Comparison of Properties of Colorless and Transparent Polyimide Nanocomposites Containing Chemically Modified Nanofillers: Functionalized-Graphene and Organoclay
Abstract
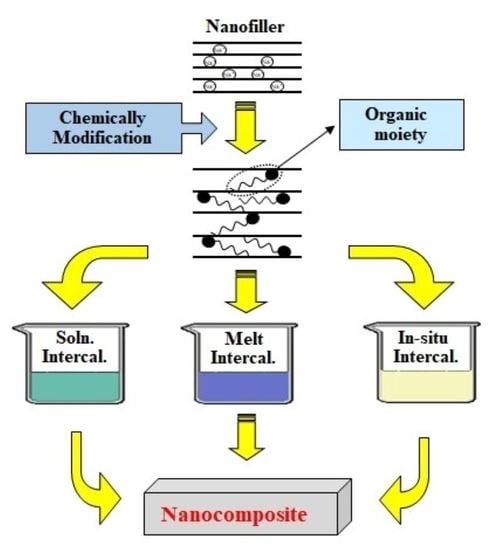
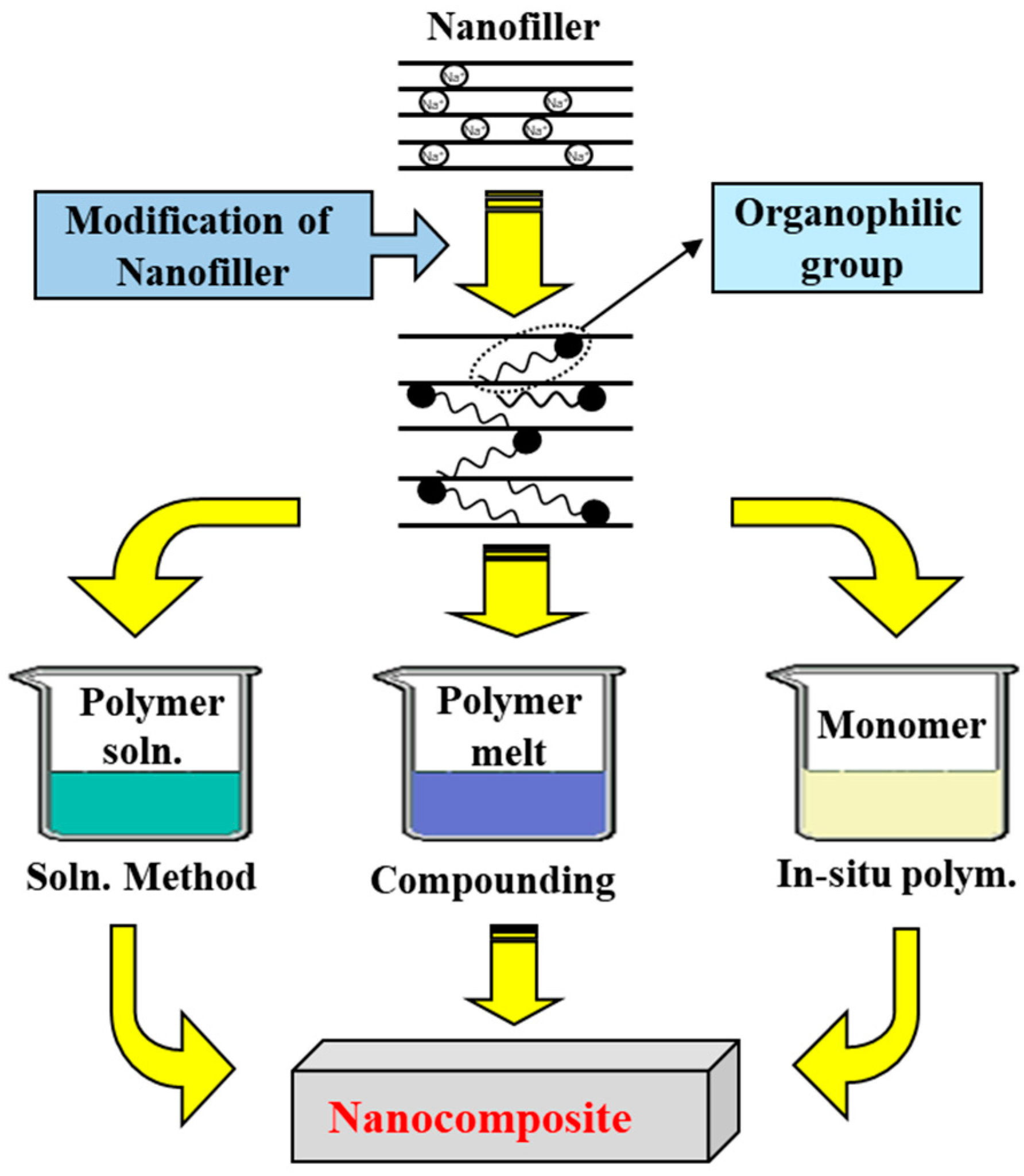
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses of Nanofillers
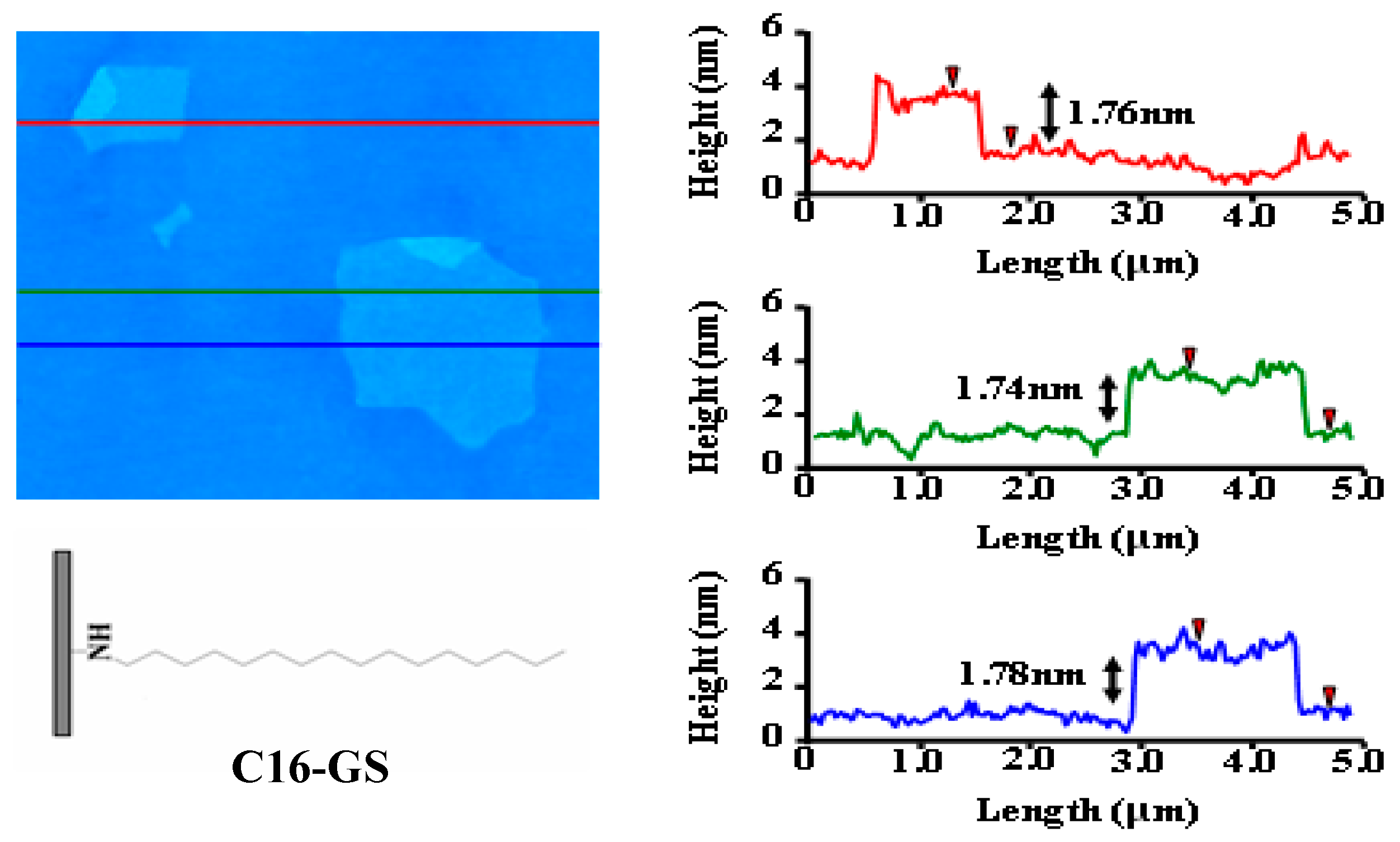
2.2.1. Synthesis of C16-GS
2.2.2. Synthesis of C16-MMT
2.3. Synthesis of PAA
2.4. Preparation of the CPI Hybrid Film
2.5. Characterization
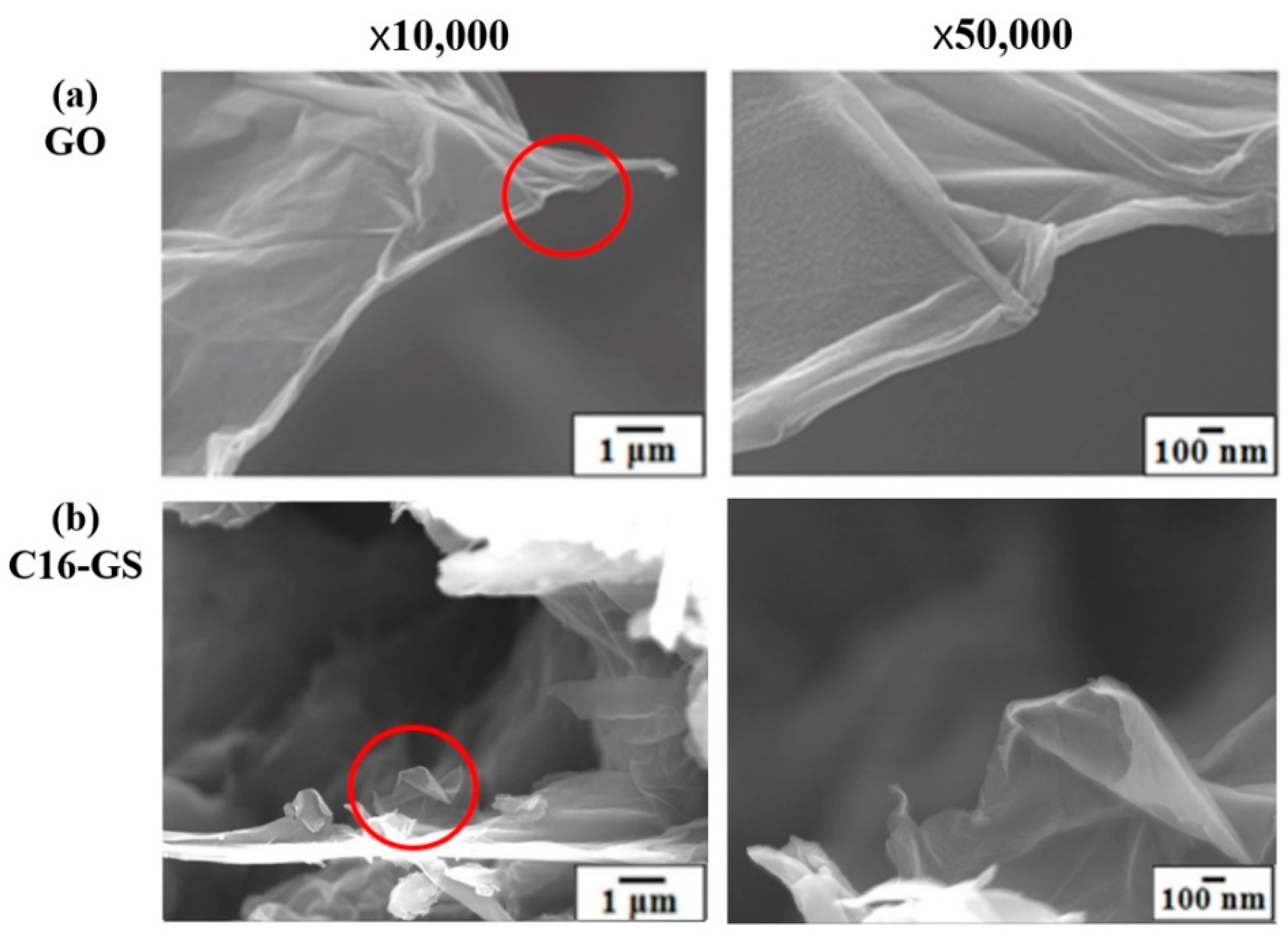
2.5.1. Atomic Force Microscopy (AFM), Field Emission Scanning Electron Microscopy (FE-SEM), and Transmission Electron Microscopy (TEM) Analyses
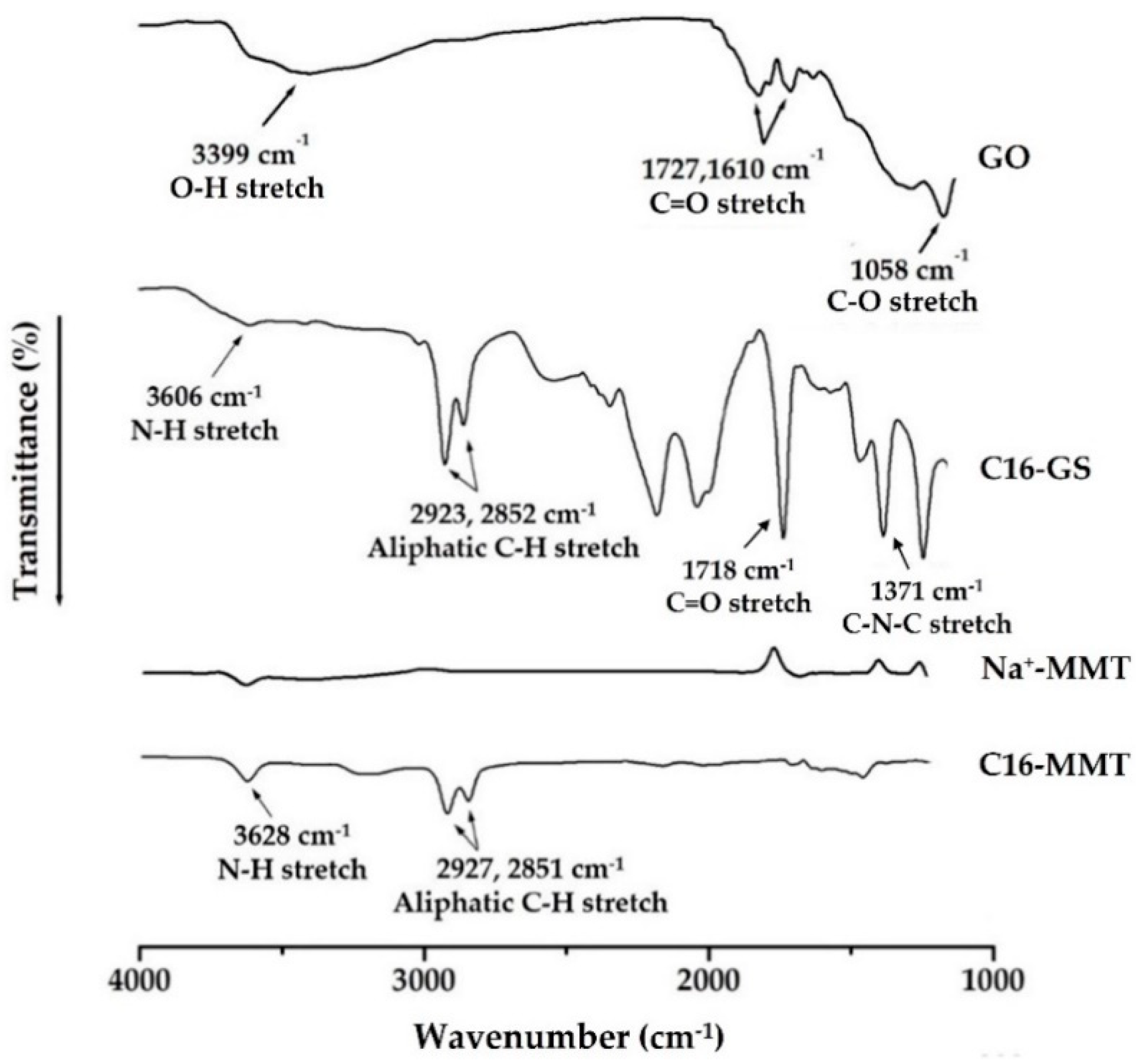
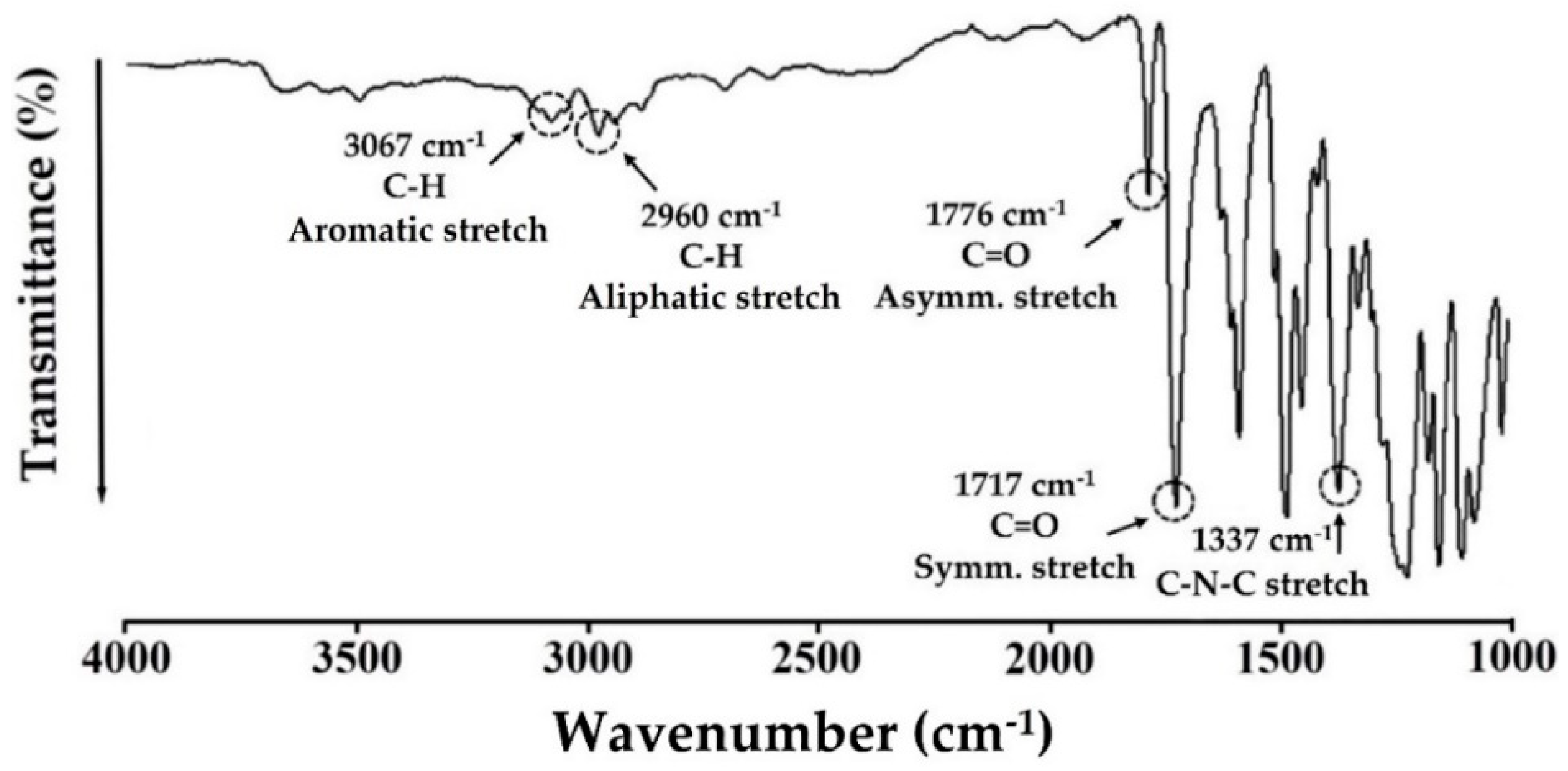
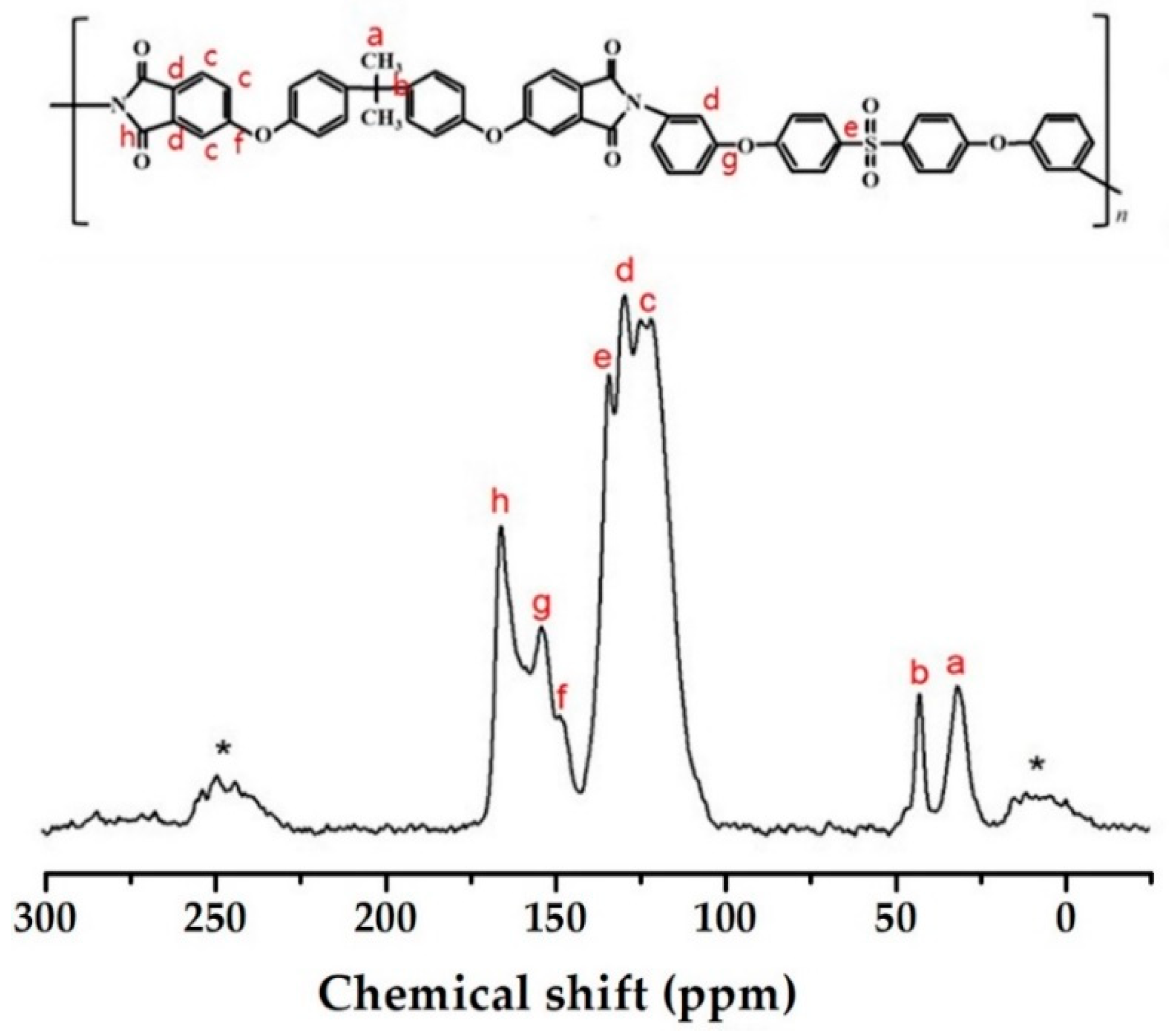
2.5.2. Fourier Transform Infrared (FT-IR) and 13C Magic Angle Spinning-Nuclear Magnetic Resonance (MAS NMR) Spectroscopy Analyses
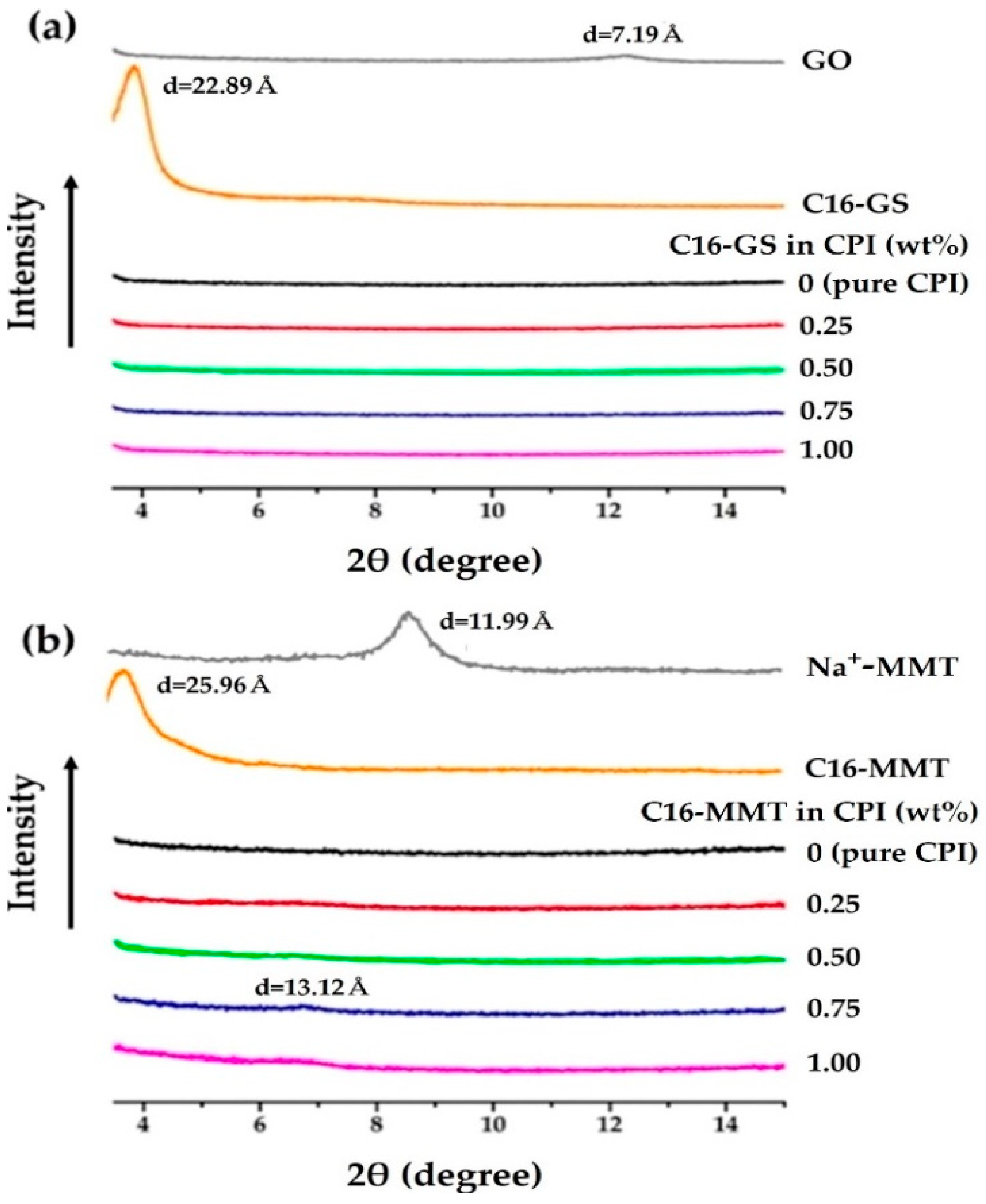
2.5.3. X-ray Diffraction (XRD) Analysis
2.5.4. Thermal Analysis
2.5.5. Optical Properties
3. Results and Discussion
3.1. Nanofillers
3.2. CPI Structure
3.3. XRD Analysis
3.4. Morphology
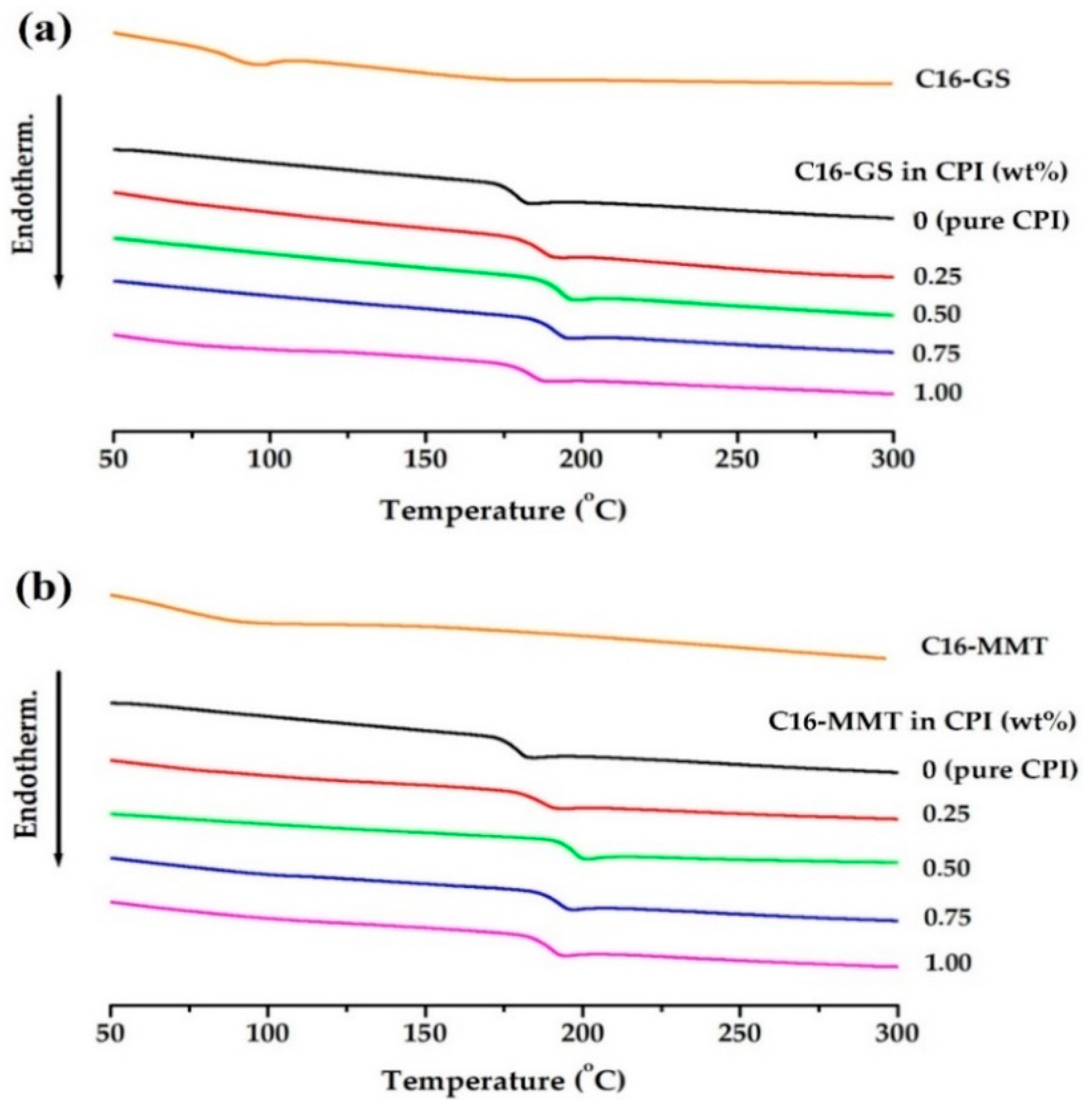
3.5. Thermal Properties
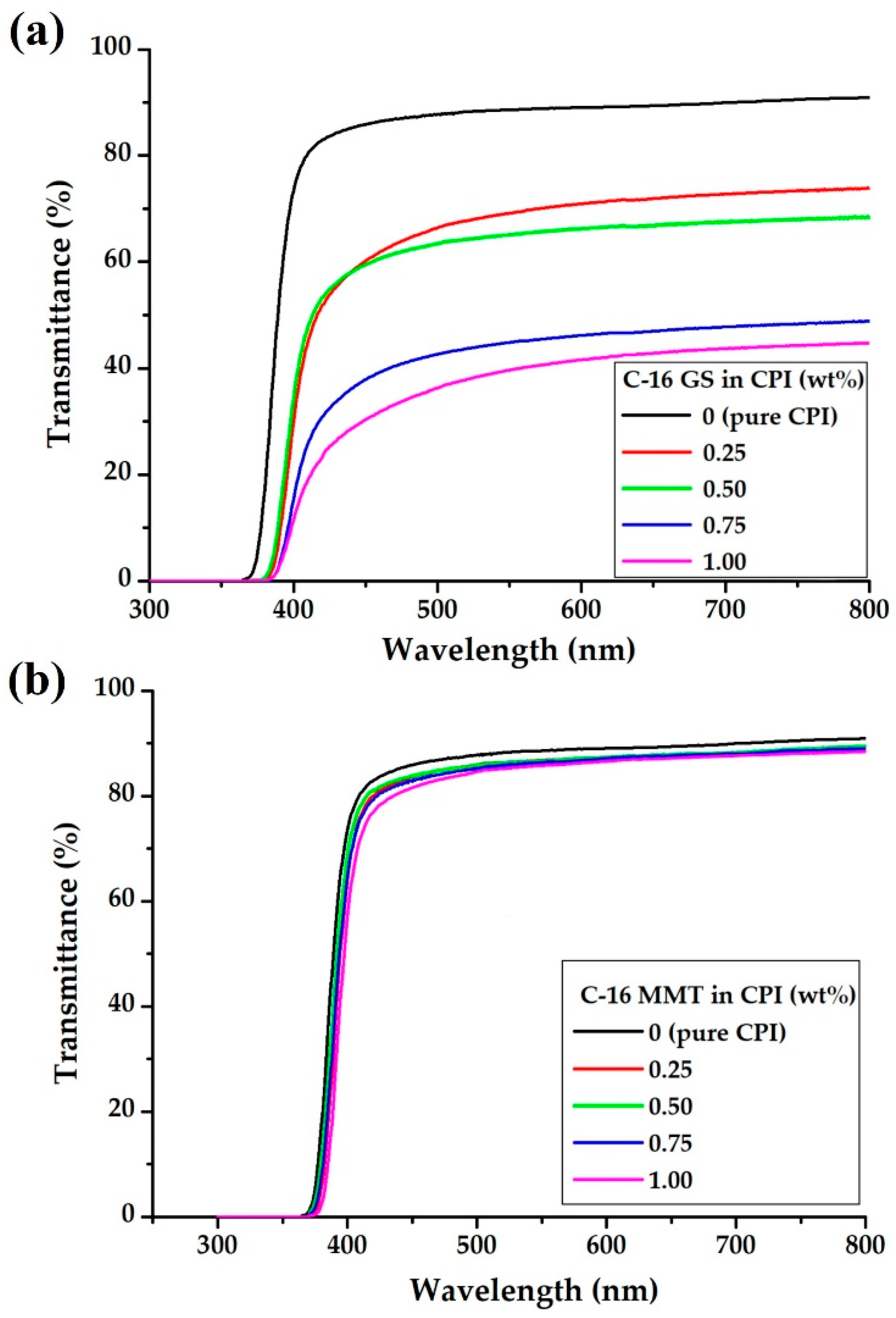
3.6. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ho, J.S.; Greenbaum, S.G. Polymer capacitor dielectrics for high temperature applications. ACS Appl. Mater. Interfaces 2018, 10, 29189–29218. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiu, H.; Ruan, K.; Zhang, Y.; Gu, J. Hierarchically multifunctional polyimide composite films with strongly enhanced thermal conductivity. Nano-Micro Lett. 2022, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-C.; MacDiarmid, A.G. Integration of polymer-dispersed liquid crystal composites with conducting polymer thin films toward the fabrication of flexible display devices. Displays 2007, 28, 101–104. [Google Scholar] [CrossRef]
- Chen, C.-J.; Yen, H.-J.; Hu, Y.-C.; Liou, G.-S. Novel programmable functional polyimides: Preparation, mechanism of CT induced memory, and ambipolar electrochromic behavior. J. Mater. Chem. C 2013, 1, 7623–7634. [Google Scholar] [CrossRef]
- Choi, M.-C.; Kim, Y.; Ha, C.-S. Polymers for flexible displays: From material selection to device applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- Nishihara, M.; Christiani, L.; Staykov, A.; Sasaki, K. Experimental and theoretical study of charge-transfer complex hybrid polyimide membranes. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 293–298. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Kim, S.D.; Lee, S.; Heo, J.; Kim, S.Y.; Chung, I.S. Soluble polyimides with trifluoromethyl pendent groups. Polymer 2013, 54, 5648–5654. [Google Scholar] [CrossRef]
- Seino, H.; Sasaki, T.; Mochizuki, A.; Ueda, M. Synthesis of fully aliphatic polyimides. High Perform. Polym. 1999, 11, 255–262. [Google Scholar] [CrossRef]
- Kim, J.-H.; Choi, M.-C.; Kim, H.; Kim, Y.; Chang, J.-H.; Han, M.; Kim, I.; Ha, C.-S. Colorless polyimide/organoclay nanocomposite substrates for flexible organic light-emitting devices. J. Nanosci. Nanotech. 2010, 10, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.X.; Wong, J.F.; Petru, M.; Hassan, A.; Nirmal, U.; Othman, N.; Ilyas, R.A. Effect of nanofillers on tribological properties of polymer nanocomposites: A review on recent development. Polymers 2021, 13, 2867. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Huh, T.H.; Lee, S.Y.; Park, S.K.; Chang, J.-H.; Lee, Y.S.; Kwark, Y.J. Homogeneous polyimide/silica nanohybrid films adapting simple polymer blending process: Polymeric silsesquiazane precursor to inorganic silica. Macromol. Res. 2018, 26, 187–193. [Google Scholar] [CrossRef]
- Shin, H.I.; Chang, J.-H. Transparent polyimide/organoclay nanocomposite films containing different diamine monomers. Polymers 2020, 12, 135. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.-S.; Wang, X.-X.; Zhang, M.; Cao, W.-Q.; Fang, X.-Y.; Yuan, J. Variable-temperature electron transport and dipole polarization turning flexible multifunctional microsensor beyond electrical and optical energy. Adv. Mater. 2020, 32, 1907156. [Google Scholar] [CrossRef]
- Cao, M.-S.; Shu, J.-C.; Wen, B.; Wang, X.-X.; Cao, W.-Q. Genetic dielectric genes inside 2D carbon-based materials with tunable electromagnetic function at elevated temperature. Small Struct. 2021, 2, 2100104. [Google Scholar] [CrossRef]
- Ibrahim, A.; Klopocinska, A.; Horvat, K.; Hamid, Z.A. Graphene-based nanocomposites: Synthesis, mechanical properties, and characterizations. Polymers 2021, 13, 2869. [Google Scholar] [CrossRef]
- Heo, C.; Moon, H.G.; Yoon, C.S.; Chang, J.-H. ABS nanocomposites films based on functionalized-graphenes sheets. J. Appl. Polym. Sci. 2012, 124, 4663–4670. [Google Scholar] [CrossRef]
- Zeng, C.; Lu, S.; Xiao, X.; Gao, J.; Pan, L.; He, Z.; Yu, J. Enhanced thermal and mechanical properties of epoxy composites by mixing noncovalently functionalized graphene sheets. Polym. Bull. 2014, 72, 453–472. [Google Scholar] [CrossRef]
- Heo, C.; Chang, J.H. Syntheses and characterizations of position specific functionalized graphenes. Polymer 2013, 37, 218–224. [Google Scholar]
- Murray, H.H. Overview-clay mineral applications. Appl. Clay Sci. 1991, 5, 379–395. [Google Scholar] [CrossRef]
- Abulyazied, D.E.; Ene, A. An Investigative Study on the Progress of Nanoclay-reinforced polymers: Preparation, Properties, and Applications: A Review. Polymers 2021, 13, 4401. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.I.; Kwark, Y.-J.; Chang, J.-H. Colorless and Transparent Copolyimides and Their Nanocomposites: Thermo-Optical Properties, Morphologies, and Gas Permeabilities. Polymers 2019, 11, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, K.; Chang, J.-H. Comparison of the properties of polyimide nanocomposites containing three different nanofillers: Organoclay, functionalized graphene, and organoclay/functionalized graphene complex. J. Comp. Mater. 2015, 49, 3031–3044. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Z.-K.; Yin, J.; Wang, X.-Y.; Qi, Z.-E. Preparation and properties of hybrids of organo-soluble polyimide and montmorillonite with various chemical surface modification methods. Polymer 1999, 40, 4407–4414. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A.; Krrauchi, T.; Kamigaito, O. Synthesis a properties of polyimide-clay hybrid. J. Polym. Sci. Part A Polym Chem. 1993, 31, 2493–2498. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2008; Chapters 2 and 4; pp. 14–95; 146–183. [Google Scholar]
- Heo, C.; Chang, J.H. Polyimide nanocomposites based on functionalized graphene sheets: Morphologies, thermal properties, and electrical and thermal conductivities. Sol. State Sci. 2013, 24, 6–14. [Google Scholar] [CrossRef]
- Chang, J.-H.; An, Y.U.; Sur, G.S. Poly(lactic acid) nanocomposites with various organoclays. I. Thermomechan-ical properties, morphology, and gas permeability. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 94–103. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Porter, D.; Metcalfe, E.; Thomas, M.J.K. Nanocomposite fire retardants—A review. Fire Mater. 2000, 24, 45–52. [Google Scholar] [CrossRef]
- Vaia, R.A.; Giannelis, E.P. Polymer melt intercalation in organically-modified layered silicates: Model predictions and experiment. Macromolecules 1997, 30, 8000–8009. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. Characterization of polymer-layered silicate (clay) nanocomposites by transmission electron microscopy and X-ray diffraction: A comparative study. J. Appl. Polym. Sci. 2003, 87, 1329–1338. [Google Scholar] [CrossRef]
- Ma, J.; Xu, J.; Ren, J.-H.; Yu, Z.-Z.; Mai, Y.-W. A new approach to polymer/montmorillo-nite nanocomposites. Polymer 2003, 44, 4619–4624. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, H.M. Enhancement of thermal, mechanical, and barrier properties of ethylene vinyl alcohol copolymer by incorporation of graphene nanosheets: Effect of functionalization of graphene oxide. High Perform. Polym. 2015, 27, 694–704. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Polybenzoxazine–montmorillonite hybrid nanocomposites: Synthesis and characterization. Polymer 2000, 41, 7083–7090. [Google Scholar] [CrossRef]
- Chang, J.-H.; Seo, B.-S.; Hwang, D.-H. An exfoliation of oganoclay in thermotropic liquid crystalline polyester nanocomposites. Polymer 2002, 43, 2969–2974. [Google Scholar] [CrossRef]
- Xu, H.; Kuo, S.-W.; Lee, J.-S.; Chang, F.-C. Preparations, thermal properties, and Tg increase mechanism of inorganic/organic hybrid polymers based on polyhedral oligomeric silsesquioxanes. Macromolecules 2002, 35, 8788–8793. [Google Scholar] [CrossRef]
- Becker, O.; Varley, R.J.; Simon, G.P. Thermal stability and water uptake of high performance epoxy layered silicate nanocomposites. Eur. Polym. J. 2004, 40, 187–195. [Google Scholar] [CrossRef]
- Zhu, J.; Uhl, F.M.; Morgan, A.B.; Wilkie, C.A. Studies on the mechanism by which the formation of nanocomposites enhances thermal stability. Chem. Mater. 2001, 13, 4649–4654. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Polymer–clay nanocomposites: Exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer 2001, 42, 807–813. [Google Scholar] [CrossRef]
- Tyan, H.-L.; Liu, Y.-C.; Wei, K.-H. Thermally and mechanically enhanced clay/polyimide nanocomposite via reactive organoclay. Chem. Mater. 1999, 11, 1942–1947. [Google Scholar] [CrossRef]
- Hsu, S.L.-C.; Wang, U.; King, J.-S.; Jeng, J.-L. Photosensitive poly(amic acid)/organoclay nanocomposites. Polymer 2003, 44, 5533–5540. [Google Scholar] [CrossRef]
- Min, U.; Kim, J.-C.; Chang, J.-H. Transparent polyimide nanocomposite films: Thermo-optical properties, morphology, and gas permeability. Polym. Eng. Sci. 2011, 51, 2143–2150. [Google Scholar] [CrossRef]






| Sample | Temperature (°C)/Time (h)/Pressure (torr) |
|---|---|
| PAA | 25/16/760 → 50/2/1 → 80/1/1 |
| CPI Hybrid | 110/0.5/1 → 140/0.5/1 → 170/0.5/1 → 195/0.8/1 → 220/0.8/1 → 235/2/1 |
| Filler in CPI (wt%) | C16-GS | C16-MMT | ||||||
|---|---|---|---|---|---|---|---|---|
| Tg (°C) | TDia (°C) | wtR600b (%) | CTE c (ppm/°C) | Tg (°C) | TDi (°C) | wtR600 (%) | CTE (ppm/°C) | |
| 0(pure CPI) | 170 | 467 | 52 | 54.7 | 170 | 467 | 52 | 54.7 |
| 0.25 | 180 | 474 | 54 | 53.2 | 180 | 508 | 56 | 49.3 |
| 0.50 | 185 | 492 | 55 | 52.0 | 191 | 511 | 56 | 49.0 |
| 0.75 | 181 | 480 | 52 | 54.2 | 185 | 504 | 55 | 49.0 |
| 1.00 | 177 | 467 | 54 | 52.2 | 183 | 483 | 55 | 47.1 |
| Filler in CPI (wt%) | C16-GS | C16-MMT | ||||||
|---|---|---|---|---|---|---|---|---|
| Thickness (μm) | λoa (nm) | 500 nmtrans (%) | YI b | Thickness (μm) | λo (nm) | 500 nmtrans (%) | YI | |
| 0(pure CPI) | 54 | 368 | 87 | 2 | 54 | 368 | 87 | 2 |
| 0.25 | 55 | 380 | 66 | 10 | 55 | 368 | 86 | 1 |
| 0.50 | 55 | 380 | 63 | 9 | 54 | 370 | 86 | 2 |
| 0.75 | 58 | 383 | 43 | 9 | 53 | 372 | 85 | 2 |
| 1.00 | 57 | 385 | 36 | 15 | 54 | 377 | 85 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Choi, M.Y.; Kwac, L.K.; Kim, H.G.; Chang, J.-H. Comparison of Properties of Colorless and Transparent Polyimide Nanocomposites Containing Chemically Modified Nanofillers: Functionalized-Graphene and Organoclay. Polymers 2022, 14, 2469. https://doi.org/10.3390/polym14122469
Lee SJ, Choi MY, Kwac LK, Kim HG, Chang J-H. Comparison of Properties of Colorless and Transparent Polyimide Nanocomposites Containing Chemically Modified Nanofillers: Functionalized-Graphene and Organoclay. Polymers. 2022; 14(12):2469. https://doi.org/10.3390/polym14122469
Chicago/Turabian StyleLee, Seon Ju, Moon Young Choi, Lee Ku Kwac, Hong Gun Kim, and Jin-Hae Chang. 2022. "Comparison of Properties of Colorless and Transparent Polyimide Nanocomposites Containing Chemically Modified Nanofillers: Functionalized-Graphene and Organoclay" Polymers 14, no. 12: 2469. https://doi.org/10.3390/polym14122469
APA StyleLee, S. J., Choi, M. Y., Kwac, L. K., Kim, H. G., & Chang, J.-H. (2022). Comparison of Properties of Colorless and Transparent Polyimide Nanocomposites Containing Chemically Modified Nanofillers: Functionalized-Graphene and Organoclay. Polymers, 14(12), 2469. https://doi.org/10.3390/polym14122469