146. [Google Scholar]
- Evdokia, K.O.; Aikaterini, B.; Georgios, B.; Joannis, K.K. Poly (sodium styrene sulfonate)-b-poly(methyl methacrylate) diblock copolymers through direct atom transfer radical polymerization: Influence of hydrophilic–hydrophobic balance on self-organization in aqueous solution. Eur. Polym. J. 2011, 47, 752–761. [Google Scholar]
- Pal, S.; Mondal, R.; Guha, S.; Chatterjee, U.; Jewrajka, S.K. Homogeneous phase crosslinked poly(acrylonitrile-co-2-acrylamido-2methyl-1-propanesulfonic acid) conetwork cation exchange membranes showing high electrochemical properties and electrodialysis performance. Polymer 2019, 180, 121680. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Reversible addition–fragmentation chain transfer (RAFT) radical polymerization and the synthesis of water-soluble (co)polymers under homogeneous conditions in organic and aqueous media. Prog. Polym. Sci. 2007, 32, 283–351. [Google Scholar] [CrossRef]
- Mincheva, R.; Paneva, D.; Mespouille, L.; Manolova, N.; Rashkov, I.; Dubois, P. Optimized water-based ATRP of an anionic monomer: Comprehension and properties characterization. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1108–1119. [Google Scholar] [CrossRef]
- Vukovic, I.; Brinke, G.T.; Loos, K. Hexagonally perforated layer morphology in PS-b-P4VP(DPP) supramolecules. Macromolecules 2012, 45, 9409–9418. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Harris, K.D.; Wu, N.L.Y.; Murphy, J.N.; Buriak, J.M. Fast assembly of ordered block copolymer nanostructures through microwave annealing. ACS Nano. 2010, 4, 7021–7029. [Google Scholar] [CrossRef]
- Jung, B.; Kim, B.; Yang, J.M. Transport of methanol and protons through partially sulfonated polymer blend membranes for direct methanol fuel cell. J. Membr. Sci. 2004, 245, 61–69. [Google Scholar] [CrossRef]
- Park, C.; Yoon, J.; Thomas, E.L. Enabling nanotechnology with self assembled block copolymer patterns. Polymer 2003, 44, 6725–6760. [Google Scholar] [CrossRef] [Green Version]
- Truong, P.V.; Black, R.L.; Coote, J.P.; Lee, B.; Ardebili, H.; Stein, G.E. Systematic approaches to tailor the morphologies and transport properties of solution-cast sulfonatetd pentablock copolymers. ACS Appl. Polym. Mater. 2019, 1, 8–17. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, S.Y. Ion transport in sulfonated polymers. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 481–493. [Google Scholar] [CrossRef]
- Aca-Aca, G.; Loría-Bastarrachea, M.I.; Ruiz-Treviño, F.A.; Aguilar-Vega, M. Transesterification of soybean oil by PAAc catalytic membrane: Sorption properties and reactive performance for biodiesel production. Renew. Energy 2018, 116, 250–257. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–717. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Chi-An, D.; Chun-Jie, C.; An-Cheng, K.; Wei-Bor, T.; Wen-Shiang, C.; Wei-Ming, L.; Wen-Pin, S.; Chien-Ching, M. Polymer Actuator Based on PVA/PAMPS Ionic Membrane: Optimization of Ionic Transport Properties. Sens. Actuator A Phys. 2009, 155, 152–162. [Google Scholar]
- Sanz, M.T.; Gmehling, J. Esterification of fatty acids to biodiesel over polymers with sulfonic pervaporation part (I): Kinetics and pervaporation studies. Chem. Eng. J. 2006, 123, 1–8. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; M’Bareck, C.O.; David, M.O.; Métayer, M.; Alexandre, S. Ion exchange membranes made of semi-interpenetrating polymer networks, used for pervaporation-assited esterification and ion transport. Mat. Res. Innov. 2003, 7, 212–219. [Google Scholar] [CrossRef]




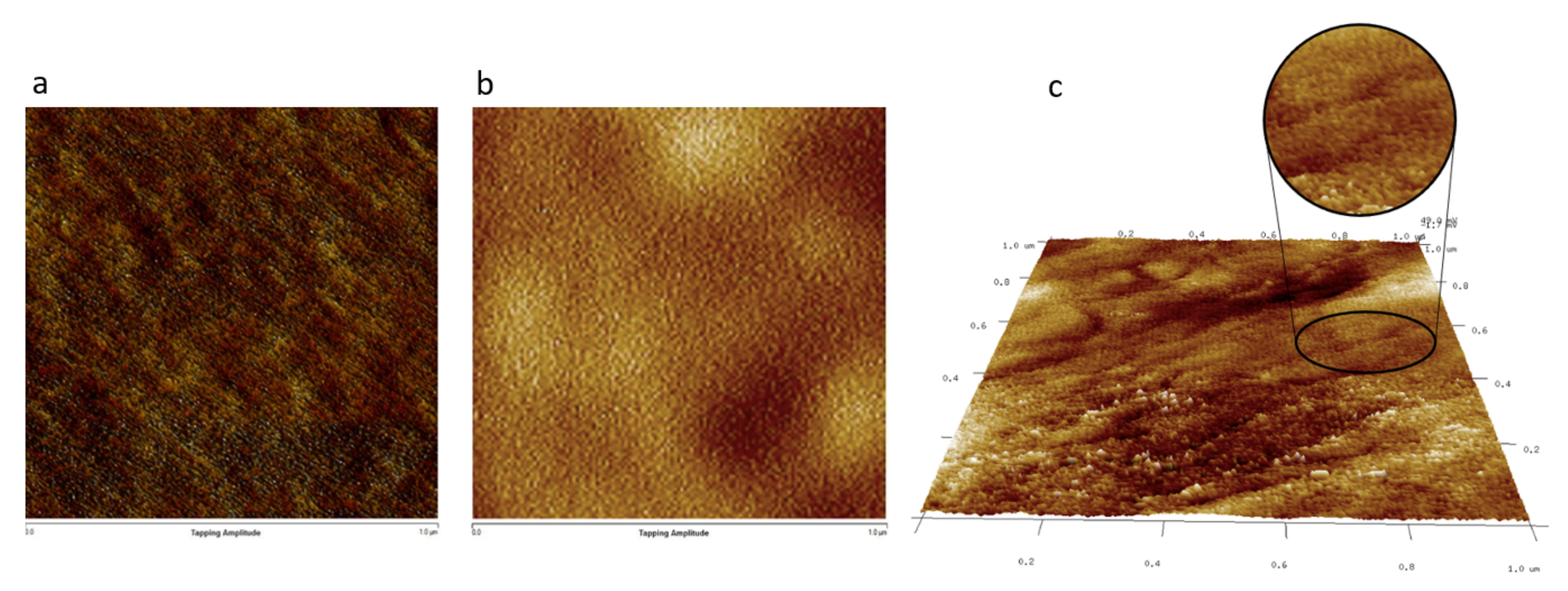


| Copolymer | PAMPS (%mmol) a | PMMA (%mmol) a | Mn × 10−4 (gmol−1) b | Mw × 10−4 (gmol−1) b | Ð b | IEC c (mmol H+g−1) |
|---|---|---|---|---|---|---|
| PAMPS-b-PMMA-1:1 | 24 | 76 | 7.85 | 11.6 | 1.48 | 1.40 |
| PAMPS-b-PMMA-1:1 | 12 | 88 | 6.80 | 9.86 | 1.45 | 0.55 |
| PAMPS-co-PMMA-1:1 | - | - | 42.5 | 81.2 | 1.91 | 2.63 |
| PAMPS-co-PMMA-1:1 | - | - | 39.7 | 74.2 | 1.87 | 1.57 |
| Material | Ref. | T (°C) | IEC mmol H+g−1 | Raw Material | Conversion (%) | Time(h) |
|---|---|---|---|---|---|---|
| Amberlyst 15 | 46 | 75 | 4.75 | Acetic acid/2-propanol | 78 | 29 |
| PVA/PSSH | 47 | 50 | - | Propanoic acid/propanol | 70 | 25 |
| PVA_SSA40 | 23 | 60 | - | Oleic acid/methanol | ~83 | 25 |
| PVA/PES/Ion-Exchange resin | 22 | 75 | - | Acetic acid/n-butanol | 68.2 | 20 |
| PAMPS-co-PMMA-1:1 | This work | 60 | 2.63 | Acetic acid/2-propanol | 85.1 | 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosiles-González, V.; Le Lagadec, R.; Varguez-Catzim, P.; Loria-Bastarrachea, M.I.; González-Díaz, A.; Hernández-Núñez, E.; Aguilar-Vega, M.; González-Díaz, M.O. Preparation and Characterization of Strongly Sulfonated Acid Block and Random Copolymer Membranes for Acetic Acid Esterification with 2-Propanol. Polymers 2022, 14, 2595. https://doi.org/10.3390/polym14132595
Rosiles-González V, Le Lagadec R, Varguez-Catzim P, Loria-Bastarrachea MI, González-Díaz A, Hernández-Núñez E, Aguilar-Vega M, González-Díaz MO. Preparation and Characterization of Strongly Sulfonated Acid Block and Random Copolymer Membranes for Acetic Acid Esterification with 2-Propanol. Polymers. 2022; 14(13):2595. https://doi.org/10.3390/polym14132595
Chicago/Turabian StyleRosiles-González, Verónica, Ronan Le Lagadec, Paulina Varguez-Catzim, María I. Loria-Bastarrachea, Abigail González-Díaz, Emanuel Hernández-Núñez, Manuel Aguilar-Vega, and María Ortencia González-Díaz. 2022. "Preparation and Characterization of Strongly Sulfonated Acid Block and Random Copolymer Membranes for Acetic Acid Esterification with 2-Propanol" Polymers 14, no. 13: 2595. https://doi.org/10.3390/polym14132595
APA StyleRosiles-González, V., Le Lagadec, R., Varguez-Catzim, P., Loria-Bastarrachea, M. I., González-Díaz, A., Hernández-Núñez, E., Aguilar-Vega, M., & González-Díaz, M. O. (2022). Preparation and Characterization of Strongly Sulfonated Acid Block and Random Copolymer Membranes for Acetic Acid Esterification with 2-Propanol. Polymers, 14(13), 2595. https://doi.org/10.3390/polym14132595









