Indirect Additive Manufacturing: A Valid Approach to Modulate Sorption/Release Profile of Molecules from Chitosan Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. 3D Printing of the Molds
2.3. Synthesis of CS Hydrogels with Different Geometries and Surface Roughness
2.4. Characterization of the Prepared CS Hydrogels
2.4.1. Chemical Analysis
2.4.2. Swelling
2.4.3. Sorption Capacity of the CS Hydrogels
2.4.4. Release Behavior of the CS Hydrogels
2.4.5. Statistical Analysis
3. Results and Discussion
3.1. 3D Printing of Molds in ABS Comprising Different Geometries with/without Macro Hierarchical Roughness
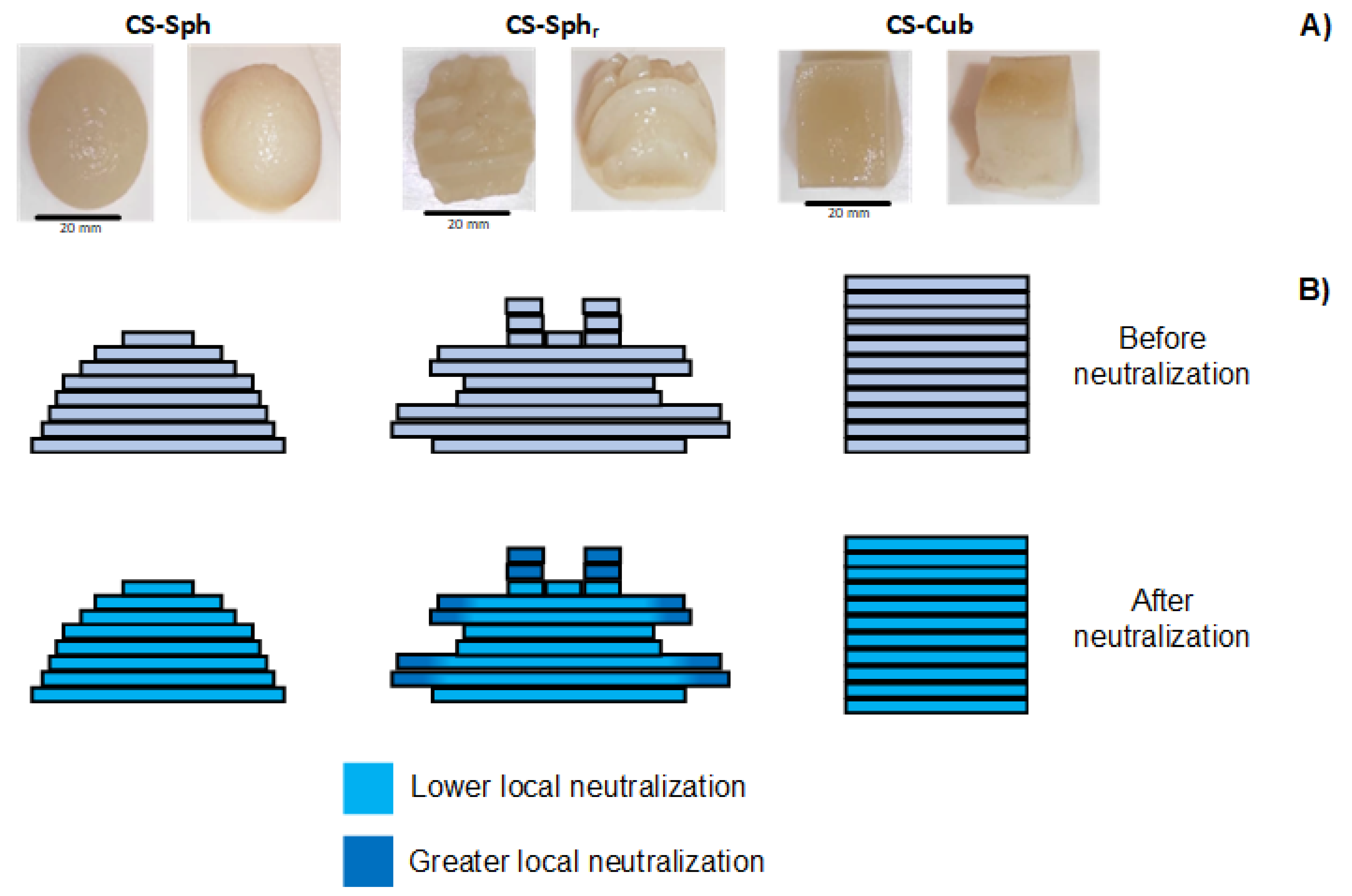
3.2. Synthesis of CS Hydrogels with Different Geometries and Surface Roughness
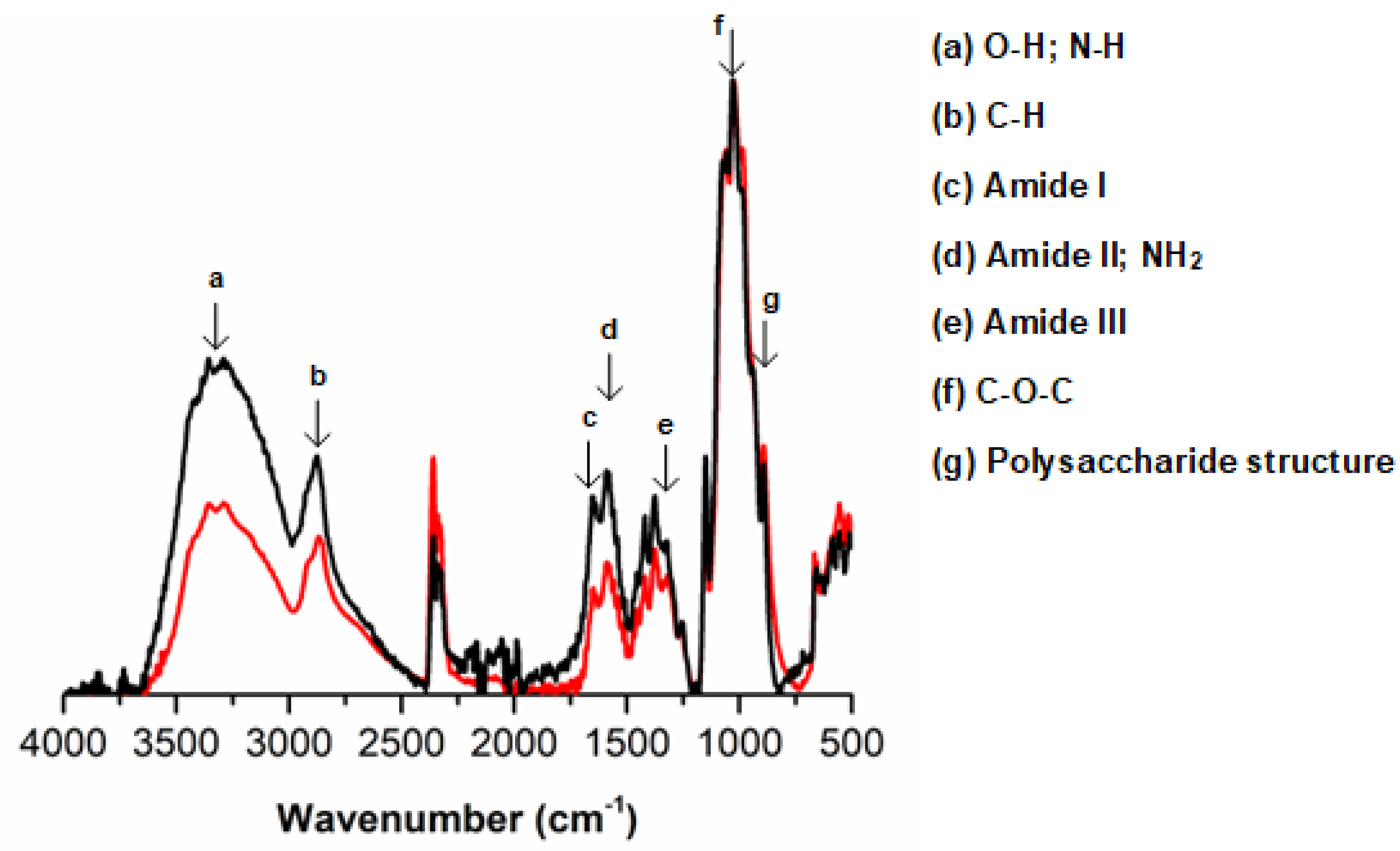
3.3. Chemical Analysis
3.4. Swelling
3.5. Sorption Capacity of the CS Hydrogels
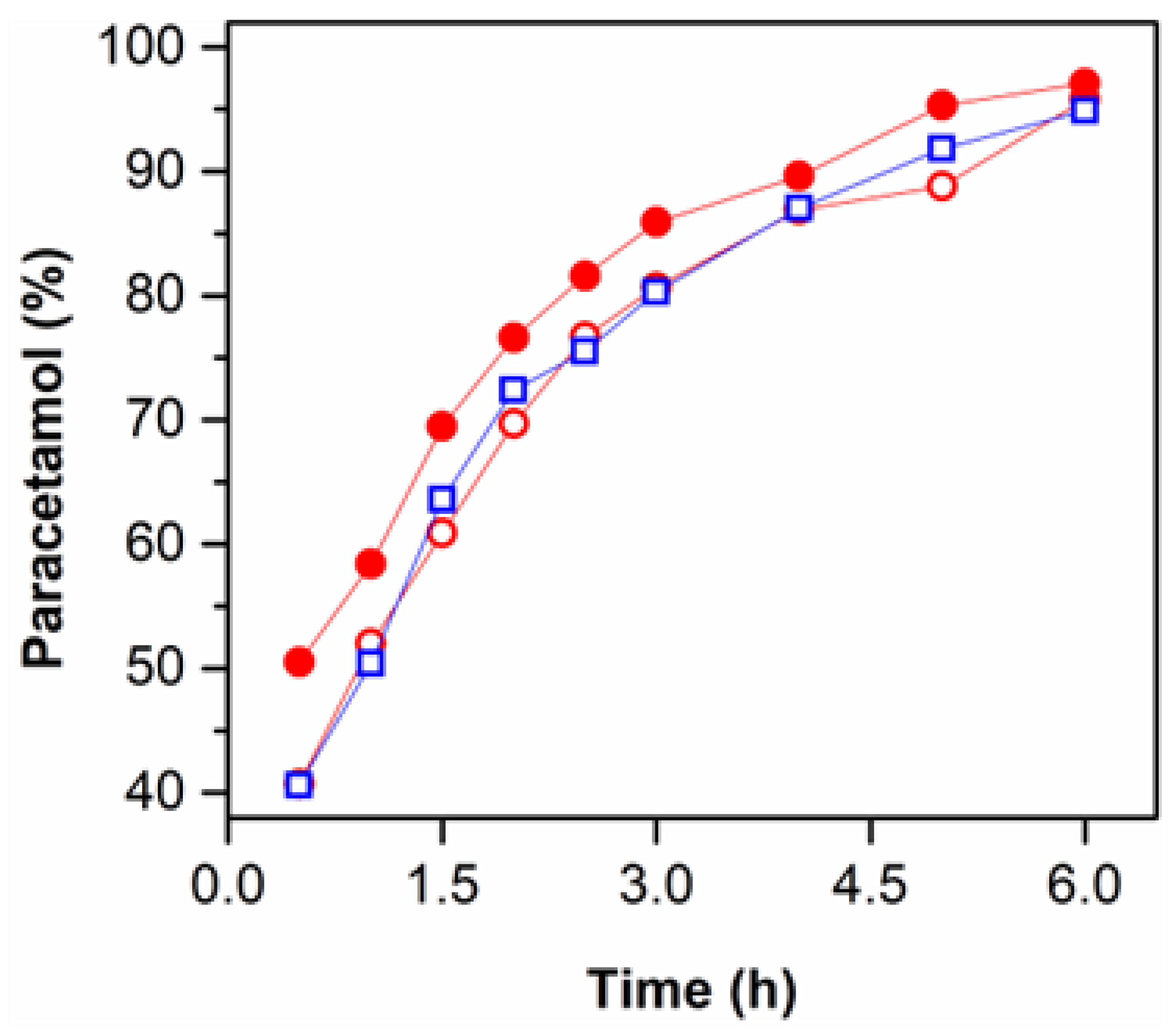
3.6. Release Profile of the Loaded CS Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haleem, A.; Javaid, M. Additive Manufacturing Applications in Industry 4.0: A Review. J. Ind. Integr. Manag. 2019, 04, 1930001. [Google Scholar] [CrossRef]
- Pinho, A.C.; Amaro, A.M.; Piedade, A.P. 3D printing goes greener: Study of the properties of post-consumer recycled polymers for the manufacturing of engineering components. Waste Manag. 2020, 118, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Vanderploeg, A.; Lee, S.-E.; Mamp, M. The application of 3D printing technology in the fashion industry. Int. J. Fash. Des. Technol. Educ. 2017, 10, 170–179. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D printing: Printing precision and application in food sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S. Application of 3D printing in medicine. Indian Heart J. 2016, 68, 108–109. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.W.J.; Le, K.Q.; Lu, Q.; Wong, C.H. An Overview of 3-D Printing in Manufacturing, Aerospace, and Automotive Industries. IEEE Potentials 2016, 35, 18–22. [Google Scholar] [CrossRef]
- Mohammed, A.; Elshaer, A.; Sareh, P.; Elsayed, M.; Hassanin, H. Additive Manufacturing Technologies for Drug Delivery Applications. Int. J. Pharm. 2020, 580, 119245. [Google Scholar] [CrossRef]
- Durga Prasad Reddy, R.; Sharma, V. Additive manufacturing in drug delivery applications: A review. Int. J. Pharm. 2020, 589, 119820. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Ginn, M.; Rastogi, V. A review of 3D printing techniques for environmental applications. Curr. Opin. Chem. Eng. 2020, 28, 173–178. [Google Scholar] [CrossRef]
- Mohd Yusoff, N.H.; Irene Teo, L.R.; Phang, S.J.; Wong, V.L.; Cheah, K.H.; Lim, S.S. Recent Advances in Polymer-based 3D Printing for Wastewater Treatment Application: An Overview. Chem. Eng. J. 2022, 429, 132311. [Google Scholar] [CrossRef]
- Piedade, A.P.; Gil, M.H.; Cavaco, M.C.; Andrade, M.E. Behaviour of catalase immobilised on poly(acrylonitrile)-g.co-hydroxyethyl methacrylate when used in a continuous system. Polym. Int. 1995, 38, 269–275. [Google Scholar] [CrossRef]
- Oliveira Brett, A.M.C.F.; Gil, M.H.; Piedade, A.P. An electrochemical bienzyme membrane sensor for free cholesterol. Bioelectrochem. Bioenerg. 1992, 28, 105–115. [Google Scholar] [CrossRef]
- Da Silva, M.A.; Gil, M.H.; Piedade, A.P.; Redinha, J.S.; Brett, A.M.O.; Costa, J.M.C. Immobilization of catalase on membranes of poly(ethylene)-g-co-acrylic acid and poly(tetrafluoroethylene)-g-co-acrylic acid and their application in hydrogen peroxide electrochemical sensors. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 269–274. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Loreti, G.; Maroni, A.; Gazzaniga, A.; Zema, L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 360–367. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Ballentine, M.L.; Das, A.; Griggs, C.S.; Klaus, K.; Bortner, M.J. Additive Manufacturing for Contaminants: Ammonia Removal Using 3D Printed Polymer-Zeolite Composites. ACS ES&T Water 2021, 1, 621–629. [Google Scholar]
- Kumbhakar, P.; Ambekar, R.S.; Mahapatra, P.L.; Sekhar Tiwary, C. Quantifying instant water cleaning efficiency using zinc oxide decorated complex 3D printed porous architectures. J. Hazard. Mater. 2021, 418, 126383. [Google Scholar] [CrossRef]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Goyanes, A.; Chang, H.; Sedough, D.; Hatton, G.B.; Wang, J.; Buanz, A.; Gaisford, S.; Basit, A.W. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int. J. Pharm. 2015, 496, 414–420. [Google Scholar] [CrossRef]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef]
- Czölderová, M.; Behúl, M.; Filip, J.; Zajíček, P.; Grabic, R.; Vojs-Staňová, A.; Gál, M.; Kerekeš, K.; Híveš, J.; Ryba, J.; et al. 3D printed polyvinyl alcohol ferrate(VI) capsules: Effective means for the removal of pharmaceuticals and illicit drugs from wastewater. Chem. Eng. J. 2018, 349, 269–275. [Google Scholar] [CrossRef]
- Pei, R.; Fan, L.; Zhao, F.; Xiao, J.; Yang, Y.; Lai, A.; Zhou, S.F.; Zhan, G. 3D-Printed metal-organic frameworks within biocompatible polymers as excellent adsorbents for organic dyes removal. J. Hazard. Mater. 2020, 384, 121418. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Y.; Zhang, X.; Zhao, X.; Ma, J.; Pu, X.; Wang, Y.; Ran, F.; Wang, Y.; Leng, F.; et al. Snakegourd root/Astragalus polysaccharide hydrogel preparation and application in 3D printing. Int. J. Biol. Macromol. 2019, 121, 309–316. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Fu, Q.; Xie, X.; Song, Y.; Xu, M.; Li, J. 3D-printed polycaprolactone-chitosan based drug delivery implants for personalized administration. Mater. Des. 2022, 214, 110394. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Barroso, N.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. 3D printable self-healing hyaluronic acid/chitosan polycomplex hydrogels with drug release capability. Int. J. Biol. Macromol. 2021, 188, 820–832. [Google Scholar] [CrossRef]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, J.; Guo, Q.; Yang, J. 3D-printed highly porous and reusable chitosan monoliths for Cu(II) removal. J. Mater. Sci. 2019, 54, 6728–6741. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Hosseini Rad, S.; Naderi, G.; Saeb, M.R.; et al. Chitosan-based inks for 3D printing and bioprinting. Green Chem. 2022, 24, 62–101. [Google Scholar] [CrossRef]
- Bergonzi, C.; Di Natale, A.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R.; Elviri, L. Study of 3D-printed chitosan scaffold features after different post-printing gelation processes. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Gholamali, I. Stimuli-Responsive Polysaccharide Hydrogels for Biomedical Applications: A Review. Regen. Eng. Transl. Med. 2021, 7, 91–114. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef] [PubMed]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Bikiaris, D.N.; Mitropoulos, A.C. Chitosan adsorbents for dye removal: A review. Polym. Int. 2017, 66, 1800–1811. [Google Scholar] [CrossRef]
- Appuhamillage, G.A.; Berry, D.R.; Benjamin, C.E.; Luzuriaga, M.A.; Reagan, J.C.; Gassensmith, J.J.; Smaldone, R.A. A biopolymer-based 3D printable hydrogel for toxic metal adsorption from water. Polym. Int. 2019, 68, 964–971. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.M.; Pinho, A.C.; Piedade, A.P. Mechanical properties of 3D printed mouthguards: Influence of layer height and device thickness. Mater. Des. 2021, 203, 109624. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Z.; Hu, Q. Chitosan Hydrogel Structure Modulated by Metal Ions. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Han, J.; Lin, H. Fabrication and characterization of a self-crosslinking chitosan hydrogel under mild conditions without the use of strong bases. Carbohydr. Polym. 2017, 156, 372–379. [Google Scholar] [CrossRef]
- Nie, J.; Lu, W.; Ma, J.; Yang, L.; Wang, Z.; Qin, A.; Hu, Q. Orientation in multi-layer chitosan hydrogel: Morphology, mechanism, and design principle. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ben Difallah, B.; Kharrat, M.; Dammak, M.; Monteil, G. Mechanical and tribological response of ABS polymer matrix filled with graphite powder. Mater. Des. 2012, 34, 782–787. [Google Scholar] [CrossRef]
- Gabriele, F.; Donnadio, A.; Casciola, M.; Germani, R.; Spreti, N. Ionic and covalent crosslinking in chitosan-succinic acid membranes: Effect on physicochemical properties. Carbohydr. Polym. 2021, 251, 117106. [Google Scholar] [CrossRef]
- Mauricio-Sánchez, R.A.; Salazar, R.; Luna-Bárcenas, J.G.; Mendoza-Galván, A. FTIR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vib. Spectrosc. 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Özbaş, Z.; Gürdaʇ, G. Swelling kinetics, mechanical properties, and release characteristics of chitosan-based semi-IPN hydrogels. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Noriega, S.E.; Subramanian, A. Consequences of Neutralization on the Proliferation and Cytoskeletal Organization of Chondrocytes on Chitosan-Based Matrices. Int. J. Carbohydr. Chem. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Beil, S.; Schamberger, A.; Naumann, W.; MacHill, S.; Van Pée, K.H. Determination of the degree of N-acetylation (DA) of chitin and chitosan in the presence of water by first derivative ATR FTIR spectroscopy. Carbohydr. Polym. 2012, 87, 117–122. [Google Scholar] [CrossRef]
- Takara, E.A.; Marchese, J.; Ochoa, N.A. NaOH treatment of chitosan films: Impact on macromolecular structure and film properties. Carbohydr. Polym. 2015, 132, 25–30. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer. 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Drozdov, A.D.; Declaville Christiansen, J. Swelling of p H -sensitive hydrogels. Phys. Rev. E—Stat. Nonlinear Soft Matter Phys. 2015, 91, 1–15. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, M.F.; Kanaan, A.F.; Piedade, A.P. Indirect Additive Manufacturing: A Valid Approach to Modulate Sorption/Release Profile of Molecules from Chitosan Hydrogels. Polymers 2022, 14, 2530. https://doi.org/10.3390/polym14132530
Moreira MF, Kanaan AF, Piedade AP. Indirect Additive Manufacturing: A Valid Approach to Modulate Sorption/Release Profile of Molecules from Chitosan Hydrogels. Polymers. 2022; 14(13):2530. https://doi.org/10.3390/polym14132530
Chicago/Turabian StyleMoreira, Mariana F., Akel F. Kanaan, and Ana P. Piedade. 2022. "Indirect Additive Manufacturing: A Valid Approach to Modulate Sorption/Release Profile of Molecules from Chitosan Hydrogels" Polymers 14, no. 13: 2530. https://doi.org/10.3390/polym14132530
APA StyleMoreira, M. F., Kanaan, A. F., & Piedade, A. P. (2022). Indirect Additive Manufacturing: A Valid Approach to Modulate Sorption/Release Profile of Molecules from Chitosan Hydrogels. Polymers, 14(13), 2530. https://doi.org/10.3390/polym14132530







