Ultralong-Lived Up-Conversional Room-Temperature Afterglow Materials with a Polyvinyl Alcohol Substrate
Abstract
:1. Introduction
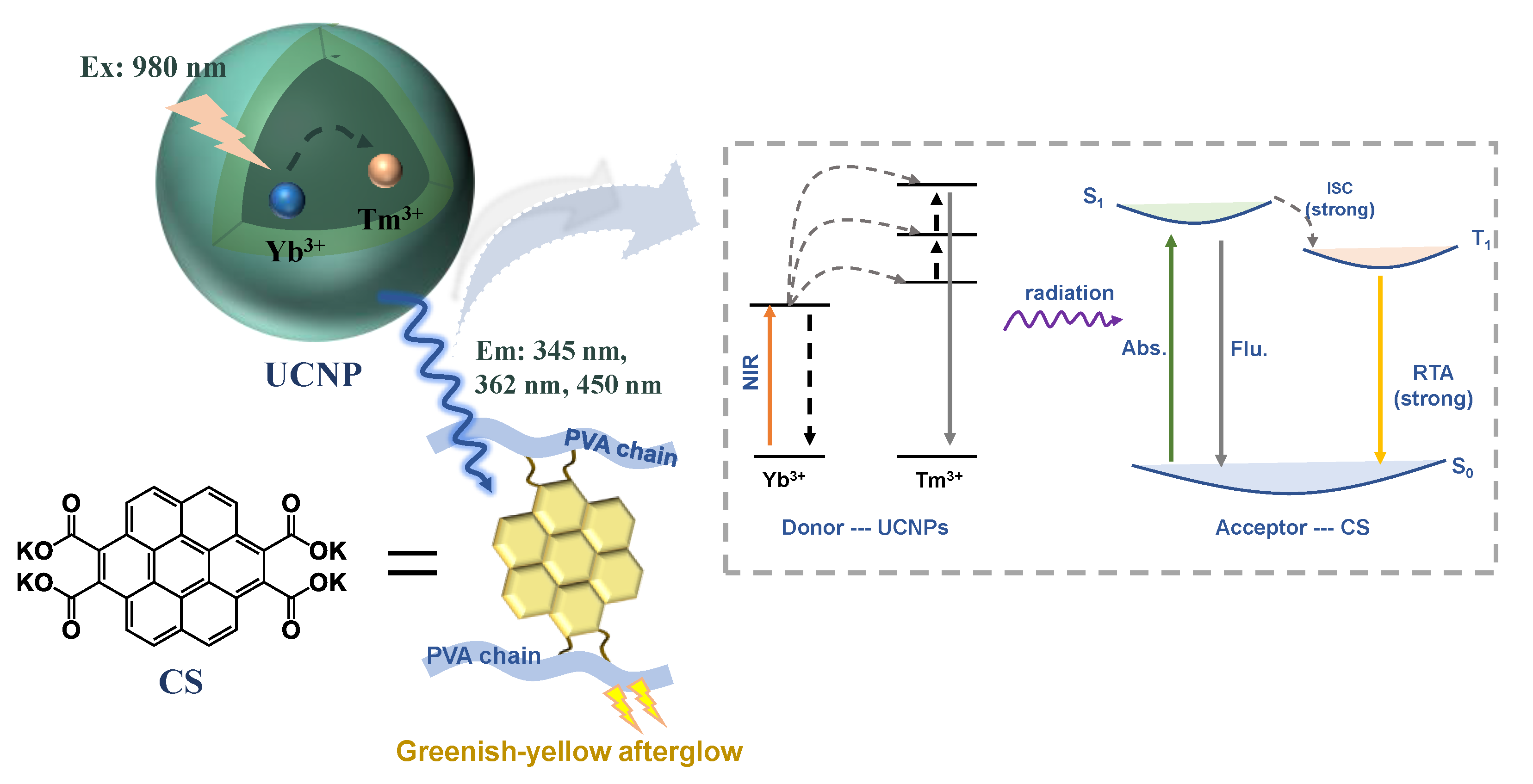
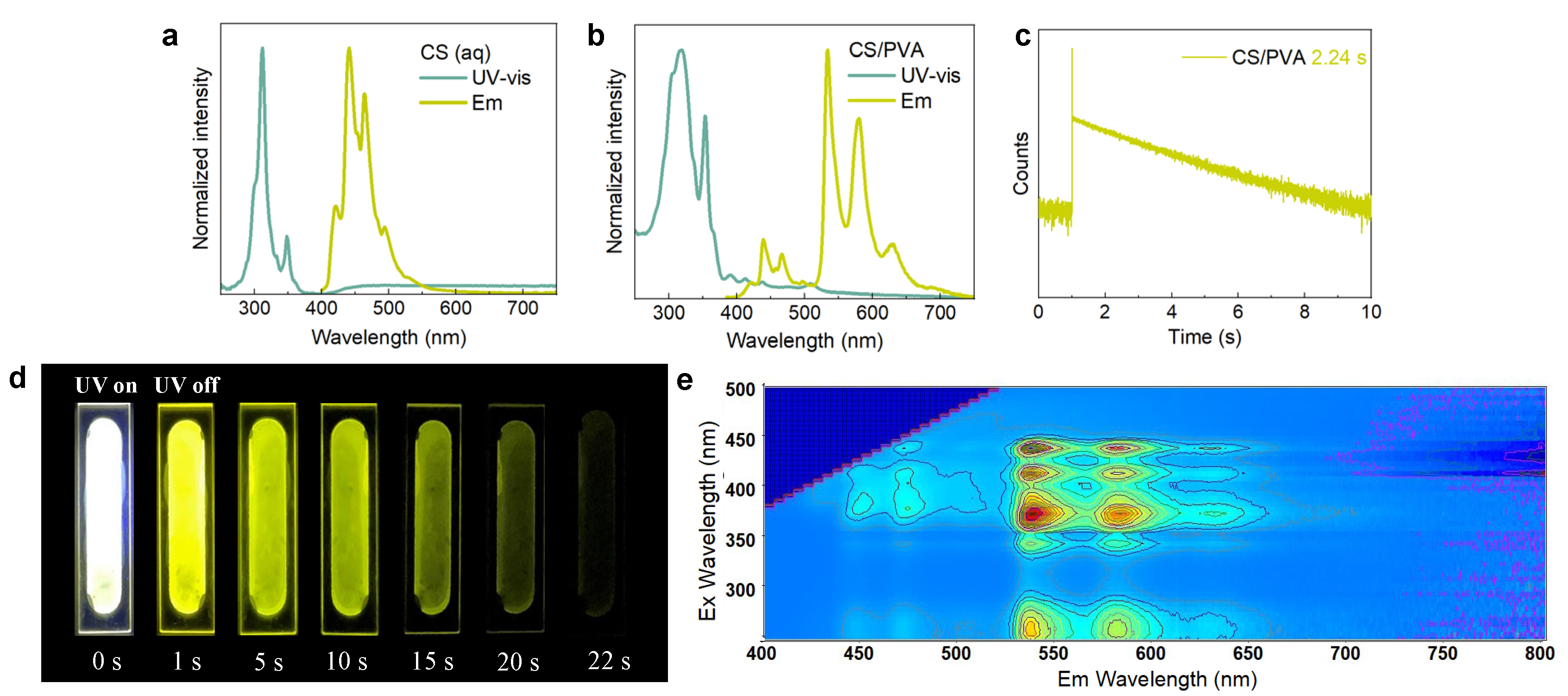
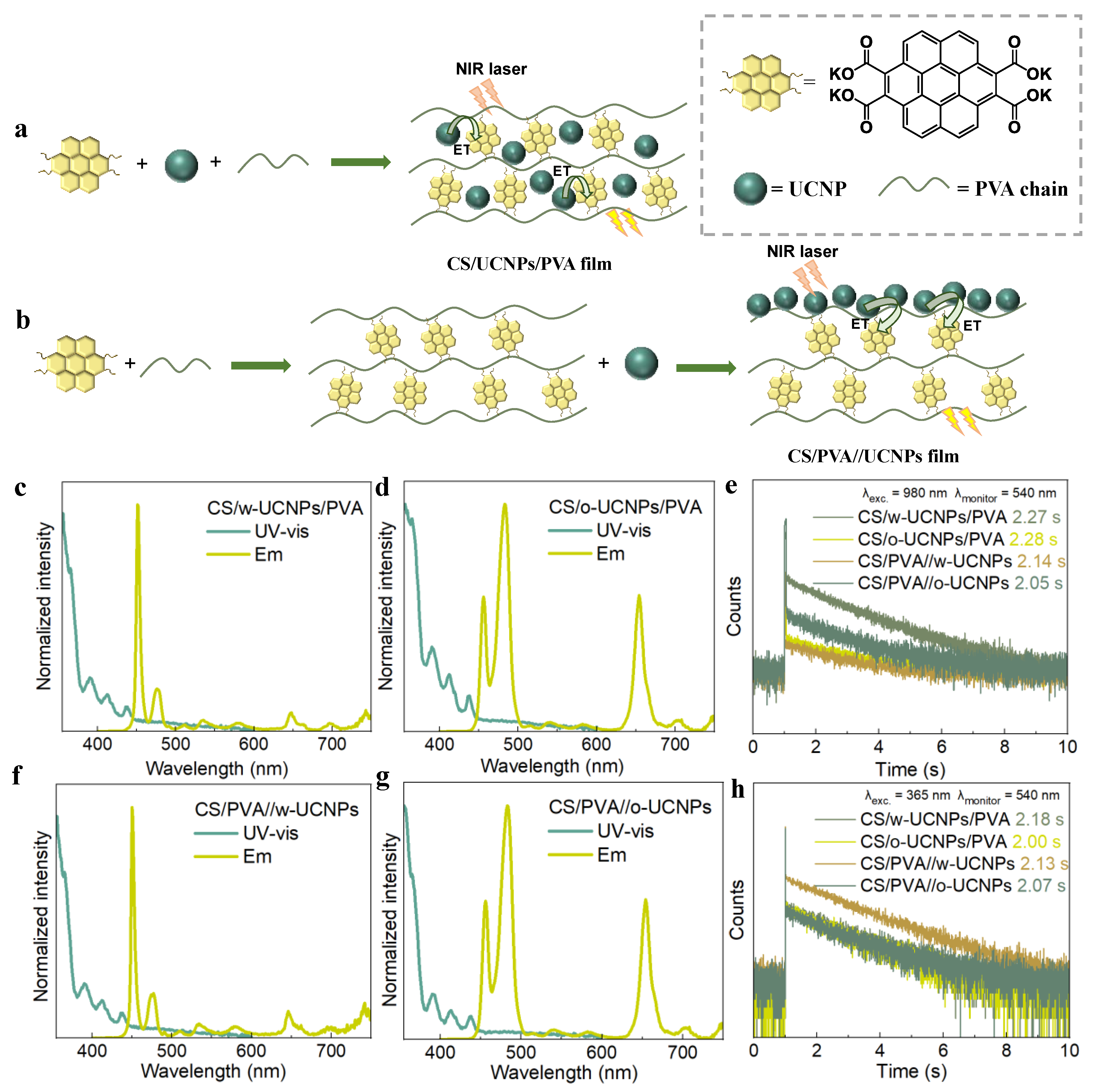
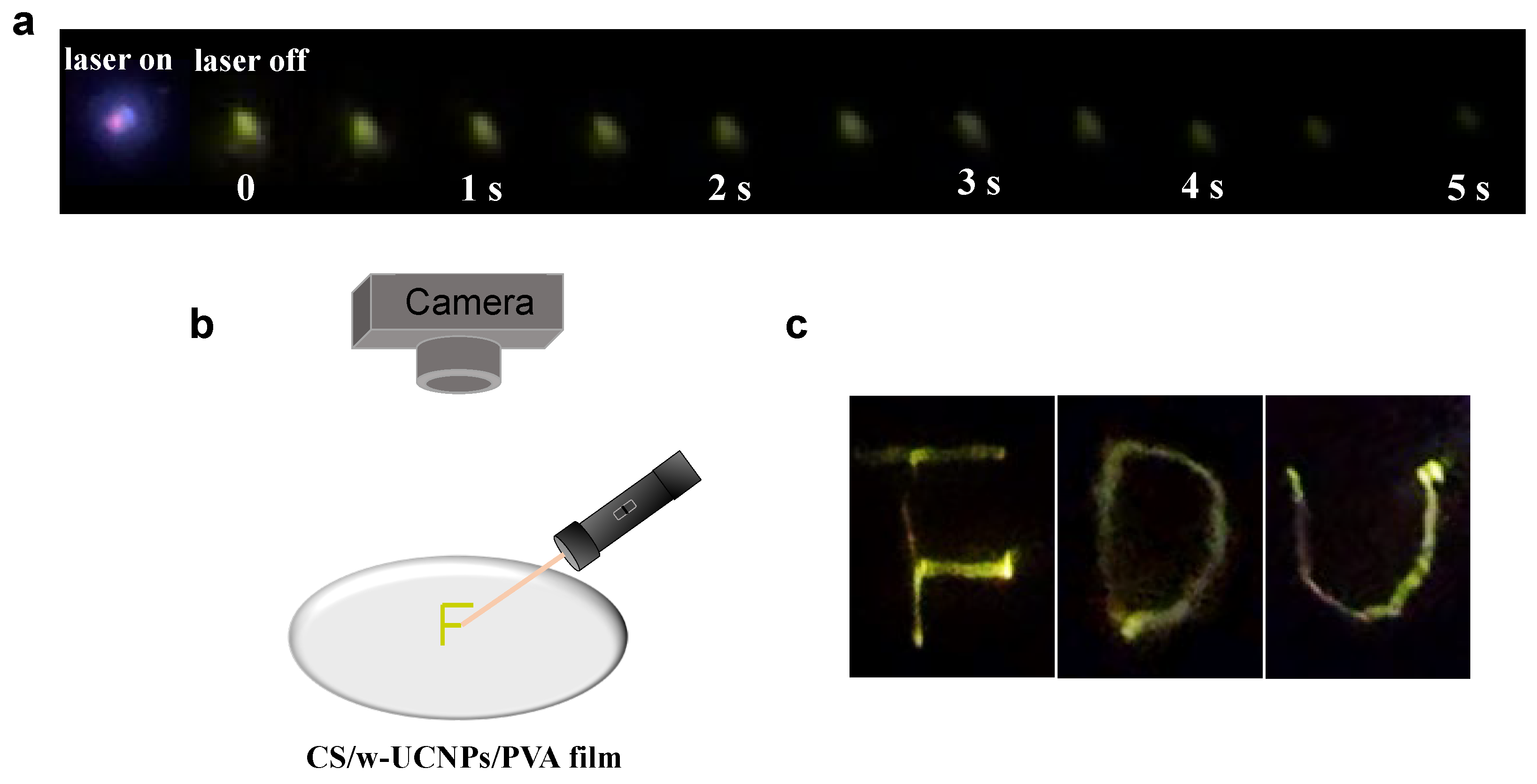
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kabe, R.; Adachi, C. Organic long persistent luminescence. Nature 2017, 550, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; He, Z.; Tang, B.Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 2020, 5, 869–885. [Google Scholar] [CrossRef]
- Baryshnikov, G.; Minaev, B.; Agren, H. Theory and Calculation of the Phosphorescence Phenomenon. Chem. Rev. 2017, 117, 6500–6537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, X.; Wu, H.; Zhu, L.; Zhao, Y.; Tian, H. Molecular Engineering for Metal-Free Amorphous Materials with Room-Temperature Phosphorescence. Angew. Chem. Int. Ed. Engl. 2020, 59, 11206–11216. [Google Scholar] [CrossRef]
- Gan, N.; Shi, H.; An, Z.; Huang, W. Recent Advances in Polymer-Based Metal-Free Room-Temperature Phosphorescent Materials. Adv. Funct. Mater. 2018, 28, 1802657. [Google Scholar] [CrossRef]
- Xu, S.; Chen, R.; Zheng, C.; Huang, W. Excited State Modulation for Organic Afterglow: Materials and Applications. Adv. Mater. 2016, 28, 9920–9940. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A new long phosphorescent phosphor with high brightness, SrAl2O4:Eu2+, Dy3+. J. Electrochem. Soc. 1996, 144, L243–L245. [Google Scholar]
- Wang, P.; Zheng, D.; Liu, S.; Luo, M.; Li, J.; Shen, S.; Li, S.; Zhu, L.; Chen, Z. Producing long afterglow by cellulose confinement effect: A wood-inspired design for sustainable phosphorescent materials. Carbon 2021, 171, 946–952. [Google Scholar] [CrossRef]
- Bredas, J.L.; Sargent, E.H.; Scholes, G.D. Photovoltaic concepts inspired by coherence effects in photosynthetic systems. Nat. Mater. 2016, 16, 35–44. [Google Scholar] [CrossRef]
- Chen-Wen, F.; Frank, S.; Sheppeck, D.C. Long-Afterglow Electroluminescent Lamp. U.S. Patent Application No. 11/163,925, 3 May 2007. [Google Scholar]
- Poulose, A.M.; Anis, A.; Shaikh, H.; Alhamidi, A.; Siva Kumar, N.; Elnour, A.Y.; Al-Zahrani, S.M. Strontium Aluminate-Based Long Afterglow PP Composites: Phosphorescence, Thermal, and Mechanical Characteristics. Polymers 2021, 13, 1373. [Google Scholar] [CrossRef]
- Pan, Z.; Lu, Y.Y.; Liu, F. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 2011, 11, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Arunkumar, P.; Kim, B.Y.; Unithrattil, S.; Kim, E.; Moon, S.H.; Hyun, J.Y.; Kim, K.H.; Lee, D.; Lee, J.S.; et al. A zero-thermal-quenching phosphor. Nat. Mater. 2017, 16, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Maldiney, T.; Bessiere, A.; Seguin, J.; Teston, E.; Sharma, S.K.; Viana, B.; Bos, A.J.; Dorenbos, P.; Bessodes, M.; Gourier, D.; et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 2014, 13, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S. Recent Advances in Materials with Room-Temperature Phosphorescence: Photophysics for Triplet Exciton Stabilization. Adv. Opt. Mater. 2017, 5, 1700116. [Google Scholar] [CrossRef]
- Zhen, X.; Tao, Y.; An, Z.; Chen, P.; Xu, C.; Chen, R.; Huang, W.; Pu, K. Ultralong Phosphorescence of Water-Soluble Organic Nanoparticles for In Vivo Afterglow Imaging. Adv. Mater. 2017, 29, 1606665. [Google Scholar] [CrossRef]
- Kabe, R.; Notsuka, N.; Yoshida, K.; Adachi, C. Afterglow Organic Light-Emitting Diode. Adv. Mater. 2016, 28, 655–660. [Google Scholar] [CrossRef]
- Xu, W.W.; Chen, Y.; Lu, Y.L.; Qin, Y.X.; Zhang, H.; Xu, X.; Liu, Y. Tunable Second-Level Room-Temperature Phosphorescence of Solid Supramolecules between Acrylamide-Phenylpyridium Copolymers and Cucurbit[7]uril. Angew. Chem. Int. Ed. Engl. 2022, 61, e202115265. [Google Scholar] [CrossRef]
- An, Z.; Zheng, C.; Tao, Y.; Chen, R.; Shi, H.; Chen, T.; Wang, Z.; Li, H.; Deng, R.; Liu, X.; et al. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 2015, 14, 685–690. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, Y.; Yin, L.; Hang, C.; Li, X.; Agren, H.; Yi, T.; Zhang, Q.; Zhu, L. Helical Self-Assembly-Induced Singlet-Triplet Emissive Switching in a Mechanically Sensitive System. J. Am. Chem. Soc. 2017, 139, 785–791. [Google Scholar] [CrossRef]
- Luo, M.; Li, X.; Ding, L.; Baryshnikov, G.; Shen, S.; Zhu, M.; Zhou, L.; Zhang, M.; Lu, J.; Agren, H.; et al. Integrating Time-Resolved Imaging Information by Single-Luminophore Dual Thermally Activated Delayed Fluorescence. Angew. Chem. Int. Ed. Engl. 2020, 59, 17018–17025. [Google Scholar] [CrossRef]
- Chen, H.; Yao, X.; Ma, X.; Tian, H. Amorphous, Efficient, Room-Temperature Phosphorescent Metal-Free Polymers and Their Applications as Encryption Ink. Adv. Opt. Mater. 2016, 4, 1397–1401. [Google Scholar] [CrossRef]
- Ma, X.; Xu, C.; Wang, J.; Tian, H. Amorphous Pure Organic Polymers for Heavy-Atom-Free Efficient Room-Temperature Phosphorescence Emission. Angew. Chem. Int. Ed. Engl. 2018, 57, 10854–10858. [Google Scholar] [CrossRef] [PubMed]
- Gahlaut, R.; Joshi, H.C.; Joshi, N.K.; Pandey, N.; Arora, P.; Rautela, R.; Suyal, K.; Pant, S. Luminescence characteristics and room temperature phosphorescence of naphthoic acids in polymers. J. Lumin. 2013, 138, 122–128. [Google Scholar] [CrossRef]
- Kuila, S.; George, S.J. Phosphorescence Energy Transfer: Ambient Afterglow Fluorescence from Water-Processable and Purely Organic Dyes via Delayed Sensitization. Angew. Chem. Int. Ed. Engl. 2020, 59, 9393–9397. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular-Level Dispersion of Graphene into Poly(vinyl alcohol) and Effective Reinforcement of their Nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Kuila, S.; Garain, S.; Bandi, S.; George, S.J. All-Organic, Temporally Pure White Afterglow in Amorphous Films Using Complementary Blue and Greenish—Yellow Ultralong Room Temperature Phosphors. Adv. Funct. Mater. 2020, 30, 2003693. [Google Scholar] [CrossRef]
- Lee, D.; Bolton, O.; Kim, B.C.; Youk, J.H.; Takayama, S.; Kim, J. Room temperature phosphorescence of metal-free organic materials in amorphous polymer matrices. J. Am. Chem. Soc. 2013, 135, 6325–6329. [Google Scholar] [CrossRef]
- Zheng, Y.; Wei, H.; Liang, P.; Xu, X.; Zhang, X.; Li, H.; Zhang, C.; Hu, C.; Zhang, X.; Lei, B.; et al. Near-Infrared-Excited Multicolor Afterglow in Carbon Dots-Based Room-Temperature Afterglow Materials. Angew. Chem. Int. Ed. Engl. 2021, 60, 22253–22259. [Google Scholar] [CrossRef]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhao, M.; Zhao, C.X.; Zhang, F. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar] [CrossRef]
- Kobayashi, H.; Nishimura, M. Method and Apparatus for Testing near Infrared-Photoimmunotherapy Treatment. U.S. Patent 17/253,671, 2021. [Google Scholar]
- Dang, Q.; Jiang, Y.; Wang, J.; Wang, J.; Zhang, Q.; Zhang, M.; Luo, S.; Xie, Y.; Pu, K.; Li, Q.; et al. Room-Temperature Phosphorescence Resonance Energy Transfer for Construction of Near-Infrared Afterglow Imaging Agents. Adv. Mater. 2020, 32, e2006752. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, K.; Du, J.; Zheng, L.; Li, Y.; Li, Z.; Lin, H. Green and Near-Infrared Dual-Mode Afterglow of Carbon Dots and Their Applications for Confidential Information Readout. Nanomicro. Lett. 2021, 13, 198. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Abbineni, G.; Clevenger, A.; Mao, C.; Xu, S. Upconversion nanoparticles: Synthesis, surface modification and biological applications. Nanomedicine 2011, 7, 710–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Wang, P.; Zhao, M.; Liu, L.; Zhou, L.; Li, B.; Albaqami, F.H.; El-Toni, A.M.; Li, X.; Xie, Y.; et al. Near-infrared rechargeable “optical battery” implant for irradiation-free photodynamic therapy. Biomaterials 2018, 163, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; George, S.J. Novel coronene-naphthalene dimide-based donor-acceptor pair for tunable charge-transfer nanostructures. Chem. Asian J. 2014, 9, 2427–2431. [Google Scholar] [CrossRef]
- Alibert-Fouet, S.; Seguy, I.; Bobo, J.F.; Destruel, P.; Bock, H. Liquid-crystalline and electron-deficient coronene oligocarboxylic esters and imides by twofold benzogenic Diels-Alder reactions on perylenes. Chemistry 2007, 13, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, P.; Lu, Y.; Wang, R.; Zhou, L.; Zheng, X.; Li, X.; Piper, J.A.; Zhang, F. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat. Nanotechnol. 2018, 13, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ma, Z.; Zhu, S.; Yue, J.; Zhang, M.; Antaris, A.L.; Yuan, J.; Cui, R.; Wan, H.; Zhou, Y.; et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat. Commun. 2017, 8, 737. [Google Scholar] [CrossRef] [Green Version]
| Abbreviations | Full Name | Abbreviations | Full Name |
|---|---|---|---|
| RTA | Room-temperature afterglow | UV light | Ultraviolet light |
| ISC | Intersystem crossing | NIR | Near infrared |
| S0 | The ground state | CS/PVA | Coronene tetra-carboxylate salt uniformly dispersed in the PVA matrix |
| S1 | The singlet state | w-UCNPs | Water-soluble up-conversion nanoparticles |
| T1 | The triplet state | o-UCNPs | Oil-soluble up-conversion nanoparticles |
| PMMA | Polymethyl methacrylate | CS/UCNPs/PVA | UCNPs mixed with CS/PVA hybrids by doping method |
| PVA | Polyvinyl alcohol | CS/PVA//UCNPs | UCNPs mixed with CS/PVA hybrids by smearing method |
| CS | Coronene tetra-carboxylate salt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Wu, B.; Shi, B.; Zhu, X.; Shen, S.; Zhu, L. Ultralong-Lived Up-Conversional Room-Temperature Afterglow Materials with a Polyvinyl Alcohol Substrate. Polymers 2022, 14, 2414. https://doi.org/10.3390/polym14122414
Zhou L, Wu B, Shi B, Zhu X, Shen S, Zhu L. Ultralong-Lived Up-Conversional Room-Temperature Afterglow Materials with a Polyvinyl Alcohol Substrate. Polymers. 2022; 14(12):2414. https://doi.org/10.3390/polym14122414
Chicago/Turabian StyleZhou, Lulu, Bin Wu, Ben Shi, Xinyan Zhu, Shen Shen, and Liangliang Zhu. 2022. "Ultralong-Lived Up-Conversional Room-Temperature Afterglow Materials with a Polyvinyl Alcohol Substrate" Polymers 14, no. 12: 2414. https://doi.org/10.3390/polym14122414
APA StyleZhou, L., Wu, B., Shi, B., Zhu, X., Shen, S., & Zhu, L. (2022). Ultralong-Lived Up-Conversional Room-Temperature Afterglow Materials with a Polyvinyl Alcohol Substrate. Polymers, 14(12), 2414. https://doi.org/10.3390/polym14122414






