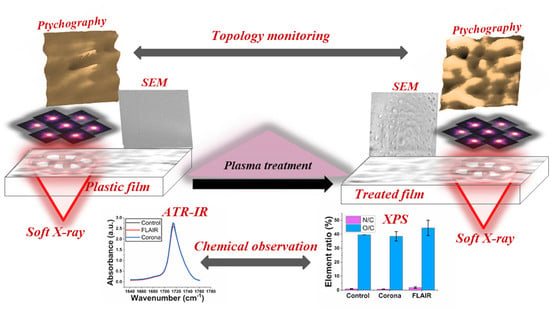
X-ray Ptychographic Imaging and Spectroscopic Studies of Plasma-Treated Plastic Films
Abstract
1. Introduction
2. Materials and Methods
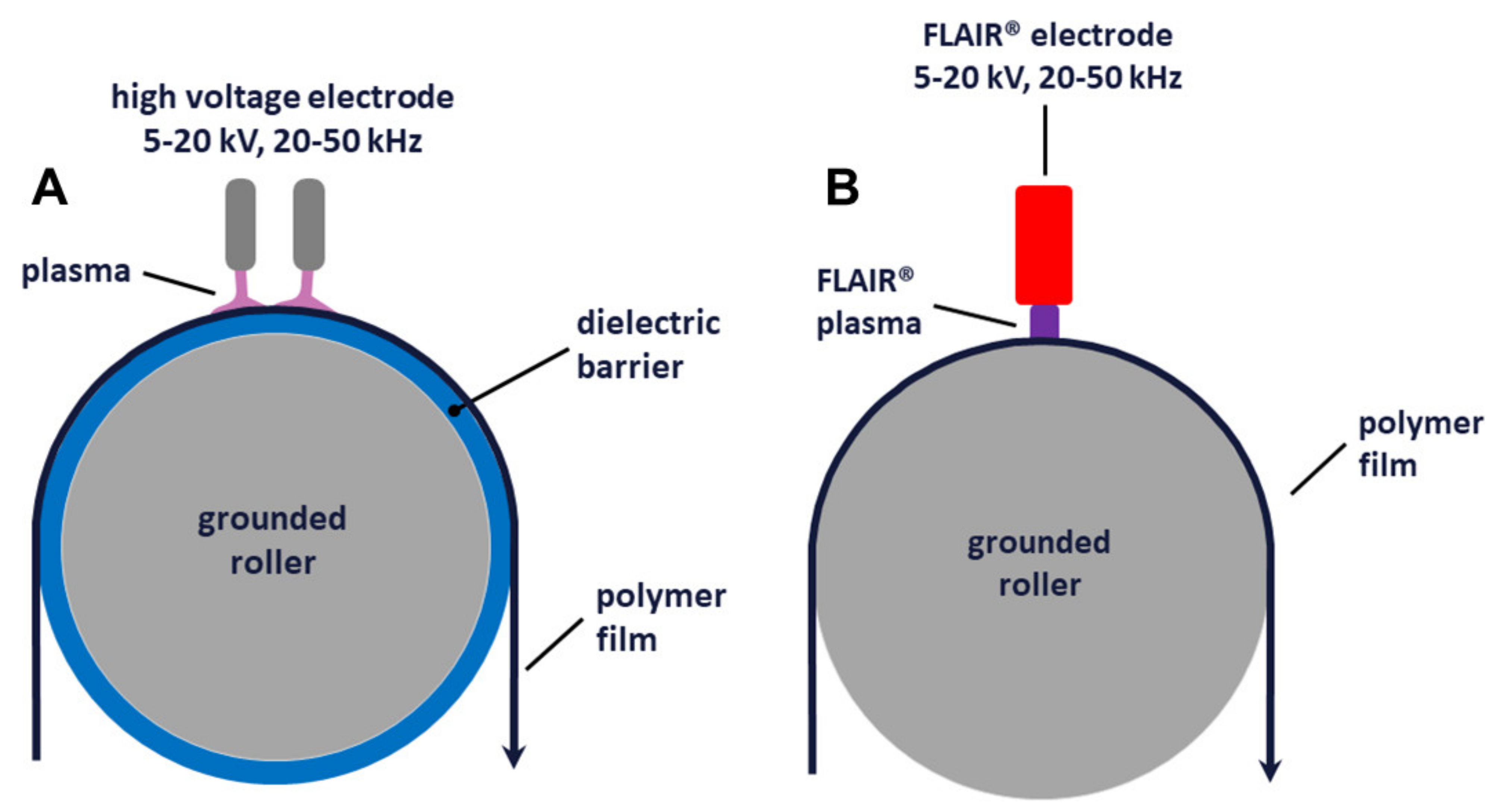
2.1. Plasma Treatments
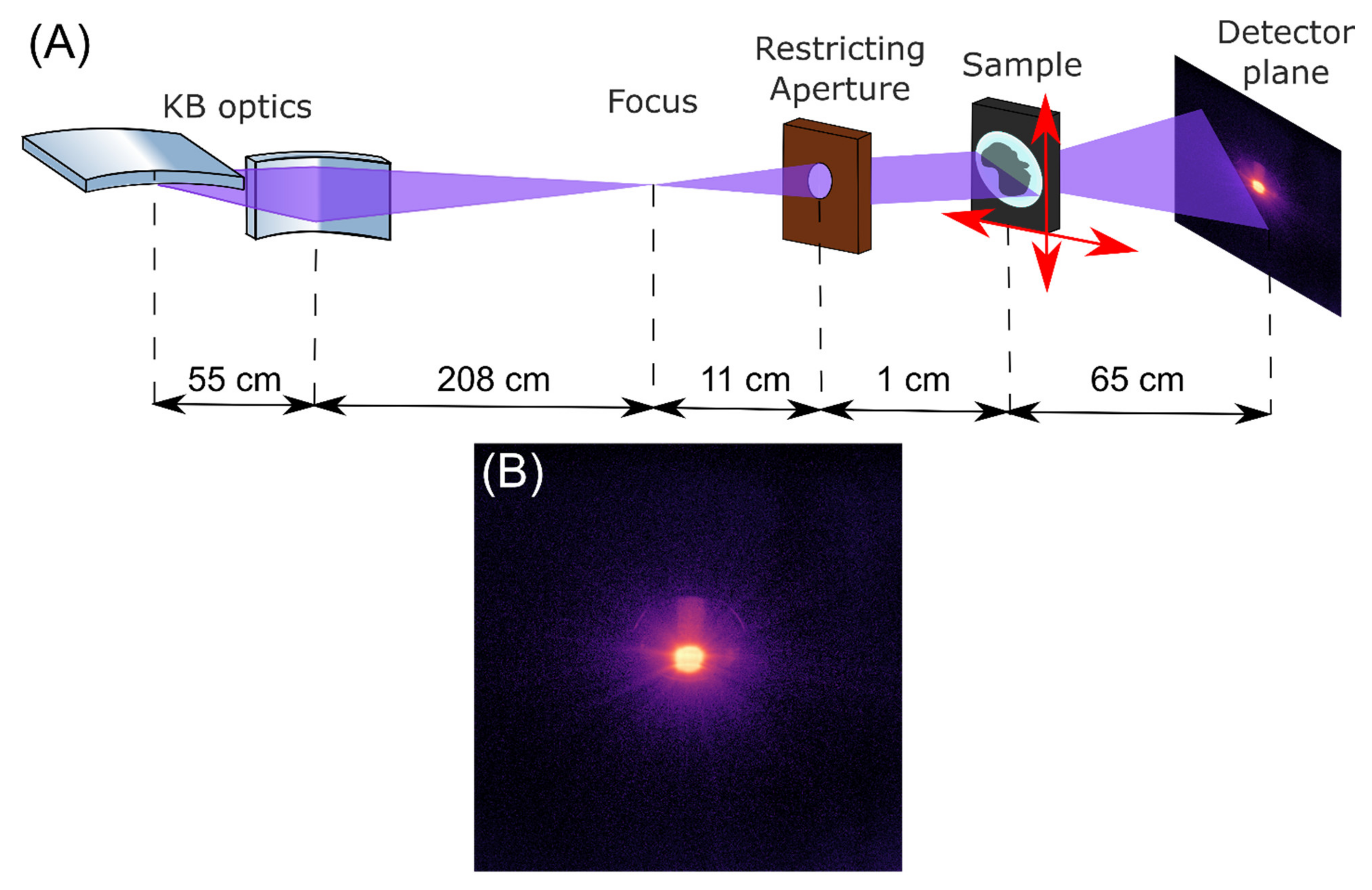
2.2. X-ray Ptychography
2.3. Scanning Electron Microscopy (SEM)
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Attenuated Total Reflection-Infrared Spectrometry (ATR-IR)
3. Results and Discussion
3.1. Surface Morphology of Plasma-Treated PET Films
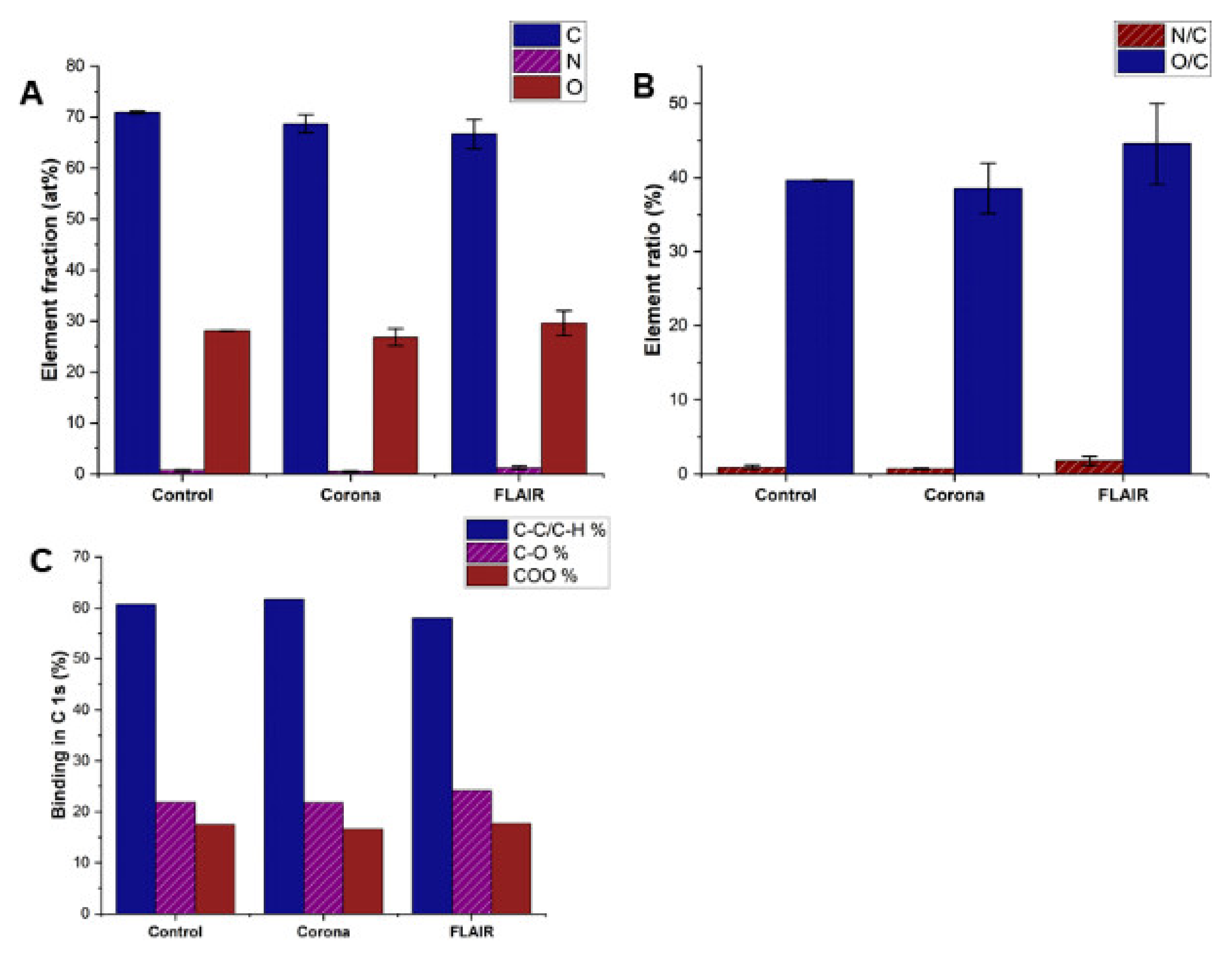
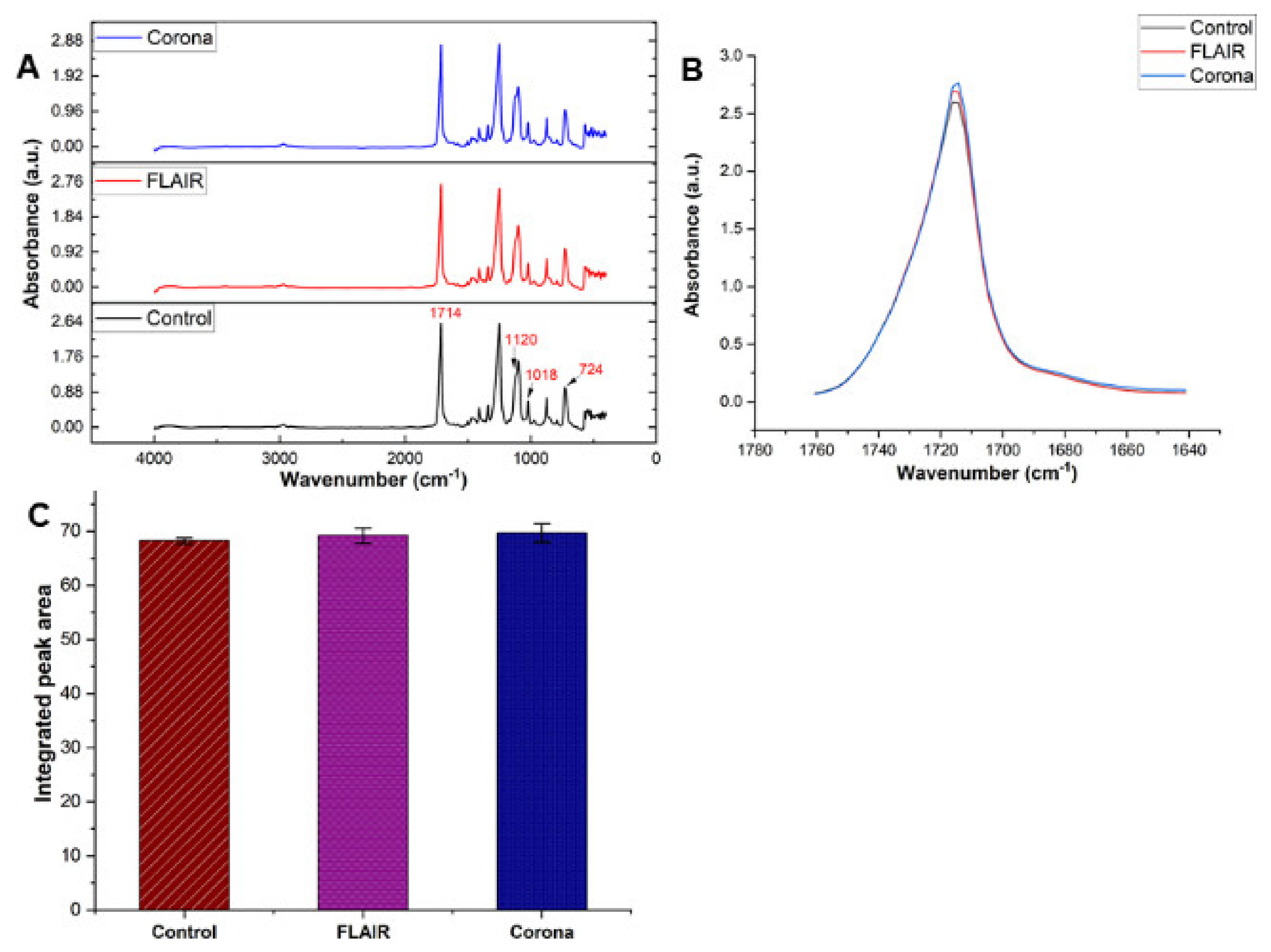
3.2. Surface Chemistry of Plasma Treated PET Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thurston, R.M.; Clay, J.D.; Schulte, M.D. Effect of atmospheric plasma treatment on polymer surface energy and adhesion. J. Plast. Film Sheet. 2007, 23, 63–78. [Google Scholar] [CrossRef]
- Gotoh, K.; Yasukawa, A.; Kobayashi, Y. Wettability characteristics of poly(ethylene terephthalate) films treated by atmospheric pressure plasma and ultraviolet excimer light. Polym. J. 2011, 43, 545–551. [Google Scholar] [CrossRef][Green Version]
- Kim, E.Y.; Kong, J.S.; An, S.K.; Kim, H.D. Surface modification of polymers and improvement of the adhesion between evaporated copper metal film and a polymer. I. Chemical modification of PET. J. Adhes. Sci. Technol. 2000, 14, 1119–1130. [Google Scholar] [CrossRef]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef] [PubMed]
- Ozaltin, K.; Lehocky, M.; Humpolicek, P.; Pelkova, J.; Di Martino, A.; Karakurt, I.; Saha, P. Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization. Polymers 2019, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Levchenko, I.; Xu, S.Y.; Baranov, O.; Bazaka, O.; Ivanova, E.P.; Bazaka, K. Plasma and Polymers: Recent Progress and Trends. Molecules 2021, 26, 4091. [Google Scholar] [CrossRef] [PubMed]
- Wefers, L.; Knittel, D.; Bosbach, D.; Rammensee, W.; Schollmeyer, E. Surface Reconstruction of Uv-Laser Irradiated Poly(Ethylene-Terephthalate) by Atomic Force Microscopy. Appl. Surf. Sci. 1992, 59, 267–271. [Google Scholar] [CrossRef]
- Walzak, M.J.; Flynn, S.; Foerch, R.; Hill, J.M.; Karbashewski, E.; Lin, A.; Strobel, M. UV and ozone treatment of polypropylene and poly(ethylene terephthalate). J. Adhes. Sci. Technol. 1995, 9, 1229–1248. [Google Scholar] [CrossRef]
- Almazan-Almazan, M.C.; Paredes, J.I.; Perez-Mendoza, M.; Dominco-Garcia, M.; Lopez-Garzon, F.J.; Martinez-Alonso, A.; Tascon, J.M.D. Surface characterisation of plasma-modified poly(ethylene terephthalate). J. Colloid Interface Sci. 2006, 293, 353–363. [Google Scholar] [CrossRef]
- Fourche, G. An Overview of the Basic Aspects of Polymer Adhesion. 2. Application to Surface Treatments. Polym. Eng. Sci. 1995, 35, 968–975. [Google Scholar] [CrossRef]
- Chang, J.S.; Lawless, P.A.; Yamamoto, T. Corona Discharge Processes. IEEE Trans. Plasma Sci. 1991, 19, 1152–1166. [Google Scholar] [CrossRef]
- Kinloch, A.J. Surface analysis and pretreatment of plastics and metals. D. M. Brewis (Editor). Applied Science, London 1982, £24, ISBN 0-35334-992-4, pp 268 + xvi. Surface Interface Anal. 1982, 4, ii. [Google Scholar] [CrossRef]
- Ahsan, H.; Ali, A.; Ali, R. Oxygen free radicals and systemic autoimmunity. Clin. Exp. Immunol. 2003, 131, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, M.; Lahti, J.; Lavonen, J.; Penttinen, T.; Rasanen, J.P.; Kuusipalo, J. The Influence of Flame, Corona and Atmospheric Plasma Treatments on Surface Properties and Digital Print Quality of Extrusion Coated Paper. J. Adhes. Sci. Technol. 2010, 24, 471–492. [Google Scholar] [CrossRef]
- Inagaki, N.; Narushim, K.; Tuchida, N.; Miyazak, K. Surface characterization of plasma-modified poly(ethylene terephthalate) film surfaces. J. Polym. Sci. B Polym. Phys. 2004, 42, 3727–3740. [Google Scholar] [CrossRef]
- Huhtamaki, T.; Tian, X.L.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements (vol 13, pg 1521, 2018). Nat. Protoc. 2019, 14, 2259. [Google Scholar] [CrossRef]
- Giewekemeyer, K.; Thibault, P.; Kalbfleisch, S.; Beerlink, A.; Kewish, C.M.; Dierolf, M.; Pfeiffer, F.; Salditt, T. Quantitative biological imaging by ptychographic x-ray diffraction microscopy. Proc. Natl. Acad. Sci. USA 2010, 107, 529–534. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, J.J.; Yao, Y.D.; Klug, J.A.; Mashrafi, S.; Roehrig, C.; Preissner, C.; Marin, F.S.; Cai, Z.H.; Lai, B.R.Y.; et al. Achieving high spatial resolution in a large field-of-view using lensless x-ray imaging. Appl. Phys. Lett. 2021, 119, 124101. [Google Scholar] [CrossRef]
- Guizar-Sicairos, M.; Thibault, P. Ptychography: A solution to the phase problem. Phys. Today 2021, 74, 42–48. [Google Scholar] [CrossRef]
- Pan, A.; Zuo, C.; Yao, B. High-resolution and large field-of-view Fourier ptychographic microscopy and its applications in biomedicine. Rep. Prog. Phys. 2020, 83, 096101. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. X-Rays. In Scanning Electron Microscopy and X-Ray Microanalysis; Goldstein, J.I., Newbury, D.E., Michael, J.R., Ritchie, N.W.M., Scott, J.H.J., Joy, D.C., Eds.; Springer: New York, NY, USA, 2018; pp. 39–63. [Google Scholar] [CrossRef]
- Pfeiffer, F. X-ray ptychography. Nat. Photonics 2018, 12, 9–17. [Google Scholar] [CrossRef]
- Enfedaque, P.; Chang, H.; Krishnan, H.; Marchesini, S. GPU-Based Implementation of Ptycho-ADMM for High Performance X-Ray Imaging. In Proceedings of the Computational Science—ICCS 2018, Cham, Switzerland, 12 June 2018; pp. 540–553. [Google Scholar]
- Maiden, A.; Johnson, D.; Li, P. Further improvements to the ptychographical iterative engine. Optica 2017, 4, 736–745. [Google Scholar] [CrossRef]
- Li, P.; Edo, T.; Batey, D.; Rodenburg, J.; Maiden, A. Breaking ambiguities in mixed state ptychography. Opt. Express 2016, 24, 9038–9052. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, K.; Mehrjoo, M.; Ruiz-Lopez, M.; Keitel, B.; Kreis, S.; Seyrich, M.; Pop, M.; Plönjes, E. Flexible ptychography platform to expand the potential of imaging at free electron lasers. Opt. Express 2021, 29, 22345–22365. [Google Scholar] [CrossRef]
- Plönjes, E.; Faatz, B.; Kuhlmann, M.; Treusch, R. FLASH2: Operation, beamlines, and photon diagnostics. AIP Conf. Proc. 2016, 1741, 020008. [Google Scholar] [CrossRef]
- Ackermann, W.; Asova, G.; Ayvazyan, V.; Azima, A.; Baboi, N.; Bähr, J.; Balandin, V.; Beutner, B.; Brandt, A.; Bolzmann, A.; et al. Operation of a free-electron laser from the extreme ultraviolet to the water window. Nat. Photonics 2007, 1, 336–342. [Google Scholar] [CrossRef]
- Faatz, B.; Plönjes, E.; Ackermann, S.; Agababyan, A.; Asgekar, V.; Ayvazyan, V.; Baark, S.; Baboi, N.; Balandin, V.; von Bargen, N.; et al. Simultaneous operation of two soft x-ray free-electron lasers driven by one linear accelerator. New J. Phys. 2016, 18, 062002. [Google Scholar] [CrossRef]
- Gay, D.M. Semiautomatic Differentiation for Efficient Gradient Computations. In Proceedings of Automatic Differentiation: Applications, Theory, and Implementations; Springer: Berlin/Heidelberg, Germany, 2006; pp. 147–158. [Google Scholar]
- Raimondi, L.; Svetina, C.; Mahne, N.; Cocco, D.; Capotondi, F.; Pedersoli, E.; Manfredda, M.; Kiskinova, M.; Keitel, B.; Brenner, G.; et al. Status of the K-B Bendable Optics at FERMI@Elettra FEL; SPIE: San Diego, CA, USA, 2014; Volume 9208. [Google Scholar]
- Attwood, D.; Sakdinawat, A. X-Rays and Extreme Ultraviolet Radiation: Principles and Applications, 2nd ed.; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Hahn, U.; Tiedtke, K. The Gas Attenuator of FLASH at DESY. AIP Conf. Proc. 2007, 879, 276–282. [Google Scholar] [CrossRef]
- Wodzinski, T.; Mehrjoo, M.; Ruiz-Lopez, M.; Keitel, B.; Kuhlmann, M.; Brachmanski, M.; Künzel, S.; Fajardo, M.; Plönjes, E. Single-shot transverse coherence measurements with Young’s double pinholes at FLASH2. J. Phys. Commun. 2020, 4, 075014. [Google Scholar] [CrossRef]
- Banterle, N.; Bui, K.H.; Lemke, E.A.; Beck, M. Fourier ring correlation as a resolution criterion for super-resolution microscopy. J. Struct. Biol. 2013, 183, 363–367. [Google Scholar] [CrossRef]
- Farr, N.T.H.; Roman, S.; Schäfer, J.; Quade, A.; Lester, D.; Hearnden, V.; MacNeil, S.; Rodenburg, C. A novel characterisation approach to reveal the mechano-chemical effects of oxidation and dynamic distension on polypropylene surgical mesh. RSC Adv. 2021, 11, 34710–34723. [Google Scholar] [CrossRef] [PubMed]
- Senge, J.F.; Astaraee, A.H.; Dłotko, P.; Bagherifard, S.; Bosbach, W.A. Extending conventional surface roughness ISO parameters using topological data analysis for shot peened surfaces. Sci. Rep. 2022, 12, 5538. [Google Scholar] [CrossRef]
- Polášková, K.; Klíma, M.; Jeníková, Z.; Blahová, L.; Zajíčková, L. Effect of Low Molecular Weight Oxidized Materials and Nitrogen Groups on Adhesive Joints of Polypropylene Treated by a Cold Atmospheric Plasma Jet. Polymers 2021, 13, 4396. [Google Scholar] [CrossRef] [PubMed]
- Dorai, R.; Kushner, M.J. Plasma surface modification of polymers using atmospheric pressure discharges. In IEEE Conference Record-Abstracts, Proceedings of the 2002 IEEE International Conference on Plasma Science (Cat. No.02CH37340), Banff, AB, Canada, 26–30 May 2002; p. 329. [Google Scholar]
- Poletti, G.; Orsini, F.; Raffaele-Addamo, A.; Riccardi, C.; Selli, E. Cold plasma treatment of PET fabrics: AFM surface morphology characterisation. Appl. Surf. Sci. 2003, 219, 311–316. [Google Scholar] [CrossRef]
- Drobota, M.; Aflori, M.; Barboiu, V. Protein Immobilization on Poly( Ethylene Terephthalate) Films Modified by Plasma and Chemical Treatments. Dig. J. Nanomater. Biostruct. 2010, 5, 35–42. [Google Scholar]
- Barbaro, G.; Galdi, M.R.; Di Maio, L.; Incarnato, L. Effect of BOPET film surface treatments on adhesion performance of biodegradable coatings for packaging applications. Eur. Polym. J. 2015, 68, 80–89. [Google Scholar] [CrossRef]
- Drobota, M.; Persin, Z.; Zemljic, L.F.; Mohan, T.; Stana-Kleinschek, K.; Doliska, A.; Bracic, M.; Ribitsch, V.; Harabagiu, V.; Coseri, S. Chemical modification and characterization of poly(ethylene terephthalate) surfaces for collagen immobilization. Cent. Eur. J. Chem. 2013, 11, 1786–1798. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Jokinen, V.; Suvanto, P.; Franssila, S. Oxygen and nitrogen plasma hydrophilization and hydrophobic recovery of polymers. Biomicrofluidics 2012, 6, 016501. [Google Scholar] [CrossRef]
- Jucius, D.; Kopustinskas, V.; Grigaliunas, V.; Guobiene, A.; Lazauskas, A.; Andrulevicius, M. Highly hydrophilic poly(ethylene terephthalate) films prepared by combined hot embossing and plasma treatment techniques. Appl. Surf. Sci. 2015, 349, 200–210. [Google Scholar] [CrossRef]
- Kirov, K.R.; Assender, H.E. Quantitative ATR-IR analysis of anisotropic polymer films: Surface structure of commercial PET. Macromolecules 2005, 38, 9258–9265. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Chan, K.L.A. ATR-FTIR spectroscopic imaging: Recent advances and applications to biological systems. Analyst 2013, 138, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.B.; Rubner, M.F.; Cohen, R.E. Depth-profiling X-ray photoelectron spectroscopy (XPS) analysis of interlayer diffusion in polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA 2013, 110, 6651–6656. [Google Scholar] [CrossRef]
- Marras, A.; Correa, J.; Lange, S.; Vardanyan, V.; Gerhardt, T.; Kuhn, M.; Krivan, F.; Shevyakov, I.; Zimmer, M.; Hoesch, M.; et al. Characterization of the Percival detector with soft X-rays. J. Synchrotron Radiat. 2021, 28, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Shanblatt, E.R.; Porter, C.L.; Gardner, D.F.; Mancini, G.F.; Karl, R.M.; Tanksalvala, M.D.; Bevis, C.S.; Vartanian, V.H.; Kapteyn, H.C.; Adams, D.E.; et al. Quantitative Chemically Specific Coherent Diffractive Imaging of Reactions at Buried Interfaces with Few Nanometer Precision. Nano Lett. 2016, 16, 5444–5450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gardner, D.F.; Seaberg, M.D.; Shanblatt, E.R.; Kapteyn, H.C.; Murnane, M.M.; Adams, D.E. High contrast 3D imaging of surfaces near the wavelength limit using tabletop EUV ptychography. Ultramicroscopy 2015, 158, 98–104. [Google Scholar] [CrossRef] [PubMed]
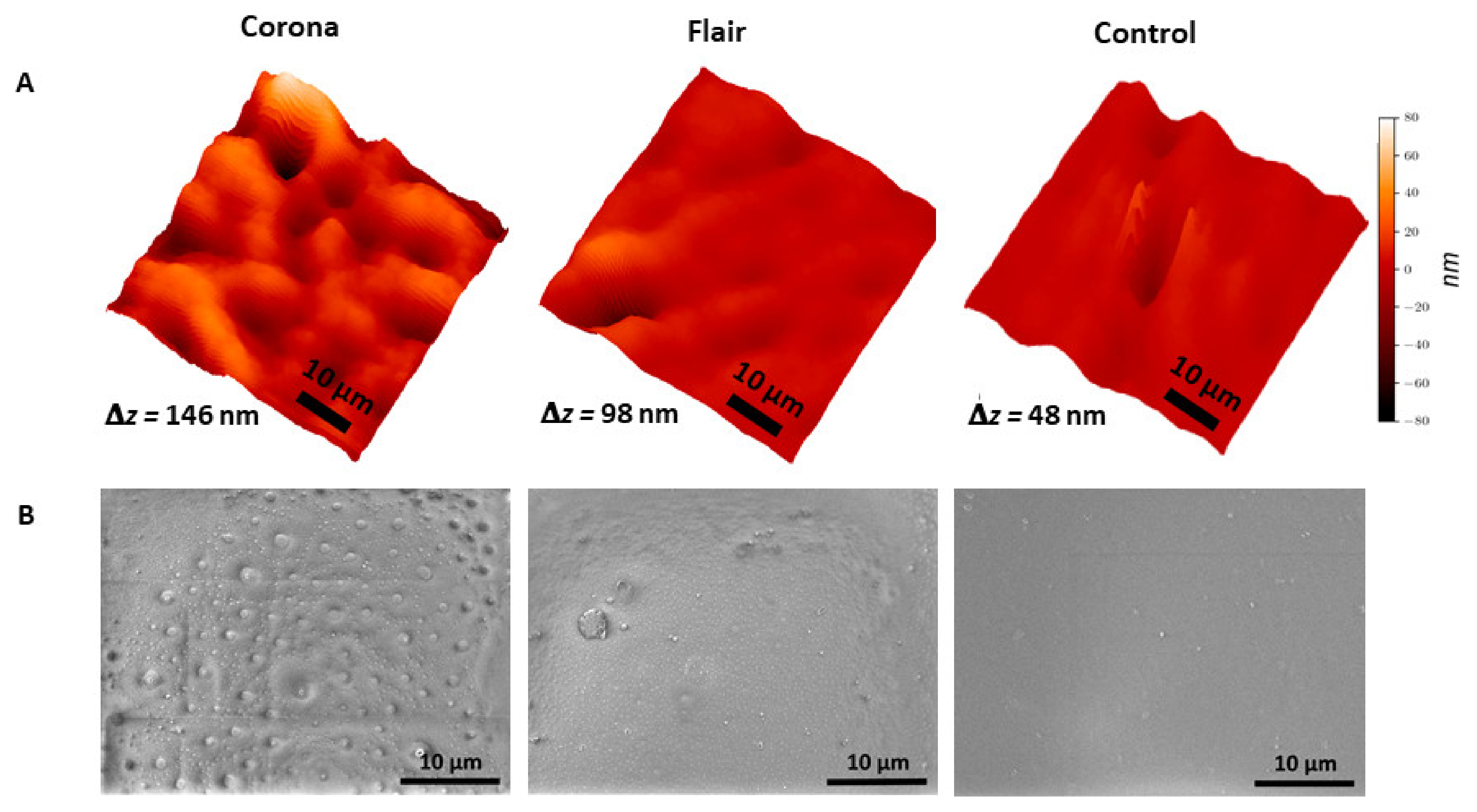
| Roughness Parameters | Corona (5 W cm−2 & 3.6 s) | FLAIR® (200 W cm−2 & 0.09 s) | Control |
|---|---|---|---|
| Rq (nm) | 20.98 ± 1.12 | 7.07 ± 1.01 | 3.83 ± 0.86 |
| Sdr | 0.45 ± 0.03 | 0.05 ± 0.004 | 0.02 ± 0.004 |
| Rku | 2.97 ± 0.67 | 18.41 ± 0.98 | 8.29 ± 0.25 |
| Rsk | −0.75 ± 0.05 | −2.76 ± 0.02 | −1.68 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravandeh, M.; Mehrjoo, M.; Kharitonov, K.; Schäfer, J.; Quade, A.; Honnorat, B.; Ruiz-Lopez, M.; Keitel, B.; Kreis, S.; Pan, R.; et al. X-ray Ptychographic Imaging and Spectroscopic Studies of Plasma-Treated Plastic Films. Polymers 2022, 14, 2528. https://doi.org/10.3390/polym14132528
Ravandeh M, Mehrjoo M, Kharitonov K, Schäfer J, Quade A, Honnorat B, Ruiz-Lopez M, Keitel B, Kreis S, Pan R, et al. X-ray Ptychographic Imaging and Spectroscopic Studies of Plasma-Treated Plastic Films. Polymers. 2022; 14(13):2528. https://doi.org/10.3390/polym14132528
Chicago/Turabian StyleRavandeh, Mehdi, Masoud Mehrjoo, Konstantin Kharitonov, Jan Schäfer, Antje Quade, Bruno Honnorat, Mabel Ruiz-Lopez, Barbara Keitel, Svea Kreis, Rui Pan, and et al. 2022. "X-ray Ptychographic Imaging and Spectroscopic Studies of Plasma-Treated Plastic Films" Polymers 14, no. 13: 2528. https://doi.org/10.3390/polym14132528
APA StyleRavandeh, M., Mehrjoo, M., Kharitonov, K., Schäfer, J., Quade, A., Honnorat, B., Ruiz-Lopez, M., Keitel, B., Kreis, S., Pan, R., Gang, S.-g., Wende, K., & Plönjes, E. (2022). X-ray Ptychographic Imaging and Spectroscopic Studies of Plasma-Treated Plastic Films. Polymers, 14(13), 2528. https://doi.org/10.3390/polym14132528