Metal Oxide Nanoparticles: An Effective Tool to Modify the Functional Properties of Thermally Stable Polyimide Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
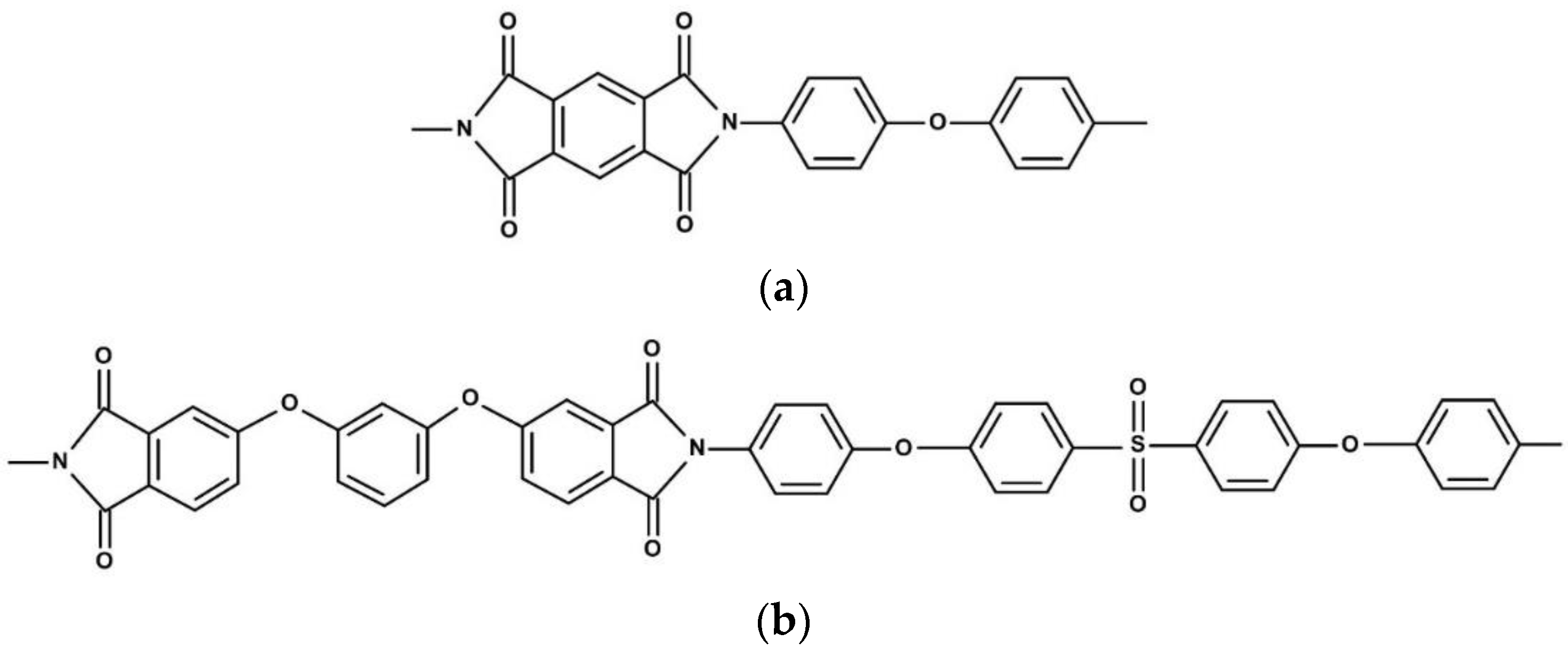
2.2. Synthesis of Nanoparticles and R-BAPS Matrix Polymer
2.3. Preparation of Pristine and Nanocomposite Films
2.4. Characterization Techniques
3. Results
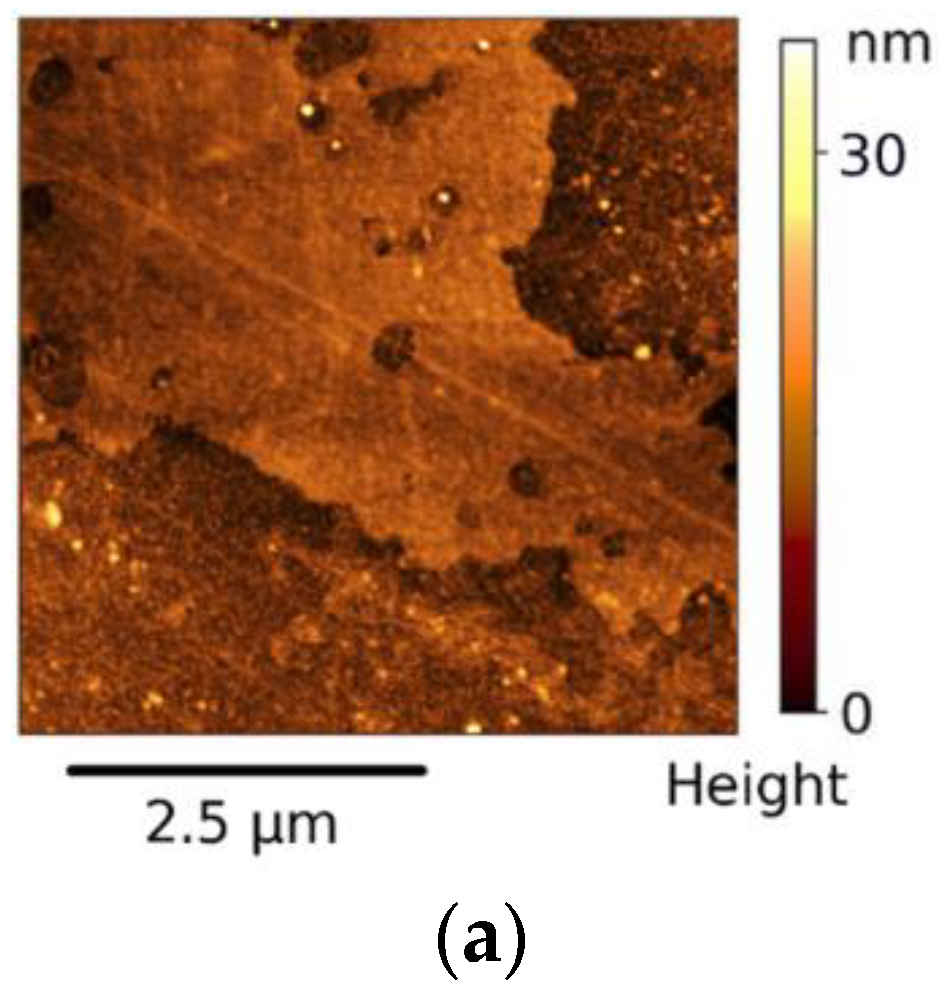
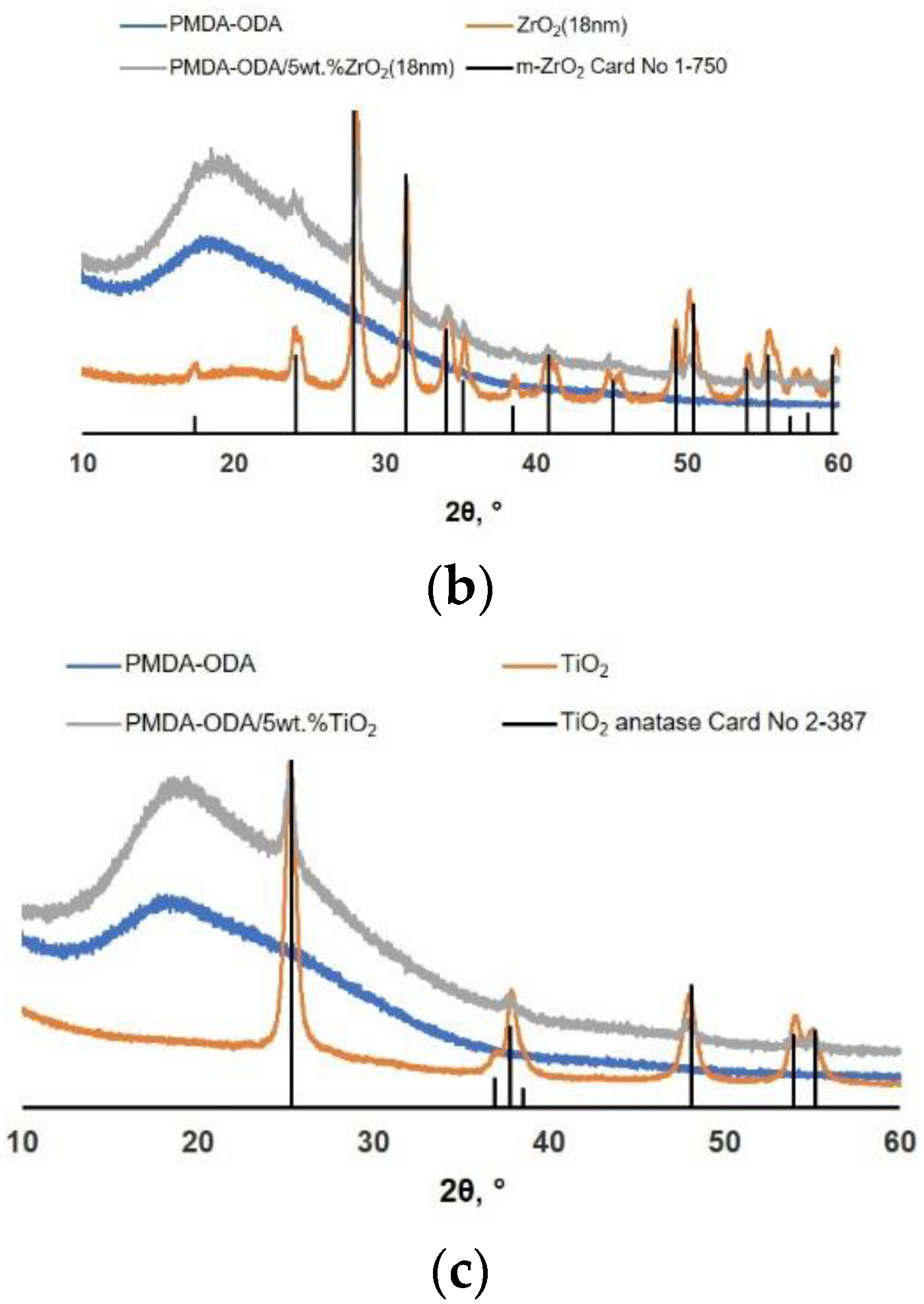
3.1. Investigation of Structure and Morphology
3.2. Thermal Properties
3.3. Mechanical Properties
3.4. Thermomechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some Basic Aspects of Polymer Nanocomposites: A Critical Review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Liu, T.; Zhong, J. Effect of Dispersion of Nano-Inorganic Particles on the Properties of Polymer Nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 563, 022026. [Google Scholar] [CrossRef]
- Meng, L.; Watson, B.W.; Qin, Y. Hybrid Conjugated Polymer/Magnetic Nanoparticle Composite Nanofibers through Cooperative Non-Covalent Interactions. Nanoscale Adv. 2020, 2, 2462–2470. [Google Scholar] [CrossRef]
- Zare, Y.; Shabani, I. Polymer/Metal Nanocomposites for Biomedical Applications. Mater. Sci. Eng. C 2016, 60, 195–203. [Google Scholar] [CrossRef]
- Faupel, F.; Zaporojtchenko, V.; Strunskus, T.; Elbahri, M. Metal-Polymer Nanocomposites for Functional Applications. Adv. Eng. Mater. 2010, 12, 1177–1190. [Google Scholar] [CrossRef]
- Diaham, S. Polyimide in Electronics: Applications and Processability Overview. In Polyimide for Electronic and Electrical Engineering Applications; BoD–Books on Demand: London, UK, 2021; p. 334. ISBN 978-1-83880-097-0. [Google Scholar]
- Sezer Hicyilmaz, A.; Celik Bedeloglu, A. Applications of Polyimide Coatings: A Review. SN Appl. Sci. 2021, 3, 363. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced Polyimide Materials: Syntheses, Physical Properties and Applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ogbonna, V.E.; Popoola, A.P.I.; Popoola, O.M.; Adeosun, S.O. A Review on Polyimide Reinforced Nanocomposites for Mechanical, Thermal, and Electrical Insulation Application: Challenges and Recommendations for Future Improvement. Polym. Bull. 2020, 79, 663–695. [Google Scholar] [CrossRef]
- Gofman, I.; Nikolaeva, A.; Yakimansky, A.; Ivanova, O.; Baranchikov, A.; Ivanov, V. Unexpected Selective Enhancement of the Thermal Stability of Aromatic Polyimide Materials by Cerium Dioxide Nanoparticles. Polym. Adv. Technol. 2019, 30, 1518–1524. [Google Scholar] [CrossRef]
- Aframehr, W.M.; Molki, B.; Bagheri, R.; Heidarian, P.; Davodi, S.M. Characterization and Enhancement of the Gas Separation Properties of Mixed Matrix Membranes: Polyimide with Nickel Oxide Nanoparticles. Chem. Eng. Res. Des. 2020, 153, 789–805. [Google Scholar] [CrossRef]
- Chiang, P.-C.; Whang, W.-T.; Tsai, M.-H.; Wu, S.-C. Physical and Mechanical Properties of Polyimide/Titania Hybrid Films. Thin Solid Film. 2004, 447, 359–364. [Google Scholar] [CrossRef]
- Dong, N.; Wang, J.; Chen, N.; Liu, B.; Tian, G.; Qi, S.; Sun, G.; Wu, D. In Situ Reinforcing: ZrO2-Armored Hybrid Polyimide Separators for Advanced and Safe Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 6250–6257. [Google Scholar] [CrossRef]
- Prasanna, S.R.V.S.; Balaji, K.; Pandey, S.; Rana, S. Metal Oxide Based Nanomaterials and Their Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–144. [Google Scholar] [CrossRef]
- Dontsova, T.A.; Nahirniak, S.V.; Astrelin, I.M. Metaloxide Nanomaterials and Nanocomposites of Ecological Purpose. J. Nanomater. 2019, 2019, 5942194. [Google Scholar] [CrossRef] [Green Version]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Nikolaeva, A.L.; Gofman, I.V.; Yakimansky, A.V.; Ivan’kova, E.M.; Gulii, N.S.; Teplonogova, M.A.; Ivanova, O.S.; Baranchikov, A.E.; Ivanov, V.K. Interplay of Polymer Matrix and Nanosized Redox Dopant with Regard to Thermo-Oxidative and Pyrolytic Stability: CeO2 Nanoparticles in a Milieu of Aromatic Polyimides. Mater. Today Commun. 2020, 22, 100803. [Google Scholar] [CrossRef]
- Meng, L.; Duwal, S.; Lane, J.M.D.; Ao, T.; Stoltzfus, B.; Knudson, M.; Park, C.; Chow, P.; Xiao, Y.; Fan, H.; et al. High Pressure Induced Atomic and Mesoscale Phase Behaviors of One-Dimensional TiO2 Anatase Nanocrystals. MRS Bull. 2022, 1–6. [Google Scholar] [CrossRef]
- Dippel, A.C.; Jensen, K.M.Ø.; Tyrsted, C.; Bremholm, M.; Bøjesen, E.D.; Saha, D.; Birgisson, S.; Christensen, M.; Billinge, S.J.L.; Iversen, B.B. Towards Atomistic Understanding of Polymorphism in the Solvothermal Synthesis of ZrO2 Nanoparticles. Acta Crystallogr. Sect. A Found. Adv. 2016, 72, 645–650. [Google Scholar] [CrossRef]
- Atabaki, F.; Ahmadizadegan, H. Fabrication of a New Polyimide/Titania (TiO2) Nanocomposite Thin Film by the Sol-Gel Route. Polym. Plast. Technol. Eng. 2015, 54, 523–531. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Smirnov, M.A.; Geydt, P.; Bugrov, A.N.; Ovaska, S.S.; Lahderanta, E.; Toikka, A.M. Structure and Transport Properties of Mixed-Matrix Membranes Based on Polyimides with ZrO2 Nanostars. Polymers 2016, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.L.; Liou, G.S. Highly Transparent and Flexible Polyimide/ZrO2 Nanocomposite Optical Films with a Tunable Refractive Index and Abbe Number. Chem. Commun. 2015, 51, 13523–13526. [Google Scholar] [CrossRef]
- Yudin, V.E.; Otaigbe, J.U.; Svetlichnyi, V.M.; Korytkova, E.N.; Almjasheva, O.V.; Gusarov, V.V. Effects of Nanofiller Morphology and Aspect Ratio on the Rheo-Mechanical Properties of Polyimide Nanocomposites. Express Polym. Lett. 2008, 2, 485–493. [Google Scholar] [CrossRef]
- Rafiee, Z.; Mallakpour, S.; Hatamvand, R. Preparation and Characterization of Heat-Resistant Polyimide/Titanium Dioxide Nanocomposite Films Containing Triptycene Side Units by Sol-Gel Processes. High Perform. Polym. 2014, 26, 373–380. [Google Scholar] [CrossRef]
- Tsai, M.H.; Chang, C.J.; Chen, P.J.; Ko, C.J. Preparation and Characteristics of Poly(Amide-Imide)/Titania Nanocomposite Thin Films. Thin Solid Films 2008, 516, 5654–5658. [Google Scholar] [CrossRef]
- Bugrov, A.N.; Smyslov, R.Y.; Zavialova, A.Y.; Kopitsa, G.P.; Khamova, T.V.; Kirilenko, D.A.; Kolesnikov, I.E.; Pankin, D.V.; Baigildin, V.A.; Licitra, C. Influence of Stabilizing Ion Content on the Structure, Photoluminescence and Biological Properties of Zr1–Xeuxo2–0.5x Nanoparticles. Crystals 2020, 10, 1038. [Google Scholar] [CrossRef]
- Bugrov, A.N.; Smyslov, R.Y.; Zavialova, A.Y.; Kopitsa, G.P. The Influence of Chemical Prehistory on the Structure, Photoluminescent Properties, Surface and Biological Characteristics of Zr0.98Eu0.02O1.99 Nanophosphors. Nanosyst. Phys. Chem. Math. 2019, 10, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Almjasheva, O.V. Heat-Stimulated Transformation of Zirconium Dioxide Nanocrystals Produced under Hydrothermal Conditions. Nanosyst. Phys. Chem. Math. 2015, 6, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Zavialova, A.Y.; Bugrov, A.N.; Smyslov, R.Y.; Kirilenko, D.A.; Khamova, T.V.; Kopitsa, G.P.; Licitra, C.; Rouchon, D. Structure and Photoluminescent Properties of TiO2:Eu3+ Nanoparticles Synthesized under Hydro and Solvothermal Conditions from Different Precursors. Nanosyst. Phys. Chem. Math. 2019, 10, 361–373. [Google Scholar] [CrossRef]
- Gofman, I.V.; Abalov, I.V.; Gladchenko, S.V.; Afanas’eva, N.V. Carbon Nanocones/Discs-a New Type of Filler to Improve the Thermal and Mechanical Properties of Polymer Films. Polym. Adv. Technol. 2012, 23, 408–413. [Google Scholar] [CrossRef]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V. Polyimides–Thermally Stable Polymers; Plenum Publishing Corp: New York, NY, USA, 1987; ISBN 978-1-4615-7636-5. [Google Scholar]
- Vonk, C.G.; Baltá-Calleja, F.J. X-Ray Scattering of Synthetic Polymers; Elsevier: Amsterdam, The Netherlands, 1989; Volume 8, p. 317. ISBN 9780444873859. [Google Scholar]
- Gambinossi, F.; Mylon, S.E.; Ferri, J.K. Aggregation Kinetics and Colloidal Stability of Functionalized Nanoparticles. Adv. Colloid Interface Sci. 2015, 222, 332–349. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown Film Extrusion of PBAT/TPS/ZnO Nanocomposites for Shelf-Life Extension of Meat Packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]
- Nikolaeva, A.L.; Gofman, I.V.; Yakimansky, A.V.; Ivan’kova, E.M.; Abalov, I.V.; Baranchikov, A.E.; Ivanov, V.K. Polyimide-Based Nanocomposites with Binary CeO2/Nanocarbon Fillers: Conjointly Enhanced Thermal and Mechanical Properties. Polymers 2020, 12, 1952. [Google Scholar] [CrossRef] [PubMed]
- Phothisarattana, D.; Harnkarnsujarit, N. Characterisations of Cassava Starch and Poly(Butylene Adipate-co-terephthalate) Blown Film with Silicon Dioxide Nanocomposites. Int. J. Food Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Ahmadizadegan, H.; Tahriri, M.; Tahriri, M.; Padam, M.; Ranjbar, M. Polyimide-TiO2 Nanocomposites and Their Corresponding Membranes: Synthesis, Characterization, and Gas Separation Applications. Solid State Sci. 2019, 89, 25–36. [Google Scholar] [CrossRef]
- Yudin, V.E.; Bugrov, A.N.; Didenko, A.L.; Smirnova, V.E.; Gofman, I.V.; Kononova, S.V.; Kremnev, R.V.; Popova, E.N.; Svetlichnyi, V.M.; Kudryavtsev, V.V. Composites of Multiblock (Segmented) Aliphatic Poly(Ester Imide) with Zirconia Nanoparticles: Synthesis, Mechanical Properties, and Pervaporation Behavior. Polym. Sci. Ser. B 2014, 56, 919–926. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Han, C.C. Understanding the Viscosity Decline of Poly(Amic Acid) Solution from the Correlation Point of View. Polymer 2020, 202, 122728. [Google Scholar] [CrossRef]
- Kreuz, J.A. Hydrolyses of Polyamic-acid Solutions. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 3787–3793. [Google Scholar] [CrossRef]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. Polyimides Thermally Stable Polymers; Springer: Berlin/Heidelberg, Germany, 1987; ISBN 978-1-4615-7636-5. [Google Scholar]
- Gofman, I.V.; Balik, K.; Cerny, M.; Zaloudkova, M.; Goikhman, M.J.; Yudin, V.E. Peculiarities of the Initial Stages of Carbonization Processes in Polyimide-Based Nanocomposite Films Containing Carbon Nanoparticles. Cogent Chem. 2015, 1, 1076712. [Google Scholar] [CrossRef]
- Sidorovich, A.V.; Kallistov, O.V.; Kydryavtsev, V.V.; Lavrent’ev, V.K.; Svetlichnyi, V.M. Nature of Fluidity of Some Polyimides. Vysok. Soedin. 1985, 27, 1254–1261. [Google Scholar]




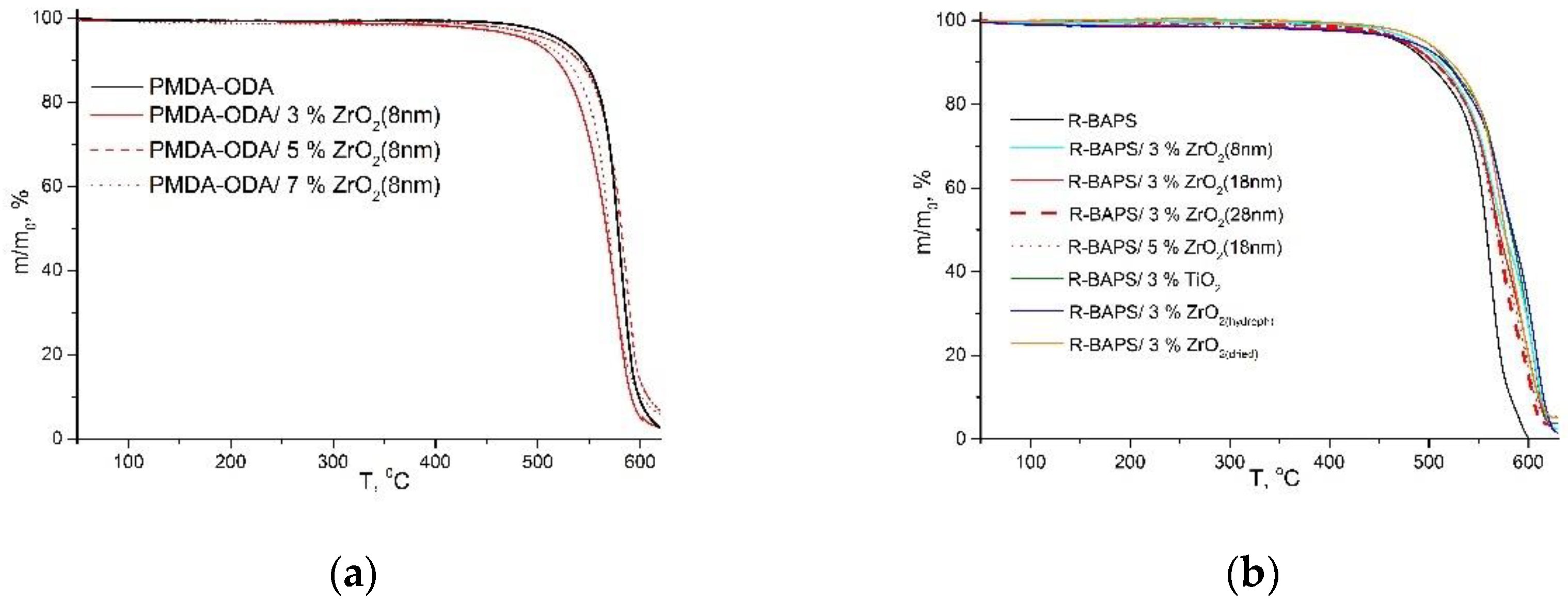

| Sample | τ10, °C | τ50, °C |
|---|---|---|
| PMDA-ODA | 546 | 578 |
| PMDA-ODA/3 wt.%ZrO2(8 nm) | 521 | 567 |
| PMDA-ODA/5 wt.%ZrO2(8 nm) | 541 | 577 |
| PMDA-ODA/7 wt.%ZrO2(8 nm) | 532 | 569 |
| PMDA-ODA/5 wt.%ZrO2(18 nm) | 553 | 581 |
| PMDA-ODA/5 wt.%ZrO2(28 nm) | 550 | 583 |
| PMDA-ODA/5 wt.%ZrO2(hydroph) | 540 | 584 |
| PMDA-ODA/5 wt.%ZrO2(dried) | 535 | 574 |
| PMDA-ODA/3 wt.%TiO2 | 548 | 584 |
| PMDA-ODA/5 wt.%TiO2 | 550 | 589 |
| R-BAPS | 504 | 557 |
| R-BAPS/3 wt.%ZrO2(8 nm) | 512 | 575 |
| R-BAPS/3 wt.%ZrO2(18 nm) | 512 | 572 |
| R-BAPS/3 wt.%ZrO2(28 nm) | 507 | 569 |
| R-BAPS/5 wt.%ZrO2(18 nm) | 509 | 568 |
| R-BAPS/3 wt.%ZrO2(hydroph) | 522 | 575 |
| R-BAPS/3 wt.%ZrO2(dried) | 524 | 585 |
| R-BAPS/3 wt.%TiO2 | 516 | 582 |
| R-BAPS/5 wt.%TiO2 | 523 | 583 |
| Sample | E, GPa | σy, MPa | σb, MPa | εb, % |
|---|---|---|---|---|
| PMDA-ODA | 2.45 (0.06) * | 100 (3) | 176 (6) | 105 (7) |
| PMDA-ODA/3 wt.%ZrO2(8 nm) | 2.63 (0.06) | 91 (3) | 110 (7) | 43 (8) |
| PMDA-ODA/5 wt.%ZrO2(8 nm) | 2.47 (0.02) | 81 (1) | 103 (2) | 28 (2) |
| PMDA-ODA/7 wt.%ZrO2(8 nm) | 2.44 (0.04) | 79 (2) | 103 (3) | 34 (3) |
| PMDA-ODA/5 wt.%ZrO2(18 nm) | 2.73 (0.09) | 102 (5) | 138 (12) | 65 (15) |
| PMDA-ODA/5 wt.%ZrO2(28 nm) | 2.67 (0.04) | 98 (2) | 124 (3) | 58 (5) |
| PMDA-ODA/5 wt.%ZrO2(hydroph) | 2.02 (0.12) | 66 (3) | 80 (7) | 26 (5) |
| PMDA-ODA/5 wt.%ZrO2(dried) | 2.60 (0.06) | 97 (2) | 115 (7) | 48 (7) |
| PMDA-ODA/3 wt.%TiO2 | 2.72 (0.09) | 92 (6) | 120 (10) | 53 (9) |
| PMDA-ODA/5 wt.%TiO2 | 2.81 (0.08) | 100 (2) | 119 (5) | 48 (5) |
| R-BAPS | 2.67 (0.06) | 96 (7) | N/A | 23 (3) |
| R-BAPS/3 wt.%ZrO2(8 nm) | 2.45 (0.09) | N/A | N/A | 9 (1) |
| R-BAPS/3 wt.%ZrO2(18 nm) | 2.67 (0.09) | 95 (3) | N/A | 14 (5) |
| R-BAPS/3 wt.%ZrO2(28 nm) | 2.71 (0.07) | 110 (3) | N/A | 11 (1) |
| R-BAPS/5 wt.%ZrO2(18 nm) | 2.10 (0.10) | 97 (1) | N/A | 14 (2) |
| R-BAPS/3 wt.% ZrO2(hydroph) | 1.90 (0.08) | 96 (4) | N/A | 12 (1) |
| R-BAPS/3 wt.%ZrO2(dried) | 2.88 (0.06) | 100 (0.2) | N/A | 7 (1) |
| R-BAPS/3 wt.%TiO2 | 2.90 (0.10) | 97 (1) | N/A | 8.2 (0.3) |
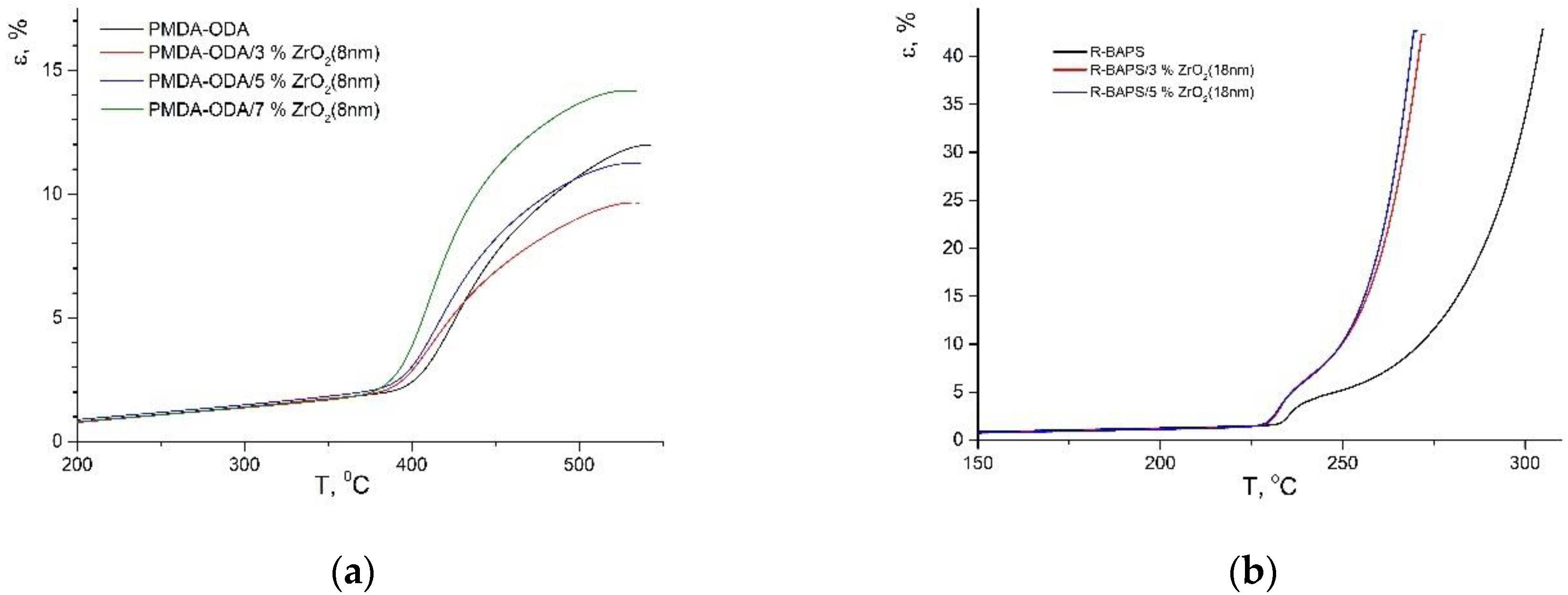
| Sample | Tg, °C | Δε (εmax − εTg) *, % |
|---|---|---|
| PMDA-ODA | 401 | 9.89 |
| PMDA-ODA/3 wt.%ZrO2(8 nm) | 391 | 7.64 |
| PMDA-ODA/5 wt.%ZrO2(8 nm) | 393 | 9.16 |
| PMDA-ODA/7 wt.%ZrO2(8 nm) | 390 | 12.30 |
| PMDA-ODA/5 wt.%ZrO2(18 nm) | 393 | 12.55 |
| PMDA-ODA/5 wt.%ZrO2(28 nm) | 393 | 12.96 |
| PMDA-ODA/3 wt.%TiO2 | 387 | 7.45 |
| PMDA-ODA/5 wt.%TiO2 | 387 | 6.21 |
| Sample | Tg, °C (TMA) | Tfl, * °C | Tg, °C (DTA) |
|---|---|---|---|
| R-BAPS | 232 | 285 | 224 |
| R-BAPS/3 wt.%ZrO2(8 nm) | 229 | 257 | 221 |
| R-BAPS/3 wt.%ZrO2(18 nm) | 230 | 259 | 223 |
| R-BAPS/3 wt.%ZrO2(28 nm) | 229 | 259 | 224 |
| R-BAPS/5 wt.%ZrO2(18 nm) | 229 | 258 | 223 |
| R-BAPS/3 wt.%TiO2 | 230 | 260 | 223 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaeva, A.L.; Bugrov, A.N.; Sokolova, M.P.; Ivan’kova, E.M.; Abalov, I.V.; Vlasova, E.N.; Gofman, I.V. Metal Oxide Nanoparticles: An Effective Tool to Modify the Functional Properties of Thermally Stable Polyimide Films. Polymers 2022, 14, 2580. https://doi.org/10.3390/polym14132580
Nikolaeva AL, Bugrov AN, Sokolova MP, Ivan’kova EM, Abalov IV, Vlasova EN, Gofman IV. Metal Oxide Nanoparticles: An Effective Tool to Modify the Functional Properties of Thermally Stable Polyimide Films. Polymers. 2022; 14(13):2580. https://doi.org/10.3390/polym14132580
Chicago/Turabian StyleNikolaeva, Alexandra L., Alexander N. Bugrov, Maria P. Sokolova, Elena M. Ivan’kova, Ivan V. Abalov, Elena N. Vlasova, and Iosif V. Gofman. 2022. "Metal Oxide Nanoparticles: An Effective Tool to Modify the Functional Properties of Thermally Stable Polyimide Films" Polymers 14, no. 13: 2580. https://doi.org/10.3390/polym14132580
APA StyleNikolaeva, A. L., Bugrov, A. N., Sokolova, M. P., Ivan’kova, E. M., Abalov, I. V., Vlasova, E. N., & Gofman, I. V. (2022). Metal Oxide Nanoparticles: An Effective Tool to Modify the Functional Properties of Thermally Stable Polyimide Films. Polymers, 14(13), 2580. https://doi.org/10.3390/polym14132580









